Abstract
We applied the dopaminergic (DA) neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to the guinea pig cochlear perilymph. Immunolabeling of lateral olivocochlear (LOC) neurons using antibodies against synaptophysin was reduced after the MPTP treatment. In contrast, labeling of the medial olivocochlear innervation remained intact. As after brainstem lesions of the lateral superior olive (LSO), the site of origin of the LOC neurons, the main effect of disrupting LOC innervation of the cochlea via MPTP was a depression of the amplitude of the compound action potential (CAP). CAP amplitude depression was similar to that produced by LSO lesions. Latency of the N1 component of the CAP, and distortion product otoacoustic emission amplitude and adaptation were unchanged by the MPTP treatment. This technique for selectively lesioning descending LOC efferents provides a new opportunity for examining LOC modulation of afferent activity and behavioral measures of perception.
Keywords: MPTP, olivocochlear efferent, compound action potential
Introduction
The lateral olivocochlear (LOC) pathway originates in, or near, the lateral superior olive (LSO) and terminates in the ipsilateral cochlea at chemically complex synapses on dendrites of the auditory nerve (AN), and, sometimes, inner hair cells (IHCs) (for reviews, see Warr 1992; Warr et al. 1986; Eybalin 1993; Puel 1995; Le Prell et al. 2001). Given the difficulties in selectively disrupting or stimulating the LOC pathway (for review, see Le Prell et al. 2003a), little was known about LOC function until recently. One early hypothesis was that LOC efferents adjust spontaneous and sound-driven AN fiber (ANF) activity (Liberman 1990; Walsh et al. 1998; Zheng et al. 1999). Confidence in putative LOC effects was limited by simultaneous disruption of medial olivocochlear (MOC) neurons during the knife cuts to disrupt LOC neurons however. Recently, we directly demonstrated LOC modulation of AN activity by disrupting LOC neurons using LSO lesions (Le Prell et al. 2003b). Other evidence that LOC neurons modulate AN activity comes from Groff and Liberman (2003), who report electrical current at some locations within the LSO (or the ventrolateral inferior colliculus) modulates AN response amplitude. Finally, the data of McMahon et al. (2004), who cooled the cochlear nucleus, suggest LOC modulation of ANFs. The LOC modulation of AN activity may also be involved in the protective phenomena termed “conditioning” (see Niu and Canlon 2002; Niu et al. 2004).
Immunocytochemical evidence indicates LOC neurons contain acetylcholine (ACh), γ-aminobutyric acid (GABA), dopamine (DA), dynorphin (dyn), enkephalin (enk), and calcitonin-gene-related peptide (CGRP). Various neurotransmitters have been colocalized in cell bodies in the LSO (Altschuler et al. 1983, 1984, 1986; Abou-Madi et al. 1987; Altschuler et al. 1988; Safieddine et al. 1997) and LOC terminals (Altschuler et al. 1985; Safieddine and Eybalin 1992). While some suggest chemically distinct LOC subpopulations (Satake and Liberman 1996), others believe most LOC efferents contain all six putative LOC neurotransmitters (Safieddine et al. 1997). Consistent with broad transmitter colocalization, dopaminergic LOC neurons originate from the medial limb of the LSO (Safieddine et al. 1997; Mulders and Robertson 2004) as do neurons that show glutamate decarboxylase, CGRP, and choline acetyltransferase immunoreactivity (Safieddine et al. 1997). The medial limb of the LSO projects to the hook region and basal and second turns of the cochlea (following Robertson et al. 1987).
A neurotoxin that damages neurons containing any single LOC transmitter might produce a virtually complete LOC degeneration if LOC transmitters are colocalized. Infusing the cholinotoxin ethylcholine mustard aziridinium ion (AF64A) into the chinchilla middle ear widely disrupted LOC neurons (Smith et al. 1989; Morley et al. 1991; Smith and Mount 1993). A cholinotoxin does not selectively disrupt LOC efferents, however, as ACh is the primary MOC neurotransmitter. To disrupt LOC innervation without damaging MOC neurons, the neurotoxin should target a transmitter not contained by the MOC neurons; one such substance is DA.
The two most commonly used DA neurotoxins are 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). 6-OHDA damages catecholamine-producing neurons in the guinea pig cochlea (Eybalin et al. 1993; d’Aldin et al. 1995a; Niu and Canlon 2002). MPTP is similarly toxic to DA neurons (see Mikkelsen et al. 1999; Da Cunha et al. 2001; Petroske et al. 2001; Sedelis et al. 2001). We disrupted LOC innervation by applying MPTP to the cochlear perilymph. Synaptophysin immunolabeling revealed a reduction in LOC innervation with no disruption of MOC innervation. The functional effect of this selective loss of LOC efferents was depressed amplitude of the sound-evoked whole-nerve compound action potential (CAP). Functional measures that reflect the status of outer hair cells (OHCs) and the strength of the MOC reflex were not disrupted by MPTP.
Methods
Subjects
Male and female guinea pigs (Elm Hill Breeding Labs, Chelmsford, MA) were used in these experiments. Functional measures included CAP and distortion product otoacoustic emissions (DPOAE; amplitude and adaptation). Tissues were harvested from a subset of these animals, as well as a small number of additional animals that did not undergo functional testing. All tissues were harvested 45 min post-MPTP. Animals were maintained with free access to food (Guinea Pig Chow, PMI Nutrition International Inc., Brentwood, MO) and water. The animal care program was AALAC-accredited. Husbandry met or exceeded all applicable standards, including the Guide for the Use and Care of Laboratory Animals, prepared by the National Research Council (1996). The University Committee on Use and Care of Animals at the University of Michigan approved all animal care and testing protocols.
Apparatus and procedures
CAP
CAP was recorded in anesthetized (108 mg/kg ketamine, 14 mg/kg xylazine) animals (N = 6) as described in Le Prell et al. (2003b). During the experiments, the tympanic membranes were examined, a tracheal tube inserted, and a platinum–iridium wire ball electrode (diameter = 0.2–0.25 mm) was placed through the wall of the cochlea into scala tympani from a postauricular surgical approach. A ball of silastic 0.5 mm distal to the end of the electrode prevented overinsertion of the electrode and loss of cochlear perilymph (as in Le Prell et al. 2004b). CAP was assessed immediately after securing the electrode in place, 30 min after round window application of an artificial perilymph solution (145 mM NaCl, 2.7 mM KCl, 2.0 mM MgSO4, 1.2 mM CaCl2, 5.0 mM HEPES; pH = 7.40, osmolality = 280–285 mosM), and 30 min after applying 50 mM MPTP (dissolved in artificial perilymph, pH adjusted to 7.4 ± 0.02) to the round window membrane. A subset of the animals was tested at additional longer post-MPTP time points in 30-min intervals (60 min: N = 2; 180 min: N = 1) to verify that the effects of MPTP treatment do not change within this temporal window. All round window applications were approximately 6 μl, and the middle ear was carefully dried prior to each CAP test.
Acoustic stimuli were generated using Tucker–Davis Technology (TDT; Alachua, FL) System II/System III hardware and SigGen 3.2 software. Signals were converted to analog (DA1), filtered (FT6-2, Fc = 40 kHz), attenuated (PA5), and presented using a 200-Ω transducer (Beyer Dynamic, Farmingdale, NY) coupled to the animals’ ear canal via vinyl tubing. CAP input–output functions were determined for brief pure-tone stimuli (2–18 kHz; 2-kHz increments) presented at levels ranging from 0 to 100 dB SPL in 5-dB increments (5-ms duration, 0.5-ms rise–fall; 10/s). Evoked potentials were filtered (300–3,000 Hz) and amplified (1,000×) using in-house constructed equipment. BioSig 3.2 (TDT) was used to average 25 presentations within each frequency/level combination. CAP threshold was defined using linear interpolation to determine the sound level needed to produce a 10-μV response.
DPOAE adaptation
Animals (N = 4) were anesthetized using 1.5 g/kg urethane, then paralyzed (1.25 mg/kg tubocurarine, i.m., redosed at 2-h intervals) and artificially respirated while body temperature was maintained at 38 ± 1°C. To verify that a surgical depth of anesthesia was maintained, heart rate and blood pressure were continually monitored throughout the experiment using a digital pulse oximeter (SurgiVet Inc., Waukesha, WI).
A constant microphone position was maintained throughout drug delivery experiments. In a single animal, we compared pretreatment baseline adaptation to measures obtained after artificial perilymph application to verify that artificial perilymph applied to the round window membrane does not influence DPOAE adaptation. For all other subjects, baseline DPOAE adaptation was assessed after applying artificial perilymph to the round window membrane. In one animal, we applied three repeated treatments of artificial perilymph to verify that repeated delivery of fluids to the round window membrane did not influence DPOAE adaptation. All subjects had strong (>20 dB) baseline adaptation. We then applied 50 mM MPTP to the round window membrane (one application per animal) and reassessed DPOAE adaptation immediately as well as 30 min post-MPTP application. After confirming that MPTP did not disrupt DPOAE adaptation, we cut the OCB using a lateral brainstem cut in one animal (as in Rajan 1995; see also Liberman et al. 1996). In a second animal, MOC function was disrupted, as in Brown et al. (1969), by slow injection of strychnine hydrochloride (0.15 mg/kg, in 2.0 ml artificial perilymph solution) into the jugular vein. Strychnine is a potent antagonist of MOC function (Desmedt and Monaco 1961; Brown et al. 1969; Bobbin and Konishi 1974; Desmedt and Robertson 1975; Rajan 1988; Kujawa et al. 1992, 1993, 1994; Sridhar et al. 1995; Dolan et al. 1999; Ota and Dolan 2000).
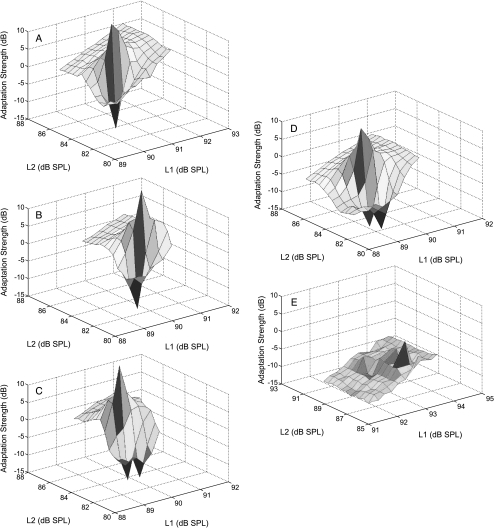
Onset adaptation of the cubic distortion product was measured, as in Halsey et al. (2004), by using procedures modified from those of Kujawa and Liberman (2001). Our primary tone frequencies (F1, F2) were fixed at 8 kHz (F1) and 9.6 kHz (F2); the cubic distortion product (2F1 − F2) was 6.4 kHz. F1 and F2 levels (L1, L2) were initially set to approximately 92 dB SPL; L2 was then systematically decreased over a 12-dB range in 1-dB steps. This procedure was repeated for at least six levels of F1, with F1 decreasing in 1-dB steps, until the levels producing maximum DPOAE adaptation were determined for both positive and negative deflections. To more finely resolve changes in DPOAE adaptation as a consequence of F1 and F2 levels, additional DPOAE tests were conducted with L1 and L2 level increments changing in 0.4-dB increments over at least six levels of F1, with 12 F2 levels presented for each level of F1. A MatLab program was used to control stimulus generation (TDT hardware) and presentation (Beyer sound drivers), as well as data collection.
As in Le Prell et al. (2003b), primary tones were 1 s in duration, with a 1.5-s pause between presentations. Sound pressure levels for F1, F2, and the DPOAE were determined during each level series using Fourier transform of the microphone input (Etymotic Research, ER-10B+ Low Noise Microphone). For each level combination, responses to four stimulus presentations were collected and averaged. DPOAE amplitude was sampled at 50-ms intervals during the 1-s primary tone duration. If standard deviations exceeded 2 dB at any time point, the data were excluded and the level combination was repeated. Adaptation of the DPOAE response was defined as the difference between DPOAE amplitude at the onset of the primary tones and the steady-state amplitude of the DPOAE (defined as the average DPOAE amplitude during the final four time points).
DPOAE, amplitude
Animals (N = 4) were anesthetized (40 mg/kg ketamine, 10 mg/kg xylazine), and the amplitude of the cubic distortion product was measured as in Le Prell et al. (2004b). The primary tones were centered at 8, 12, and 16 kHz and spaced such that F2 = 1.2 × F1. The frequency of the distortion product was equal to 2F1 − F2. Thus when the primary tones were centered at 16 kHz, F1 was 14.6 kHz, F2 was 17.4 kHz, and the DPOAE was 11.7 kHz. Initially, the level of F1 was fixed at 80 dB SPL and the level of F2 was adjusted to be 10 dB quieter. The 10-dB difference in F1 and F2 sound levels was maintained as the level of F1 was decreased in 5-dB steps, to a minimum of 25 dB SPL. DPOAE amplitude and noise floor amplitude were measured relative to F1.
Histology
At the conclusion of the electrophysiological testing, i.e., 45 min following the delivery of MPTP to the round window membrane, animals were deeply anesthetized with an overdose of sodium pentobarbital and decapitated. The temporal bones were quickly removed, dissected open at the round window and the apex, and gently perfused with 4% paraformaldehyde in phosphate buffer. Immunolabeling with antisynaptophysin mouse monoclonal antibody (1:10 dilution; IGN Pharmaceuticals, Inc.) was conducted using procedures modeled after those of Burgess et al. (1997; as in Le Prell et al. 2003b). The tissues were carefully dissected for surface preparations and mounted on glass slides. Some tissues were lost due to trauma associated with the initial gross dissection; immunolabeling was quantified in all available tissues.
For each animal, we photographed three to six regions from each available cochlear turn at two different focal planes. First, we imaged the focal plane in which LOC innervation was the densest. We then imaged MOC immunolabeling by adjusting the focal plane until MOC puncta at the bases of the OHCs were in focus. In general, within the single focal plane selected, the puncta corresponding to OHC rows 1 and 2 were the most sharply focused and the sparser MOC innervation corresponding to OHC row 3 was less sharply focused but nonetheless clearly discernable. We quantified LOC and MOC innervation using Metamorph image analysis software (version 4.6r9, Universal Imaging Corporation) as in Le Prell et al. (2003b). The LOC surface area was assessed within a 48.83 × 16.56-μm segment (total area = 800.723 μm2) from each digitized image. The MOC immunolabeled area was assessed within a larger 76.71 × 38.83-μm segment (total area = 2,978.96 μm2) in order to capture all three rows of MOC puncta. For both LOC and MOC puncta, we used a variable color/intensity exclusion criterion within Metamorph to measure labeling within each of the three to six sections imaged for each cochlear turn in each animal. To obtain final estimates of LOC and MOC labeling, we averaged the results from all sampled LOC or MOC regions for each animal from each cochlear turn, prior to averaging across animal data.
Results
Immunocytochemistry
MPTP treatment resulted in a striking reduction in LOC terminals (38% decrease) with little or no effect on MOC terminals (10% increase). Examples of synaptophysin immunolabeling of LOC and MOC terminals in control and MPTP-treated cochleae are depicted in Figures 1 and 2. Quantitative evaluation of cochlear tissue immunolabeling is presented in Figure 3.
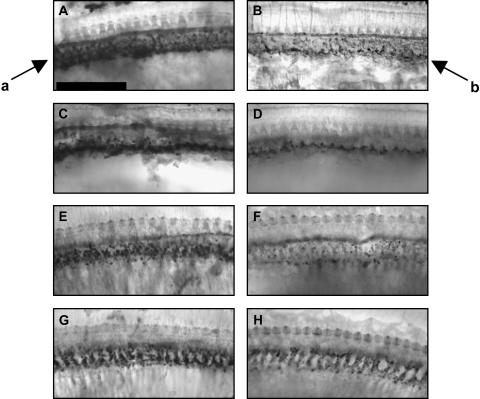
Fig. 1.
Cochlear tissues had less dense immunolabeling of lateral olivocochlear (LOC) efferents after MPTP. Tissues were immunolabeled with antisynaptophysin, and are from base (A, B), second (C, D) and third (E, F) turns, and apex (G, H). Control tissues are depicted in the left panels (A, C, E, G). MPTP-treated tissues are depicted in the right panels (B, D, F, H). Arrows indicate normal LOC immunolabeling below the inner hair cells in the region of the inner spiral bundle (arrow a) and sparse LOC immunolabeling after MPTP treatment (arrow b). Scale bar = 50 μm.
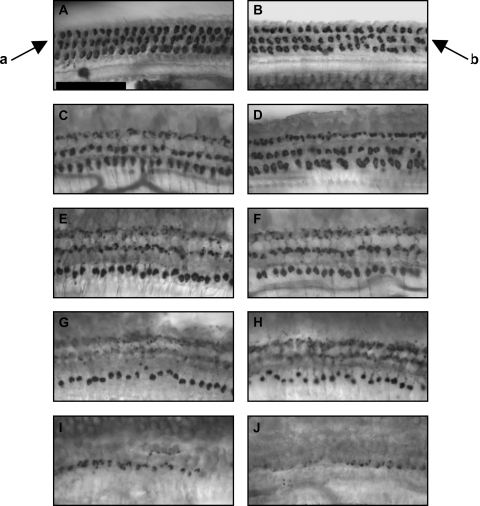
Fig. 2.
Medial olivocochlear (MOC) immunolabeling was not affected by MPTP. Tissues were immunolabeled with antisynaptophysin, and are from base (A, B), second (C, D) and third (E, F) turns, lower apex (G, H) and upper apex (I, J). Control tissues are depicted in the left panels (A, C, E, G, H). MPTP-treated tissues are depicted in the right panels (B, D, F, H, J). Arrows indicate normal MOC immunolabeling below the outer hair cells (arrow a), and unchanged immunolabeling of MOC puncta after MPTP treatment (arrow b). Scale bar = 50 μm.
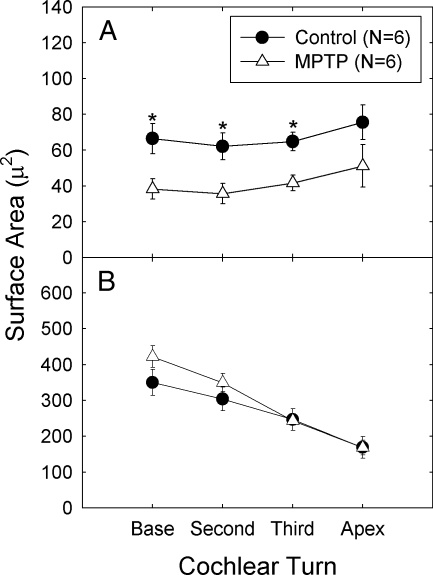
Fig. 3.
A. Surface area of synaptophysin immunolabeling of the lateral olivocochlear (LOC) neurons was reduced in MPTP-treated cochlear tissues.
B. Surface area of medial olivocochlear (MOC) neurons was not reliably changed. Asterisks indicate statistically reliable differences between control and MPTP-treated ears. The amount of tissue from apical turns was not sufficient for the conduct of statistical comparisons, and all hook region tissues were excluded from analysis as the majority of hook region tissues from both the control and MPTP groups were damaged during dissection. For all other tissues, labeled area was measured in three to six regions of each cochlear turn for each animal. Labeling was first averaged within each animal; average labeling across animals is depicted.
Statistical comparisons were conducted separately for the LOC and MOC synaptophysin quantification. Comparisons were conducted using analysis of variance (ANOVA) with between- and within-subject measures. Group means were substituted for the small number of missing data points, and the Greenhouse–Geisser correction for sphericity was applied. The within-subject measure was cochlear turn (base, turn 2, turn 3, and apex) and the between-subject variable was group (control or MPTP).
Statistical comparisons revealed LOC immunolabeling (Fig. 3A) was significantly decreased in MPTP-treated ears (p = 0.005); there were no reliable changes in the amount of immunolabeling as a function of turn (p = 0.134) and we failed to find a groups × turn interaction (p = 0.932). In contrast, MOC immunolabeling (Fig. 3B) was not reliably affected by MPTP treatment (p = 0.170); MOC immunolabeling significantly decreased as a function of turn (p < 0.001) but there was no groups × turn interaction (p = 0.380). Taken together, LOC immunolabeled area decreased as a function of MPTP treatment but did not reliably change from base to apex, whereas MOC immunolabeled area decreased from base to apex for both groups, but there was no difference between the control and MPTP-treated groups.
Electrophysiology
MPTP depressed CAP amplitude over the entire range of frequencies tested (see Fig. 4). The statistical reliability of drug-induced differences in CAP amplitude was evaluated using three-way repeated-measures ANOVAs. The three repeated-measures factors consisted of condition (baseline, artificial perilymph, and MPTP), stimulus frequency (2, 4, 6, 8, 10, 12, 14, 16, and 18 kHz), and stimulus intensity (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, and 100 dB SPL). Pairwise comparisons were conducted using Bonferroni corrections.
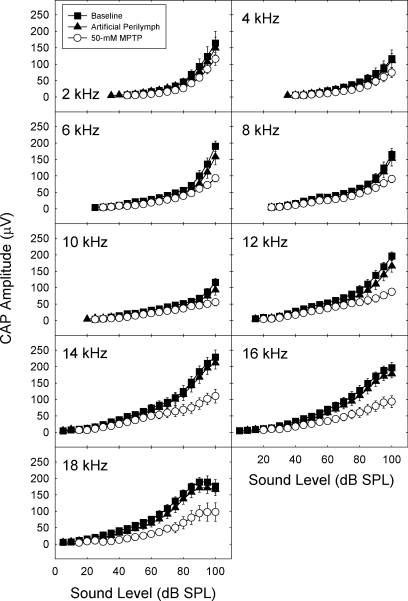
Fig. 4.
Amplitude of the compound action potential (CAP) amplitude, defined as the amplitude of the N1–P1 component, was depressed after MPTP treatment. CAP amplitude was determined from 0- to 100-dB SPL at frequencies extending from 2 to 18 kHz. Mean CAP amplitude (±SE) is depicted immediately after cementing the recording electrode in place, 30 min after applying artificial perilymph to the round window membrane, and 30 min after applying 50 mM MPTP to the round window membrane.
The overall analysis of CAP amplitude revealed a significant interaction of condition with frequency and intensity (F = 3.53; df = 320,1280; p < 0.001). When the interaction was broken down by condition, it was observed that baseline and artificial perilymph conditions were not significantly different from each other and did not interact with frequency or intensity (p > 0.60). Hence baseline and artificial perilymph conditions were combined and compared with the MPTP condition at each level of stimulus frequency. The contrasts of MPTP and combined control conditions interacted with intensity at all frequencies (p < 0.01 after applying Bonferroni correction). Thus CAP amplitude was depressed at higher intensities for the entire frequency range tested. The magnitude of the effect was smaller at the lower frequencies.
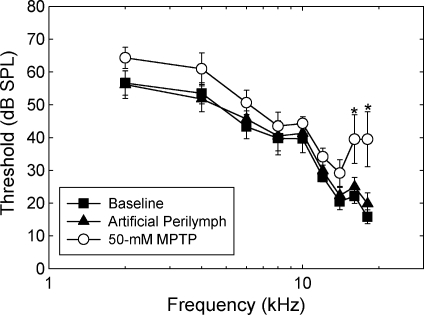
In contrast to depressions in CAP amplitude, which were observed across frequencies, MPTP induced significant elevations in CAP threshold only at higher frequencies (see Fig. 5). The frequency × condition interaction was significant (F = 4.22; df = 16,80; p < 0.001). After applying the Bonferroni correction, statistically reliable threshold elevations were limited to 16 and 18 kHz (p < 0.05).
Fig. 5.
Threshold (±SE) of the compound action potential (CAP) was elevated by MPTP treatment only at the highest frequencies tested. Asterisks indicate statistically reliable differences between control and MPTP-treated ears. CAP threshold was determined immediately after cementing the recording electrode in place, 30 min after applying artificial perilymph to the round window membrane, and 30 min after applying 50 mM MPTP to the round window membrane.
Because threshold differences were observed at the highest test frequencies, we normalized the data to threshold by expressing CAP amplitude in decibel sensation level (SL), where 0 dB SL is the lowest signal that elicits a 10-μv neural response. The same basic effect was seen when using sensation level measures as with sound pressure level measures (not depicted). After normalizing to threshold, CAP amplitude was still depressed by MPTP at higher intensities (F = 1.67; df = 40,200; p =0.012); however, this effect no longer significantly varied as a function of frequency (F < 1).
MPTP did not affect N1 latency (not depicted). Analysis of the latency data only revealed a significant frequency × intensity interaction (F = 26.38; df = 160,800; p < 0.001) that did not interact with condition.
DPOAE adaptation
Baseline testing with the bulla opened and artificial perilymph applied to the round window membrane indicated strong (≥20 dB) DPOAE adaptation (as described by Kujawa and Liberman 2001). DPOAE adaptation, a measure of the strength of the MOC reflex (see Liberman et al. 1996; Maison and Liberman 2000; Kujawa and Liberman 2001), was essentially the same after artificial perilymph, or MPTP (see Fig. 6). Across three applications of artificial perilymph in a single animal, we observed a 2.3-dB variation in DPOAE adaptation (see Fig. 6A, B, and C). Across the four animals tested, changes in DPOAE adaptation after MPTP ranged from an increase of 4.2 dB to a decrease of 7.3 dB (mean ± SD = −2.1 ± 4.8; Fig. 6D illustrates a 3.1-dB decrease in adaptation 30 min after MPTP). In contrast to the lack of reliable changes induced by MPTP, i.v. strychnine caused a profound depression of DPOAE adaptation in the one animal tested (a 20.4-dB decrease in response amplitude; see Fig. 6E), as did OCB transection in a second animal (19.1 dB decrease in response amplitude; not illustrated).
Fig. 6.
Onset adaptation of distortion product otoacoustic emissions (DPOAEs) was not reliably changed by MPTP treatment. DPOAEs show a rapid level-dependant adaptation shortly after signal onset when the medial olivocochlear (MOC) pathway is intact. DPOAE adaptation is depicted 30 min after sequential applications of artificial perilymph (panels A, B, C), 30 min after applying 50 mM MPTP to the round window membrane (D), and after i.v. strychnine (0.15 mg/kg, see panel E).
DPOAE amplitude
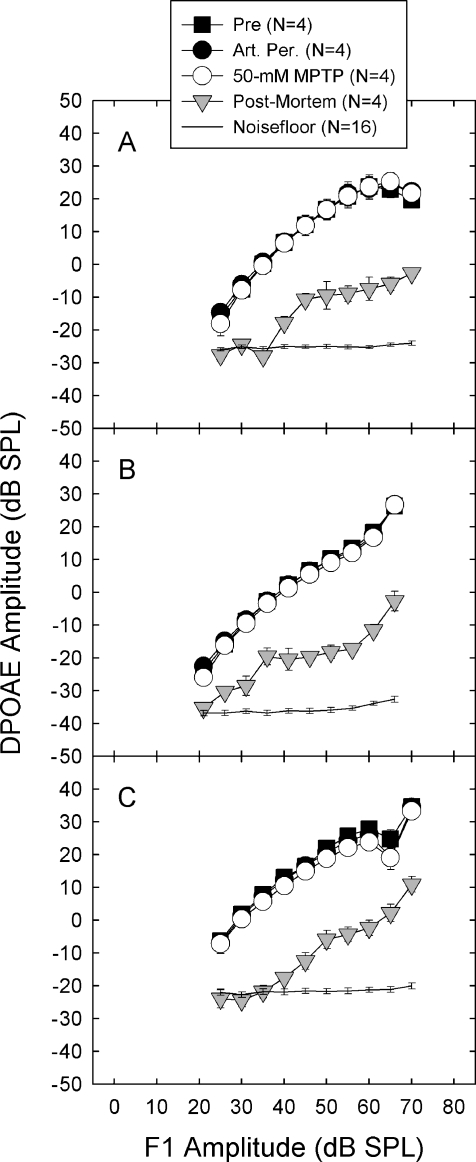
DPOAE amplitude, a measure of the functional integrity of the OHC population, was not affected by MPTP (see Fig. 7). The lack of effect of MPTP on DPOAE amplitude indicates that OHC function was not disrupted by MPTP applied to the round window membrane. In contrast, euthanasia with an overdose of sodium pentobarbital profoundly depressed DPOAE steady state amplitude. The DPOAE amplitudes assessed in postmortem ears are consistent with postmortem data from rabbits (Whitehead et al. 1992) and guinea pigs (Frolenkov et al. 1998). Similar data are available from mutant (hyt/hyt) mice with OHC abnormalities (Li et al. 1999), rats treated with kanamycin (Mills et al. 1999), and guinea pigs treated with neomycin (Le Prell et al. 2004b).
Fig. 7.
Steady-state amplitude of distortion product otoacoustic emissions (DPOAE) was unchanged by MPTP treatment. Steady-state DPOAE amplitude is a measure of the integrity of the outer hair cell population. DPOAE steady-state amplitude is depicted after opening the bulla (“Pre”), 30 min after applying artificial perilymph to the round window membrane, and 30 min after applying 50 mM MPTP to the round window membrane. The levels of F1 and F2 were systematically varied such that F2 was always 10 dB quieter than F1. DPOAE frequencies were 5.8 kHz (A), 8.7 kHz (B), and 11.7 kHz (C).
Discussion
These results demonstrate that MPTP, a dopaminergic neurotoxin, selectively damages LOC neurons. This is a significant technical achievement as other LOC manipulations are accompanied by simultaneous disruption of MOC neurons (i.e., Liberman 1990; Zheng et al. 1999) or auditory pathways ascending from the LSO (i.e., Le Prell et al. 2003a, b). The unmyelinated LOC neurons traveling in the OCB do not respond well to electrical stimulation (e.g., Guinan and Gifford 1988), and a recent effort to electrically stimulate LOC neurons via electrodes placed into the inferior colliculus or LSO yielded mixed results (Groff and Liberman 2003) with some stimulation sites producing fast MOC-like effects and others producing putative LOC effects that included site-dependent slow suppression or enhancement of CAP amplitude.
To further our understanding of LOC function, we evaluated AN activity before and after MPTP-induced LOC disruption. Consistent with the report that LSO lesions depressed CAP amplitude (Le Prell et al. 2003b), we report that LOC disruption induced by intracochlear MPTP depressed CAP amplitude. We extend our earlier data by now reporting that selective LOC disruption is not accompanied by changes in DPOAE onset adaptation or steady-state amplitude. Together, these data provide direct evidence that the LOC neurons normally modulate AN activity.
MPTP selectively disrupts LOC efferents: anatomical evidence
Anatomical evidence that MPTP selectively damages LOC neurons included a significant reduction in synaptophysin immunolabeling of LOC neurons, with no reduction in MOC labeling. Decreased LOC immunolabeling at the 45-min post-MPTP time point is not surprising as decreases in metabolic activity (Palacios and Wiederhold 1984) and mitochondrial function (Mizuno et al. 1988) as well as behavioral changes (Chiueh et al. 1984; for review, see Sedelis et al. 2001) are frequently reported within 5–30 min of MPTP injection. High-performance liquid chromatography showed MPTP to be quickly metabolized to its active form (Shinka et al. 1987), and microdialysis revealed MPTP-induced changes in DA efflux within 10 min (Wu et al. 2000). Rapid and pronounced disruption to metabolic energy presumably reduces the ability of LOC neurons to package new LOC transmitter stores, resulting in the observed decrease in immunolabeling of the protein synaptophysin (which is found in the walls of synaptic vesicles).
Lateral olivocochlear immunolabeling remaining after MPTP might indicate a small population of functionally intact LOC neurons that did not contain DA. Alternatively, MPTP treatment may have induced a functional disruption of all LOC innervation and the remaining LOC immunolabeling might be a population of neurons with intact puncta but degraded function as a consequence of the MPTP-induced mitochondrial dysfunction and oxidative stress that precede neuronal death (for reviews, see Sayre 1989; Tipton and Singer 1993; Przedborski and Jackson-Lewis 1998). Another possibility is that the remaining LOC population was preserved by one or more of the many trophic factors that reduce death of DA neurons after treatment with a DA neurotoxin. The protective effects of neurotrophic factors on AN survival (e.g., Staecker et al. 1996a, b; Miller et al. 1997; Ylikoski et al. 1998; Shinohara et al. 2002) and the presence of neurotrophic factors in the mature guinea pig cochlea (Malgrange et al. 1998; Qun et al. 1999; Stover et al. 2000, 2001; Stankovic and Corfas 2003) are well known. Glial cell-derived neurotrophic factor (GDNF) is one of the most potent trophic factors in preserving cellular function post-MPTP in the striatum and substantia nigra (for review, see Eberhardt and Schulz 2003), and GDNF is also potent in the cochlea (for review, see Altschuler et al. 1999). Resolving the chemical distribution of the intact puncta in the remaining LOC neurons, as well as overall functional status, presents an interesting direction for future investigations.
The finding that changes in LOC innervation were greatest in the lower turns is consistent with reports by other groups. For example, Mulders and Robertson (2004) report that TH-labeled neurons in the LSO were primarily limited to the medial limb of the LSO, which projects to the hook, basal (first) turn, and second turn of the cochlea. Thus they predict that an absence of dopaminergic LOC effects would be visible only on fibers well below 6 kHz. Our electrophysiological data are clearly consistent with this prediction; we report that the greatest effects were observed at and above 6 kHz. Although Mulders and Robertson (2004) report little to no TH labeling in the third turn and the apex of the cochlea, other investigations describe TH labeling in these higher turns (i.e., Jones et al. 1987; Usami et al. 1988; d’Aldin et al. 1995a; Niu and Canlon 2002). Niu and Canlon (2002) more precisely quantified TH immunolabeling in the cochlea. They reported very sparse TH immunoreactivity in the most apical turn and very dense TH immunoreactivity in the regions corresponding to 8 kHz and above (i.e., 10–11 mm from the apex). In the intermediate midcochlear regions corresponding to 1–6 kHz (i.e., 4–9 mm from the apex), TH immunoreactivity was obvious, although less dense than those regions corresponding to 8 kHz and above.
The substantial reduction in immunolabeled LOC neurons we observed, while consistent with the broad TH immunolabeling previously described in the cochlea (i.e., Jones et al. 1987; Usami et al. 1988; Niu and Canlon 2002), is seemingly contradictory to reports that the number of TH-positive neurons in the LSO is small (i.e., Mulders and Robertson 2004; Niu et al. 2004). Although the number of TH-positive neurons in the LSO is small, it is well known that at least one class of LSO neurons (termed shell neurons, in the rat) travel long distances within the cochlea, project with numerous bifurcations, and end with many terminal boutons. These neurons have been well described in the guinea pig (Brown 1987), rat (Vetter and Mugnaini 1992; Warr et al. 1997; Warr and Boche 2003), chinchilla (Azeredo et al. 1999), and hamster (Sanchez-Gonzalez et al. 2003), and may exist in the cat as well (Warr et al. 2002). Although it has not been definitively shown that TH-positive neurons in the cochlea fall within this bifurcating class of neurons, Niu et al. (2004) argue that the dense TH staining they previously observed in the cochlea (Niu and Canlon 2002) is clearly consistent with bifurcating distribution given the small number of TH-positive neurons in the LSO. Taken together, the anatomical evidence suggests the dense network of TH-positive LOC neurons in the cochlea originates from a smaller population of neurons in the LSO. Although many LOC transmitters are known to be colocalized at their site of origin (Altschuler et al. 1983, 1984, 1986; Abou-Madi et al. 1987; Altschuler et al. 1988; Safieddine et al. 1997) or their site of termination within the cochlea (Altschuler et al. 1985; Safieddine and Eybalin 1992), the precise chemical distribution within the LOC population remains to be definitively identified.
MPTP selectively disrupts LOC efferents: physiological evidence
DPOAE adaptation and amplitude were used to show MOC neurons and OHCs were functionally intact after MPTP treatment. In three of the four animals tested, we observed a small reduction in DPOAE adaptation post-MPTP, an effect that was within the variability observed across repeat applications of artificial perilymph. Although the small reductions in MOC reflex strength are within the “noise” of the repeated artificial perilymph tests, this small reduction in the MOC reflex could be predicted based on the observed reduction in CAP amplitude (i.e., Fig. 4). Depressed CAP amplitude decreases the ascending neural input that drives the descending MOC reflex loop. There were no consistent changes in OHC function, as shown in DPOAE steady-state amplitude. We previously reported no effect of MPTP on endocochlear potential (EP), indicating that the stria vascularis, which contains DA receptors (Jones et al. 1987; Usami et al. 1988; Kanoh 1995), is functionally intact (Le Prell and Bledsoe 2003).
Functional consequences of LOC disruption: MPTP vs. LSO lesion
Similar to LSO lesions (Le Prell et al. 2003b), threshold changes with MPTP were limited to a small subset of frequencies. One important difference is that LSO lesions, which consistently disrupted the lateral (low frequency) limb of the LSO, elevated thresholds at 2 kHz, whereas MPTP (applied to the round window membrane in the high frequency region, from which it diffused to lower frequency regions) elevated thresholds at 16 and 18 kHz. Threshold elevations after selective LOC disruption are an important finding. The functional status of the OHCs and the MOC neurons (which innervate the OHCs) are typically believed to set cochlear sensitivity as electrical stimulation of the crossed OCB depresses cochlear sensitivity (Galambos 1956; Wiederhold 1970; Gifford and Guinan 1987; Murugasu and Russell 1996; for reviews, see Ulfendahl 1997; Robles and Ruggero 2001). We hypothesize that LOC efferents further maintain threshold sensitivity by modulating ANF spontaneous rate. Support for this hypothesis comes from the small but statistically significant ANF threshold elevations, at a subset of test frequencies, induced by cutting both the MOC and LOC pathways (see Liberman 1990; Walsh et al. 1998; Zheng et al. 1999).
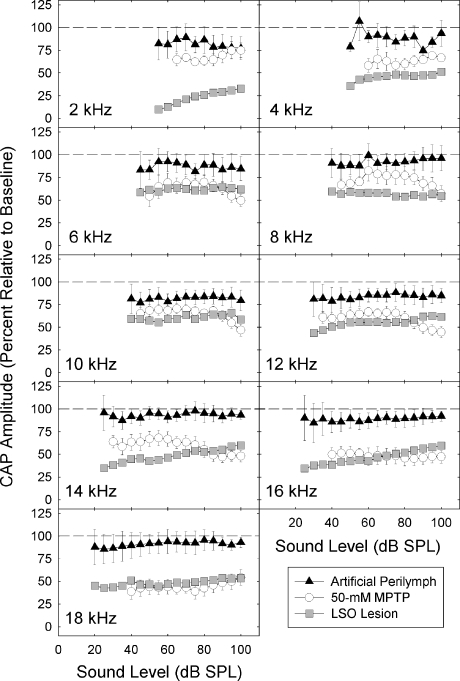
After disrupting LOC innervation, CAP amplitude was depressed. Depressed CAP amplitude was evident after normalizing the data to remove any effects of threshold changes. To directly compare the effects of LOC disruption induced by MPTP and LSO lesions, we normalized CAP amplitude for the data set shown in Figure 4 and the data reported by Le Prell et al. (2003b). The functional consequences of these manipulations were similar, particularly from 12 to 18 kHz (see Fig. 8). That the effects of MPTP were reduced at lower frequencies is not unexpected. Zheng et al. (1996) reported a similar basal-to-apical gradient of functional effects after applying kainic acid to the round window membrane in chinchillas. Similar to LSO lesions, MPTP applied to the round window did not affect N1 latency.
Fig. 8.
Depression of the amplitude of the compound action potential (CAP) was generally equivalent in animals treated with intracochlear MPTP and animals in which the lateral superior olive (LSO) was lesioned. Amplitude of the CAP 30 min after applying artificial perilymph or 50 mM MPTP to the round window membrane was normalized to pretreatment baseline. We also normalized CAP amplitude assessed 1 week after lesioning the LSO to baseline measures from animals in which the LSO was intact (original data are presented in Le Prell et al. 2003b). Dashed lines indicate 100% of baseline; decreasing values indicate CAP was depressed post-treatment.
Pharmacology of LOC disruption: excitatory and inhibitory transmitter substances
The population of LOC neurons contains a combination of excitatory and inhibitory substances. We have proposed a model in which LOC neurons set the sensitivity of the ANFs via manipulation of a “set point,” a task accomplished by balancing the release of the excitatory and inhibitory transmitter substances (see Le Prell et al. 2003a). Specifically, we proposed that delivering putative LOC transmitter substances such as ACh and/or dyn selectively lowers the cochlear set point, thereby enhancing neural activity. In contrast, release of substances such as DA and enk selectively raises the set point of the cochlea, thereby decreasing cochlear activity. Our prediction is that this tonic input from the LOC neurons maintains the distribution of spontaneous activity and sensitivity of the ANFs in conditions of quiet and noise.
The finding that LOC disruption depresses CAP amplitude is consistent with suggestions that the net effect of LOC innervation in the cochlea is excitatory, and is consistent with the first part of the above model. Specifically, the results indicate intact LOC neurons enhance neural activity, and loss of the LOC innervation has the effect of a net depression of activity. Several LOC transmitters are candidates for enhancing AN output. ACh excites ANFs when ejected by microiontophoresis into the dendritic region beneath the IHCs (Felix and Ehrenberger 1992). We speculated that dyn may play an important excitatory role in cochlear afferent transmission (e.g., Le Prell et al. 2001, 2003a, b) based on reports that i.v. administration of the kappa agonist (−)pentazocine enhanced CAP amplitude and improved threshold sensitivity (Sahley et al. 1991; Sahley and Nodar 1994). In contrast to these earlier reports, we recently presented data showing several kappa opioid receptor agonists depress AN activity when delivered directly to the cochlea (Le Prell et al. 2004a). Thus we consider the effects of dyn in the cochlea to be unresolved, as are the effects of intracochlear CGRP. In the amphibian lateral line, CGRP increases spontaneous activity (Adams et al. 1987; Sewell and Starr 1991; Bailey and Sewell 2000a, b) and suppresses driven activity (Bailey and Sewell 2000a). There are no published reports on the functional consequences of CGRP agonists in the cochlea, although Maison et al. (2003) recently reported ABR amplitude was depressed by 20–25% in αCGRP-null mice. Depression of the ABR is consistent with an excitatory effect of CGRP on AN activity.
There is also evidence consistent with the second part of the above model. Dopamine and DA agonists inhibit AN activity (d’Aldin et al. 1995a, b; Oestreicher et al. 1997; Ruel et al. 2001; Sun and Salvi 2001). Both GABA (Felix and Ehrenberger 1992; Malgrange et al. 1997; Arnold et al. 1998) and enkephalin (Burki et al. 1993) similarly inhibit AN activity. Many hypothesize that one (or more) of these inhibitory substances are upregulated during periods of excessive (traumatic) stimulation, and LOC neurons thus protect ANFs from trauma (Pujol et al. 1993; Pujol 1994; d’Aldin et al. 1995a, b; Gil-Loyzaga 1995; Puel 1995; Gaborjan et al. 1999; Gaborjan and Vizi 1999; Halmosa et al. 2000). In fact, the DA agonist piribedil protects against hearing loss induced by exposure to loud sounds (d’Aldin et al. 1995a). In addition, limited evidence indicates that sound-evoked trauma is more pronounced in animals with LSO lesions (Le Prell et al. 2003a). In that investigation, acoustic overstimulation depressed CAP amplitude to a greater extent in lesioned animals than intact controls, an effect that was primarily limited to test frequencies lower than the sound exposure frequency.
Future directions
There is now a substantial body of evidence that the net effect of LOC innervation of the mammalian cochlea is excitatory. The pharmacological basis of this overall effect remains unclear, although several candidate transmitters have been identified. In addition, interactions among the excitatory and inhibitory substances remain to be determined. Identifying specific mechanisms through which LOC transmitters interact remains a significant challenge for future research. Our prediction is that tonic input from the LOC neurons provides a mix of excitatory and inhibitory influences that maintain the distribution of spontaneous activity and sensitivity of the ANFs. If the LOC neurons adjust the level of AN activity under varying conditions of acoustic stimulation (e.g., quiet and noise), they may provide a mechanism for the AN to maintain and accurately convey dynamic range information to the central auditory system thus maintaining an optimal set point for MOC modulation of basilar membrane mechanics.
Acknowledgments
Financial support for this study was provided by NIH-NIDCD grants, including P01-DC00078 (D. Dolan, S. Bledsoe), RO1-DC004194 (D. Dolan), and P30-DC05188. We thank R. Altschuler, G. Atkin, R. Diener, L. Grant, R. Griffith, A. Kanicki, J. McLaren, S. Shubert, N. Wys, and W. Zhang for their invaluable help and assistance.
References
- Abou-Madi L, Pontarotti P, Tramu G, Cupo A, Eybalin M. Coexistence of putative neuroactive substances in lateral olivocochlear neurons of rat and guinea pig. Hear. Res. 1987;30:135–146. doi: 10.1016/0378-5955(87)90131-6. [DOI] [PubMed] [Google Scholar]
- Adams JC, Mroz EA, Sewell WF. A possible neurotransmitter role for CGRP in a hair-cell sensory organ. Brain Res. 1987;419:347–351. doi: 10.1016/0006-8993(87)90606-8. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Parakkal MH, Fex J. Localization of enkephalin-like immunoreactivity in acetylcholinesterase-positive cells in the guinea-pig lateral superior olivary complex that project to the cochlea. Neuroscience. 1983;9:621–630. doi: 10.1016/0306-4522(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Fex J, Parakkal MH, Eckenstein F. Colocalization of enkephalin-like and choline acetyltransferase-like immunoreactivities in olivocochlear neurons of the guinea pig. J. Histochem. Cytochem. 1984;32:839–843. doi: 10.1177/32.8.6379037. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Hoffman DW, Reeks KA, Fex J. Localization of dynorphin B-like and alpha-neoendorphin-like immunoreactivities in the guinea pig organ of Corti. Hear. Res. 1985;17:249–258. doi: 10.1016/0378-5955(85)90069-3. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Hoffman DW, Wenthold RJ. Neurotransmitters of the cochlea and cochlear nucleus: immunocytochemical evidence. Am. J. Otolaryngol. 1986;7:100–106. doi: 10.1016/s0196-0709(86)80038-2. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Reeks KA, Fex J, Hoffman DW. Lateral olivocochlear neurons contain both enkephalin and dynorphin immunoreactivities: immunocytochemical co-localization studies. J. Histochem. Cytochem. 1988;36:797–801. doi: 10.1177/36.7.2898496. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann. N.Y. Acad. Sci. 1999;884:305–311. doi: 10.1111/j.1749-6632.1999.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Arnold T, Oestreicher E, Ehrenberger K, Felix D. GABA(A) receptor modulates the activity of inner hair cell afferents in guinea pig cochlea. Hear. Res. 1998;125:147–153. doi: 10.1016/s0378-5955(98)00144-0. [DOI] [PubMed] [Google Scholar]
- Azeredo WJ, Kliment ML, Morley BJ, Relkin E, Slepecky NB, Sterns A, Warr WB, Weekly JM, Woods CI. Olivocochlear neurons in the chinchilla: a retrograde fluorescent labelling study. Hear. Res. 1999;134:57–70. doi: 10.1016/s0378-5955(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Bailey GP, Sewell WF. Calcitonin gene-related peptide suppresses hair cell responses to mechanical stimulation in the Xenopus lateral line organ. J. Neurosci. 2000a;20:5163–5169. doi: 10.1523/JNEUROSCI.20-13-05163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GP, Sewell WF. Pharmacological characterization of the CGRP receptor in the lateral line organ of Xenopus laevis. J. Assoc. Res. Otolaryngol. 2000b;1:82–88. doi: 10.1007/s101620010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbin RP, Konishi T. Action of cholinergic and anticholinergic drugs at the crossed olivocochlear bundle-hair cell junction. Acta Oto-laryngol. (Stockh.) 1974;77:56–65. doi: 10.3109/00016487409124598. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J. Comp. Neurol. 1987;260:605–618. doi: 10.1002/cne.902600412. [DOI] [PubMed] [Google Scholar]
- Brown RD, Daigneault EA, Pruett JR. The effects of selected cholinergic drugs and strychnine on cochlear responses and olivo-cochlear inhibition. J. Pharmacol. Exp. Ther. 1969;165:300–309. [PubMed] [Google Scholar]
- Burgess BJ, Adams JC, Nadol JB. Morphologic evidence for innervation of Deiters’ and Hensen’s cells in the guinea pig. Hear. Res. 1997;108:74–82. doi: 10.1016/s0378-5955(97)00040-3. [DOI] [PubMed] [Google Scholar]
- Burki C, Felix D, Ehrenberger K. Enkephalin suppresses afferent cochlear neurotransmission. ORL, J. Oto-rhino-laryngol. Relat. Spec. 1993;55:3–6. doi: 10.1159/000276344. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Markey SP, Burns RS, Johannessen JN, Jacobowitz DM, Kopin IJ. Neurochemical and behavioral effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in rat, guinea pig, and monkey. Psychopharmacol. Bull. 1984;20:548–553. [PubMed] [Google Scholar]
- Cunha C, Gevaerd MS, Vital MA, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS. Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behav. Brain Res. 2001;124:9–18. doi: 10.1016/s0166-4328(01)00211-x. [DOI] [PubMed] [Google Scholar]
- d’Aldin C, Eybalin M, Puel JL, Charachon G, Ladrech S, Renard N, Pujol R. Synaptic connections and putative functions of the dopaminergic innervation of the guinea pig cochlea. Eur. Arch. Otorhinolaryngol. 1995;252:270–274. doi: 10.1007/BF00185388. [DOI] [PubMed] [Google Scholar]
- d’Aldin C, Puel JL, Leducq R, Crambes O, Eybalin M, Pujol R. Effects of a dopaminergic agonist in the guinea pig cochlea. Hear. Res. 1995;90:202–211. doi: 10.1016/0378-5955(95)00167-5. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Monaco P. Mode of action of the efferent olivo-cochlear bundle on the inner ear. Nature. 1961;192:1263–1265. doi: 10.1038/1921263a0. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Robertson D. Ionic mechanism of the efferent olivo-cochlear inhibition studied by cochlear perfusion in the cat. J. Physiol. (Lond.) 1975;247:407–428. doi: 10.1113/jphysiol.1975.sp010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan DF, Yamasoba T, Leonova E, Beyer LA, Raphael Y. Morphological and physiological effects of long duration infusion of strychnine into the organ of Corti. J. Neurocytol. 1999;28:197–206. doi: 10.1023/a:1007071905824. [DOI] [PubMed] [Google Scholar]
- Eberhardt O, Schulz JB. Apoptotic mechanisms and antiapoptotic therapy in the MPTP model of Parkinson’s disease. Toxicol. Lett. 2003;139:135–151. doi: 10.1016/s0378-4274(02)00428-9. [DOI] [PubMed] [Google Scholar]
- Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol. Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- Eybalin M, Charachon G, Renard N. Dopaminergic lateral efferent innervation of the guinea-pig cochlea: immunoelectron microscopy of catecholamine-synthesizing enzymes and effect of 6-hydroxydopamine. Neuroscience. 1993;54:133–142. doi: 10.1016/0306-4522(93)90389-w. [DOI] [PubMed] [Google Scholar]
- Felix D, Ehrenberger K. The efferent modulation of mammalian inner hair cell afferents. Hear. Res. 1992;64:1–5. doi: 10.1016/0378-5955(92)90163-h. [DOI] [PubMed] [Google Scholar]
- Frolenkov GI, Belyantseva IA, Kurc M, Mastroianni MA, Kachar B. Cochlear outer hair cell electromotility can provide force for both low and high intensity distortion product otoacoustic emissions. Hear. Res. 1998;126:67–74. doi: 10.1016/s0378-5955(98)00150-6. [DOI] [PubMed] [Google Scholar]
- Gaborjan A, Vizi ES. Characterization of voltage dependent calcium channels on the lateral olivocochlear efferent fibers of the guinea pig. Neurosci. Lett. 1999;269:49–51. doi: 10.1016/s0304-3940(99)00410-3. [DOI] [PubMed] [Google Scholar]
- Gaborjan A, Lendvai B, Vizi ES. Neurochemical evidence of dopamine release by lateral olivocochlear efferents and its presynaptic modulation in guinea-pig cochlea. Neuroscience. 1999;90:131–138. doi: 10.1016/s0306-4522(98)00461-8. [DOI] [PubMed] [Google Scholar]
- Galambos R. Suppression of auditory nerve activity by stimulation of efferent fibers to the cochlea. J. Neurophysiol. 1956;19:424–437. doi: 10.1152/jn.1956.19.5.424. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ. Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear. Res. 1987;29:179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Gil-Loyzaga PE. Neurotransmitters of the olivocochlear lateral efferent system: with an emphasis on dopamine. Acta Oto-laryngol. (Stockh.) 1995;115:222–226. doi: 10.3109/00016489509139296. [DOI] [PubMed] [Google Scholar]
- Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J. Neurophysiol. 2003;90:3178–3200. doi: 10.1152/jn.00537.2003. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions. Hear. Res. 1988;33:97–113. doi: 10.1016/0378-5955(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Halmosa G, Gaborjan A, Lendvai B, Repassy G, Szabo LZ, Vizi ES. Veratridine-evoked release of dopamine from guinea pig isolated cochlea. Hear. Res. 2000;144:89–96. doi: 10.1016/s0378-5955(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Halsey K, Kabara L, Grant LM, Dolan DF. Efferent-mediated adaptation of the DPOAE as a predictor of aminoglycoside toxicity. Assoc. Res. Otolaryngol. Abstr. 2004;27:179–180. doi: 10.1016/j.heares.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Jones N, Fex J, Altschuler RA. Tyrosine hydroxylase immunoreactivity identifies possible catecholaminergic fibers in the organ of Corti. Hear. Res. 1987;30:33–38. doi: 10.1016/0378-5955(87)90180-8. [DOI] [PubMed] [Google Scholar]
- Kanoh N. Dopamine inhibits the Na–K ATPase activity of the stria vascularis in the cochlea. In vivo ultracytochemical study. Acta Oto-laryngol. (Stockh.) 1995;115:27–30. doi: 10.3109/00016489509133341. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Effects of olivocochlear feedback on distortion product otoacoustic emissions in guinea pig. J. Assoc. Res. Otolaryngol. 2001;2:268–278. doi: 10.1007/s101620010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. Intracochlear application of acetylcholine alters sound-induced mechanical events within the cochlear partition. Hear. Res. 1992;61:106–116. doi: 10.1016/0378-5955(92)90041-k. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. Contralateral sound suppresses distortion product otoacoustic emissions through cholinergic mechanisms. Hear. Res. 1993;68:97–106. doi: 10.1016/0378-5955(93)90068-c. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. A nicotinic-like receptor mediates suppression of distortion product otoacoustic emissions by contralateral sound. Hear. Res. 1994;74:122–134. doi: 10.1016/0378-5955(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Prell CG, Bledsoe SC. Disruption of lateral olivocochlear neurons depresses compound action potential amplitude. Assoc. Res. Otolaryngol. Abstr. 2003;26:250. [Google Scholar]
- Prell CG, Bledsoe SC , Bobbin RP, Puel JL. Neurotransmission in the inner ear: functional and molecular analyses. In: Jahn AF, Santos-Sacchi J, editors. Physiology of the Ear, 2. New York: Singular Publishing; 2001. pp. 575–611. [Google Scholar]
- Prell CG, Dolan DF, Schacht J, Miller JM, Lomax MI, Altschuler RA. Pathways for protection from noise-induced hearing loss. Noise Health. 2003;5:1–17. [PubMed] [Google Scholar]
- Prell CG, Shore SE, Hughes LF, Bledsoe SC. Disruption of lateral efferent pathways: functional changes in auditory evoked responses. J. Assoc. Res. Otolaryngol. 2003;4:276–290. doi: 10.1007/s10162-002-3018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prell CG, Halsey K, Dolan DF, Bledsoe SC. Kappa opioid-receptor agonists depress compound action potential amplitude. Assoc. Res. Otolaryngol. Abstr. 2004;27:329–330. [Google Scholar]
- Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, Dolan D, Bledsoe SC, Moody DB. Chronic infusion of AMPA into the guinea pig cochlea induces temporary functional deficits and long-term morphological trauma. J. Acoust. Soc. Am. 2004;116:1044–1056. doi: 10.1121/1.1772395. [DOI] [PubMed] [Google Scholar]
- Li D, Henley CM, O’Malley BW. Distortion product otoacoustic emissions and outer hair cell defects in the hyt/hyt mutant mouse. Hear. Res. 1999;138:65–72. doi: 10.1016/s0378-5955(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Effects of chronic cochlear de-efferentation on auditory-nerve response. Hear. Res. 1990;49:209–223. doi: 10.1016/0378-5955(90)90105-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Puria S, Guinan JJ. The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1 − f2 distortion product otoacoustic emission. J. Acoust. Soc. Am. 1996;99:3572–3584. doi: 10.1121/1.414956. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J. Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Emeson RB, Adams JC, Luebke AE, Liberman MC. Loss of alpha CGRP reduces sound-evoked activity in the cochlear nerve. J. Neurophysiol. 2003;90:2941–2949. doi: 10.1152/jn.00596.2003. [DOI] [PubMed] [Google Scholar]
- Malgrange B, Rigo JM, Lefebvre PP, Coucke P, Goffin F, Xhauflaire G, Belachew S, Water TR, Moonen G. Diazepam-insensitive GABAA receptors on postnatal spiral ganglion neurones in culture. NeuroReport. 1997;8:591–596. doi: 10.1097/00001756-199702100-00003. [DOI] [PubMed] [Google Scholar]
- Malgrange B, Rogister B, Lefebvre PP, Mazy-Servais C, Welcher AA, Bonnet C, Hsu RY, Rigo JM, Water TR, Moonen G. Expression of growth factors and their receptors in the postnatal rat cochlea. Neurochem. Res. 1998;23:1133–1138. doi: 10.1023/a:1020724506337. [DOI] [PubMed] [Google Scholar]
- McMahon CM, Brown DJ, Patuzzi RB. Transient focal cooling at the round window and cochlear nucleus shows round window CAP originates from cochlear neurones alone. Hear. Res. 2004;190:75–86. doi: 10.1016/S0378-5955(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Moller A, Jensen LH, Pedersen A, Harajehi JB, Pakkenberg H. MPTP-induced Parkinsonism in minipigs: A behavioral, biochemical, and histological study. Neurotoxicol. Teratol. 1999;21:169–175. doi: 10.1016/s0892-0362(98)00037-3. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int. J. Dev. Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mills DM, Loos BM, Henley CM. Increased susceptibility of male rats to kanamycin-induced cochleotoxicity. Hear. Res. 1999;128:75–79. doi: 10.1016/s0378-5955(98)00190-7. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sone N, Suzuki K, Saitoh T. Studies on the toxicity of 1-methyl-4-phenylpyridinium ion (MPP+) against mitochondria of mouse brain. J. Neurol. Sci. 1988;86:97–110. doi: 10.1016/0022-510x(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Spangler KM, Schneider BL, Javel E. Selective degeneration of a putative cholinergic pathway in the chinchilla cochlea following infusion with ethylcholine aziridinium ion. Brain Res. 1991;544:94–100. doi: 10.1016/0006-8993(91)90889-4. [DOI] [PubMed] [Google Scholar]
- Mulders WHAM, Robertson D. Dopaminergic olivocochlear neurons originate in the high frequency region of the lateral superior olive of guinea pigs. Hear. Res. 2004;187:122–130. doi: 10.1016/s0378-5955(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J. Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Niu X, Canlon B. Activation of tyrosine hydroxylase in the lateral efferent terminals by sound conditioning. Hear. Res. 2002;174:124–132. doi: 10.1016/s0378-5955(02)00646-9. [DOI] [PubMed] [Google Scholar]
- Niu X, Bogdanovic N, Canlon B. The distribution and the modulation of tyrosine hydroxylase immunoreactivity in the lateral olivocochlear system of the guinea pig. Neuroscience. 2004;125:725–733. doi: 10.1016/j.neuroscience.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Oestreicher E, Arnold W, Ehrenberger K, Felix D. Dopamine regulates the glutamatergic inner hair cell activity in guinea pigs. Hear. Res. 1997;107:46–52. doi: 10.1016/s0378-5955(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Ota Y, Dolan DF. The effects of efferent activation on the acoustically and electrically evoked otoacoustic emission. Hear. Res. 2000;148:124–136. doi: 10.1016/s0378-5955(00)00150-7. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Wiederhold KH. Acute administration of 1-N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a compound producing parkinsonism in humans, stimulates [2-14C]deoxyglucose uptake in the regions of the catecholaminergic cell bodies in the rat and guinea pig brains. Brain Res. 1984;301:187–191. doi: 10.1016/0006-8993(84)90422-0. [DOI] [PubMed] [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V. Mechanisms of MPTP toxicity. Mov. Disord. 1998;1:35–38. [PubMed] [Google Scholar]
- Puel JL. Chemical synaptic transmission in the cochlea. Prog. Neurobiol. 1995;47:449–476. doi: 10.1016/0301-0082(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Pujol R. Lateral and medial efferents: a double neurochemical mechanism to protect and regulate inner and outer hair cell function in the cochlea. Br. J. Audiol. 1994;28:185–191. doi: 10.3109/03005369409086567. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL, Gervais d’Aldin C, Eybalin M. Pathophysiology of the glutamatergic synapses in the cochlea. Acta Oto-laryngol. (Stockh.) 1993;113:330–334. doi: 10.3109/00016489309135819. [DOI] [PubMed] [Google Scholar]
- Qun LX, Pirvola U, Saarma M, Ylikoski J. Neurotrophic factors in the auditory periphery. Ann. N.Y. Acad. Sci. 1999;884:292–304. doi: 10.1111/j.1749-6632.1999.tb08649.x. [DOI] [PubMed] [Google Scholar]
- Rajan R. Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity. I. Dependence on electrical stimulation parameters. J. Neurophysiol. 1988;60:549–568. doi: 10.1152/jn.1988.60.2.549. [DOI] [PubMed] [Google Scholar]
- Rajan R. Frequency and loss dependence of the protective effects of the olivocochlear pathways in cats. J. Neurophysiol. 1995;74:598–615. doi: 10.1152/jn.1995.74.2.598. [DOI] [PubMed] [Google Scholar]
- Robertson D, Anderson CJ, Cole KS. Segregation of efferent projections to different turns of the guinea pig cochlea. Hear. Res. 1987;25:69–76. doi: 10.1016/0378-5955(87)90080-3. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol. Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J, Nouvian R, Gervais d’Aldin C, Pujol R, Eybalin M, Puel JL. Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea. Eur. J. Neurosci. 2001;14:977–986. doi: 10.1046/j.0953-816x.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- Safieddine S, Eybalin M. Triple immunoflourescence evidence for coexistence of acetylcholine, enkephalins, and calcitonin-gene related peptide within efferent (olivocochlear) neurons of rats and guinea pigs. Eur. J. Neurosci. 1992;4:981–992. doi: 10.1111/j.1460-9568.1992.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Safieddine S, Prior AM, Eybalin M. Choline acetyltransferase, glutamate decarboxylase, tyrosine hydroxylase, calcitonin gene-related peptide and opioid peptides coexist in lateral efferent neurons of rat and guinea-pig. Eur. J. Neurosci. 1997;9:356–367. doi: 10.1111/j.1460-9568.1997.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Sahley TL, Nodar RH. Improvement in auditory function following pentazocine suggests a role for dynorphins in auditory sensitivity. Ear Hear. 1994;15:422–431. doi: 10.1097/00003446-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Sahley TL, Kalish RB, Musiek FE, Hoffman DW. Effects of opioid be drugs on auditory evoked potentials suggest a role of lateral olivocochlear dynorphins in auditory function. Hear. Res. 1991;55:133–142. doi: 10.1016/0378-5955(91)90099-u. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Warr WB, Lopez DE. Anatomy of olivocochlear neurons in the hamster studied with FluoroGold. Hear. Res. 2003;185:65–76. doi: 10.1016/s0378-5955(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Satake M, Liberman MC. Morphological subclasses of lateral olivocochlear terminals? Ultrastructural analysis of inner spiral bundle in cat and guinea pig. J. Comp. Neurol. 1996;371:621–632. doi: 10.1002/(SICI)1096-9861(19960805)371:4<621::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Sayre LM. Biochemical mechanism of action of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicol. Lett. 1989;48:121–149. doi: 10.1016/0378-4274(89)90168-9. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 2001;125:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Sewell WF, Starr PA. Effects of calcitonin gene-related peptide and efferent nerve stimulation on afferent transmission in the lateral line organ. J. Neurophysiol. 1991;65:1158–1169. doi: 10.1152/jn.1991.65.5.1158. [DOI] [PubMed] [Google Scholar]
- Shinka T, Castagnoli NJ, Wu EY, Hoag MKP, Trevor AJ. Cation-exchange high-performance liquid chromatography assay for the nigrostriatal toxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and its monoamine oxidase B generated metabolites in brain tissues. J. Chromatogr. 1987;398:279–287. doi: 10.1016/s0021-9673(01)96513-6. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc. Natl. Acad. Sci. USA. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DW, Mount RJ. Damage to cochlear efferents following AF64A intoxication. Acta Oto-Laryngol. 1993;113:512–518. doi: 10.3109/00016489309135855. [DOI] [PubMed] [Google Scholar]
- Smith DW, Mount RJ, Callahan JW. Selective damage to cochlear efferents by the choline neurotoxin ethylcholine mustard aziridinium ion (AF64A) in the chinchilla. Hear. Res. 1989;42:113–117. doi: 10.1016/0378-5955(89)90121-4. [DOI] [PubMed] [Google Scholar]
- Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of efferent stimulation on cochlear potentials in the guinea pig. J. Neurosci. 1995;15:3667–3678. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Galinovic-Schwartz V, Liu W, Lefebvre P, Kopke R, Malgrange B, Moonen G, Water TR. The role of the neurotrophins in maturation and maintenance of postnatal auditory innervation. Am. J. Otol. 1996;17:486–492. [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. NeuroReport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stankovic KM, Corfas G. Real-time quantitative RT-PCR for low-abundance transcripts in the inner ear: analysis of neurotrophic factor expression. Hear. Res. 2003;185:97–108. doi: 10.1016/s0378-5955(03)00298-3. [DOI] [PubMed] [Google Scholar]
- Stover T, Gong TL, Cho Y, Altschuler RA, Lomax MI. Expression of the GDNF family members and their receptors in the mature rat cochlea. Brain Res. Mol. Brain Res. 2000;76:25–35. doi: 10.1016/s0169-328x(99)00328-9. [DOI] [PubMed] [Google Scholar]
- Stover T, Nam Y, Gong TL, Lomax MI, Altschuler RA. Glial cell line-derived neurotrophic factor (GDNF) and its receptor complex are expressed in the auditory nerve of the mature rat cochlea. Hear. Res. 2001;155:143–151. doi: 10.1016/s0378-5955(01)00227-1. [DOI] [PubMed] [Google Scholar]
- Sun W, Salvi RJ. Dopamine modulates sodium currents in cochlear spiral ganglion neurons. NeuroReport. 2001;12:803–807. doi: 10.1097/00001756-200103260-00037. [DOI] [PubMed] [Google Scholar]
- Tipton KF, Singer TP. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J. Neurochem. 1993;61:1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- Ulfendahl M. Mechanical responses of the mammalian cochlea. Prog. Neurobiol. 1997;53:331–380. doi: 10.1016/s0301-0082(97)00040-3. [DOI] [PubMed] [Google Scholar]
- Usami S, Hozawa J, Tazawa M, Yoshihara T, Igarashi M, Thompson GC. Immunocytochemical study of catecholaminergic innervation in the guinea pig cochlea. Acta Oto-laryngol., Suppl. (Stockh.) 1988;447:36–45. doi: 10.3109/00016488809102855. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Mugnaini E. Distribution and dendritic features of three groups of rat olivocochlear neurons. A study with two retrograde cholera toxin tracers. Anat. Embryol. (Berl.) 1992;185:1–16. doi: 10.1007/BF00213596. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, McFadden SL, Liberman MC. Long-term effects of sectioning the olivocochlear bundle in neonatal cats. J. Neurosci. 1998;18:3859–3869. doi: 10.1523/JNEUROSCI.18-10-03859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr WB. Organization of olivocochlear efferent systems in mammals. In: Webster DB, Popper AN, Fay RR, editors. Mammalian Auditory Pathway: Neuroanatomy. Boston: Little, Brown, and Co.; 1992. pp. 410–448. [Google Scholar]
- Warr WB, Boche JE. Diversity of axonal ramifications belonging to single lateral and medial olivocochlear neurons. Exp. Brain Res. 2003;153:499–513. doi: 10.1007/s00221-003-1682-3. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ, White JS. Organization of efferent fibers: the lateral and medial olivocochlear systems. In: Altschuler RA, Hoffman DW, Bobbin RP, editors. Neurobiology of Hearing: The Cochlea. New York: Raven Press; 1986. pp. 333–348. [Google Scholar]
- Warr WB, Boche JEB, Neely ST. Efferent innervation of the inner hair cell region: origins and terminations of two lateral olivocochlear systems. Hear. Res. 1997;108:89–111. doi: 10.1016/s0378-5955(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Warr WB, Boche JEB, Ye Y, Kim DO. Organization of olivocochlear neurons in the cat studied with the retrograde tracer cholera toxin-B. J. Assoc. Res. Otolaryngol. 2002;3:457–478. doi: 10.1007/s10162-002-2046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead ML, Lonsbury-Martin BL, Martin GK. Evidence for two discrete sources of 2f1 − f2 distortion-product otoacoustic emission in rabbit. II. Differential physiological vulnerability. J. Acoust. Soc. Am. 1992;92:2662–2682. doi: 10.1121/1.404382. [DOI] [PubMed] [Google Scholar]
- Wiederhold ML. Variations in the effects of electric stimulation of the crossed olivocochlear bundle on cat single auditory-nerve-fiber responses to tone bursts. J. Acoust. Soc. Am. 1970;48:966–977. doi: 10.1121/1.1912235. [DOI] [PubMed] [Google Scholar]
- Wu W-R, Zhu Z-T, Zhu X-Z. Differential effects of l-deprenyl on MPP+- and MPTP-induced dopamine overflow in microdilysates of striatum and nucleus accumbens. Life Sci. 2000;67:241–250. doi: 10.1016/s0024-3205(00)00628-7. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear. Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Wang J, Salvi RJ, Henderson D. Effects of kainic acid on the cochlear potentials and distortion product otoacoustic emissions in chinchilla. Hear. Res. 1996;95:161–167. doi: 10.1016/0378-5955(96)00047-0. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Henderson D, McFadden SL, Ding DL, Salvi RJ. Auditory nerve fiber responses following chronic cochlear de-efferentation. J. Comp. Neurol. 1999;406:72–86. doi: 10.1002/(sici)1096-9861(19990329)406:1<72::aid-cne5>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]