Abstract
Developmental dysplasia of the hip (DDH) is a neonatal condition with various causes. Neuromuscular dysplasia of the hip (NDH) is a sequel of neuromuscular disease, and generally presents later in childhood than DDH. Some evidence, however, supports a concept of a neuromuscular etiology of DDH: (1) a high prevalence of spinal dysraphism in DDH; and (2) abnormal sensory evoked potentials in 31% of DDH patients. To explore this suggestion we ascertained the presence of neuromuscular disease within a cohort of DDH patients, and asked whether the neuromuscular condition is the initial etiology of the dysplasia or a coincidental finding. We retrospectively reviewed patients presenting with DDH. Only those with an initial diagnosis of DDH and a subsequent diagnosis of a neuromuscular condition were assessed. Fifteen of 560 patients fulfilled the criteria, however the presence of true DDH within this group was minimal, as several cases emerged as early presenting NDH. We therefore believe it unlikely DDH has a substantial neurological etiology.
Level of Evidence: Level III, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Developmental dysplasia of the hip (DDH), previously known as congenital dislocation of the hip (CDH), is a common and well-documented condition primarily detected in neonates. The principal abnormality is hip instability affecting both acetabular and femoral development [19]. It is a spectrum of disease, ranging from the unstable hip, where the femoral head is well centered, but which displaces when force is applied, to the fully fixed dislocated hip [1]. DDH has a complex etiology, although research suggests it is more prevalent among first children, girls, those with a positive family history, and infants who were breech presentation [5, 21, 22, 24]. DDH in the UK is screened for neonatally using the Ortolani and Barlow tests, although ultrasound has proved a useful secondary tool [2, 5, 8, 23]. Early diagnosis allows usually successful treatment in a Pavlik harness. If this is unsuccessful or the diagnosis made late surgical treatment is necessary [5, 20].
The displacement and dislocation of the hip is also a recognized sequela of neuromuscular conditions such as cerebral palsy and myelomeningocele. This is considered an entirely different clinical entity to DDH, due to the differing underlying pathology and consequently differing expected outcomes. Dysplastic changes occur as a result of altered muscle tone, which causes abnormal stresses across the hip joint. This will gradually affect the positioning of the femoral head, subsequently impairing acetabular development [7, 11, 13, 17]. Consequently, neuromuscular dysplasia of the hip (NDH) generally presents later in childhood than DDH [14]. Treatment is primarily surgical, and patients may require multiple operations due to the underlying problem of disordered muscle tone [13, 17].
Wilkinson and Sedgwick [21, 22] proposed a proportion of DDH cases, however, might be a result of neurological dysfunction. A group of patients who had DDH resistant to primary surgery was studied and a high proportion had either overt or occult spinal dysraphism. It was suggested spinal dysraphism could be a contributory factor to the development of resistant DDH. Sensory evoked potentials (SEPs) were studied in a cohort of DDH patients, and compared to that of a normal population. These assessed both the dorsal column and medial lemniscus sensory pathways of the spinal cord. Thirty-one percent of DDH patients had altered SEPs that could indicate the complex etiology of DDH may have a neurological component [21, 22].
We hypothesized there is no etiological association between neurological abnormality and DDH and asked whether it was possible to identify early NDH by studying the natural history of the two disorders. In short we investigated (1) whether the neuromuscular condition is the primary etiological agent for the acetabular dysplasia or simply a coincidental finding, and (2) the interactions of DDH and neuromuscular disorders.
Materials and Methods
We retrospectively examined the medical records of a cohort of patients who presented between 1990 and 2006 and who had an initial diagnosis of DDH that was then subsequently reassessed due to the presence of a substantial primary neuromuscular disorder. During this time 560 infants presented and of these, 15 (2.7%) children (three male and 12 female) fulfilled the inclusion criteria. Among these 15 patients there were 25 dysplastic hips: 10 patients presented with a bilateral DDH, three with left-sided DDH, and two with right-sided DDH. The median age of presentation of DDH within the cohort was 4.2 months (range, 0–9 months). There were six patients (all with bilateral dysplasia) who were diagnosed during the neonatal checks and were all treated initially using a Pavlik harness. This reduced and stabilized eight hips (six patients), however four hips (three patients) underwent subsequent surgery. Overall, 15 of the 27 hips (11 patients) underwent surgery (Table 1).
Table 1.
Patient and operative details
| Patient number | Gender | Neurological diagnosis | DDH | Pavlik harness | Other treatment required | |||
|---|---|---|---|---|---|---|---|---|
| Side | Age at Dx | Used | Success | |||||
| L | R | |||||||
| 1 | M | CP-SD | R | 3 m | N | – | – | R CR, R CR + AT, R OR |
| 2 | F | CP-SD | BL | 9 m | N | – | – | R CR, BL VDO |
| 3 | F | syndrome- S Dy + TofF | L | 9 m | N | – | – | L CR + AT |
| 4 | F | SBO/SpD | BL | 0 m | Y | Y | Y | – |
| 5 | F | GDD | BL | 0 m | Y | N | Y | L CR + AT |
| 6 | F | CP | L | 9 m | N | – | – | – |
| 7 | F | CP-SQ | BL | 8 m | N | – | – | L CR + AT, R CR + ST, R VDO, R PO + AR, L PF-VDO, L PF-VO |
| 8 | F | CP-SD | BL | 0 m | Y | N | Y | L CR + AT, L SPO |
| 9 | F | CP | BL | 3 m | N | – | – | L OR |
| 10 | M | CMD | R | 8 m | N | – | – | R OR + AR |
| 11 | F | GDD | L | 8 m | N | – | – | L OR + C + A |
| 12 | F | CS | BL | 0 m | Y | N | N | BL CR + AT, R OR |
| 13 | F | CP-SD | BL | 0 m | Y | Y | Y | – |
| 14 | F | GDD | BL | 0 m | Y | Y | Y | – |
| 15 | M | CP-SQ | L | 6 m | N | – | – | L CR + AR, B/L AR |
M = male; F = female; R = right; L = left; BL = bilateral; Y = yes; N = no; m = months; CP = cerebral palsy; SD = spastic diplegia; SH = spastic hemiplegia; SQ = spastic quadraplegia; S Dy = subtle dysmorphism; TofF = tetraology of Fallot; SBO = spina bifida occulta; GDD = global developmental delay; CMD = congenital muscular dystrophy; CS = Curriano’s syndrome; CR = closed reduction; OR = open reduction; + AT = with adductor tenotomy; +AR = with adductor release; +A+C = with acetabuloplasty and capsulorrhaphy; O = osteotomy (type); V = varus; VD = varus derotation; P = pelvic; PF = proximal femoral; S = Salter.
We reviewed the medical records with regard to the natural history and progression of the hip dysplasia, and the treatment required. We (AL and NMPC) measured the acetabular index and migration percentage on anteroposterior pelvic radiographs where possible. One-hundred nineteen anteroposterior radiographs of the pelvis were assessed, with a range of one to 16 radiographs per patient, which reflects the wide age range (0.7–16.7 years) of initial and followup radiographs. Radiographic documentation was complete in all cases. Acetabular index was classified according to Tönnis, and subluxation was defined as a migration percentage of greater than 30% [1, 3, 9, 12, 17, 18]. Radiographs taken before October 2006 were on plain film, and so could be measured on a light box using a transparent ruler and pencil on an overlying transparent sheet. Radiographs taken after October 2006 could be accessed only on the computerized Picture Archiving and Communications System (PACS), and therefore were measured using the tools provided within the software.
Results
In relation to the question of whether the neuromuscular condition is the primary etiological agent for the acetabular dysplasia or simply a coincidental finding we found severe dysplasia in 36 radiographs of the left hip (30.3% of total assessed) and 44 radiographs of the right hip (37% of total assessed), and nine left hips and six right hips (in 10 patients) had at least one radiograph showing severe dysplasia. Questionable dysplasia was seen in 16 radiographs of the left hip (13.4% of total assessed) and 10 radiographs of the right hip (8.4% of total hips assessed). Persistent severe dysplasia (defined as severe acetabular dysplasia seen in at least 80% of the radiographic evidence) was seen in six hips in five patients. Only four patients had no radiographic evidence of acetabular dysplasia with regard to acetabular index.
Subluxation (including dislocations) was seen in 27 radiographs of the left hip (22.6% of total assessed) and 42 radiographs of the right hips (35.3% of total assessed). Nine left hips and 11 right hips (in 11 patients) had subluxation on at least one radiograph. Seven hips in six patients had at least one incidence of dislocation within the radiographic evidence.
Of eight hips successfully treated in the Pavlik harness the only hip that did not show sustained resolution was in patient 5, who was diagnosed with bilateral DDH neonatally, and global developmental delay of unknown cause. The Pavlik harness reduced and stabilized the right hip, as shown in the radiographic evidence (Figs. 1A-B). The right hip remained stabilized until approximately 1 year of age, when the migration percentage began to steadily increase until subluxation occurred (Figs. 1C-D, 2). This is probably a case where the initial diagnosis of DDH was correct and well treated, however NDH subsequently developed independently of the initial dysplasia.
Fig. 1A–D.
Radiographic evidence charting the acetabular dysplasia in Patient 5 shows a (A) dislocated left hip at age 0.45 years. Arthrogram shows (B) concentric closed reduction of the left hip at age 0.47 years, (C) early subluxation and valgus orientation of the right hip at age 2.64 years, and (D) progressive migration of both hips at age 3.16 years.
Fig. 2.
Migration percentage of the right hip in patient 5 is shown to confirm progressive displacement.
It is clear there is a very high incidence of persisting dysplasia subluxation in the hips and neuromuscular disease reflecting a different natural history from a simple DDH.
Discussion
We wished to investigate the relationship between neuromuscular disorders to DDH and whether there was an association. In addition we wished to identify whether those hips subsequently diagnosed as having being part of a neuromuscular disorder presented a different natural history in terms of persistent dysplasia and subluxation and the need for surgery. The results indicate those hips retrospectively diagnosed as being part of a neuromuscular disorder do indeed behave differently in terms of displacement and dysplasia and is a major for requirement for subsequent surgery.
The question is whether or not neurological abnormalities may be a direct causal link to primary DDH. The incidence in this cohort suggests not.
There are limitations to this study. Only a few patients fit the inclusion criteria and consequently the number of patients within the cohort was not large enough to conduct a meaningful statistical analysis. The study was also limited by its retrospective nature which meant the followup period was variable for each patient, as was the frequency and timing of radiographs. Finally, there was no way to standardize the radiographic technique and therefore the positioning of the hips may have affected the values of the measurements. Any conclusions drawn from the data must be seen as suggestions for further study particularly for a longitudinal study in a multicenter setting.
However, it appears unlikely Wilkinson’s assertion [22] that DDH has a substantial neurological etiology is correct. The proportion of patients who had true DDH and were subsequently diagnosed with a neuromuscular condition was minimal, as many of the cases when assessed retrospectively were clearly examples of NDH, not DDH. Therefore, our data suggest DDH and NDH are in fact two separate clinical conditions without a shared etiology.
However, there were interesting cases that illustrate the differing presentations of acetabular dysplasia occurring within the context of a neuromuscular disorder. These have been discussed with reference to age at first presentation as this had most influence on primary treatment.
In only 40% of this cohort of hips was subsequent neurological pathology diagnosed within the first 3 months of life. In conventional DDH early presentation is usual, although there is an incidence of late presentation. In relation to treatment only 66.7% of the hips treated were treated successfully, which is far lower than the normal success rate with DDH (which is 85%) [4]. Altered muscle tone could compromise the effect of the Pavlik harness. Clearly, normal biomechanical conditions cannot be restored [7, 17]. It may be argued therefore that the successful treatment in the Pavlik harness actually indicates the true DDH. Seven of the eight hips reduced were stabilized and showed no further clinical or radiographic signs. Accordingly it may well be these cases were coincidentally true cases of DDH rather than neurologically related.
Successful treatment in a Pavlik harness appeared a good prognostic factor for acetabular development in this cohort, as seven of the eight hips reduced and stabilized by a Pavlik harness showed no further clinical or radiographic signs of developing dysplasia (although two hips were in a patient who was only 0.7 years old when the study was conducted). This probably indicates these cases represented true DDH in the cohort.
A study of children with cerebral palsy demonstrated acetabular dysplasia must be present for dislocation to occur, even if increased muscle tone was present [6]. Therefore initial treatment with the Pavlik harness allowing resolution of dysplasia could result in the successful development of the acetabulum and consequently prevent further hip dysfunction. This is illustrated well in patient 13 who was diagnosed neonatally with bilateral DDH successfully treated with a Pavlik harness. Although the patient demonstrated a mild increase in migration percentage on the right side (Fig. 3), the acetabular index is well within the ranges of normal, and is perhaps a protective factor against further subluxation.
Fig. 3.
Migration percentage of the right hip of patient 13 is shown to show mild increase in migration % (see text).
It is possible DDH could act as stimulus for the development of NDH. NDH occurs as a result of prolonged distorted stress across the hip joint, which causes a gradual development of acetabular dysplasia [7, 17]. If NDH appears to develop neonatally, it is possible DDH is initially present but its resolution is subsequently impaired due to the neuromuscular abnormality. Therefore DDH in children with neuromuscular disorders may predispose them to developing early-presenting NDH. Consequently the risk factors, which influence the development of DDH, could also affect the likelihood that NDH will occur in these patients.
The combination of DDH and a neuromuscular condition could also increase the risk of NDH developing where the neuromuscular symptoms are relatively mild. The risk of acetabular dysfunction increases in cerebral palsy with decreased gross motor function [16]. However, patient 8 had a very mild left-sided spastic hemiplegic cerebral palsy (which would be unlikely to cause NDH later in life), presented with bilateral DDH as a neonate, and was managed using a Pavlik harness. The right hip responded well, but the left hip required further surgery. This could be an example of unresponsive DDH, although the altered tone on the left side even though minimal, in combination with DDH may have facilitated the development of NDH.
In those who presented before the age of 3 months it is likely eight hips in five patients had true DDH. The remainder may have had DDH that was unresolved due to disordered muscle tone and consequently developed into NDH. They may have also simply been representatives of the small proportion of DDH that is unresponsive to treatment, although this is less probable.
Nine patients (13 dysplastic hips) presented after 3 months of age, representing 60% of the cohort. However, in a study of late-presenting DDH, only 2.1% of cases were diagnosed after 3 months [15]. The likelihood of all of the late-presenting cases in the cohort being DDH, which was missed or developed after the early screening program, is therefore minimal. This indicates acetabular dysplasia which is neuromuscular in origin was more prevalent in those who presented after 3 months than in those who presented before this time.
Late-presenting DDH would initially require a closed or open reduction, and all patients (except patient 6) were primarily treated in this manner, however five hips required further surgical therapy. It is difficult to analyze the surgical techniques due to differing trends in preferred surgical options [10]. It is clear, however, that those who presented after 3 months had a worse prognosis for treatment, requiring a mean of 2.1 surgical procedures (range, 0–6 procedures), compared to those who were diagnosed before 3 months of age who required a mean of one (range, 0–3 procedures). This again indicates the prevalence of NDH within the late-presenting group was higher than in those who presented with DDH prior to 3 months of age.
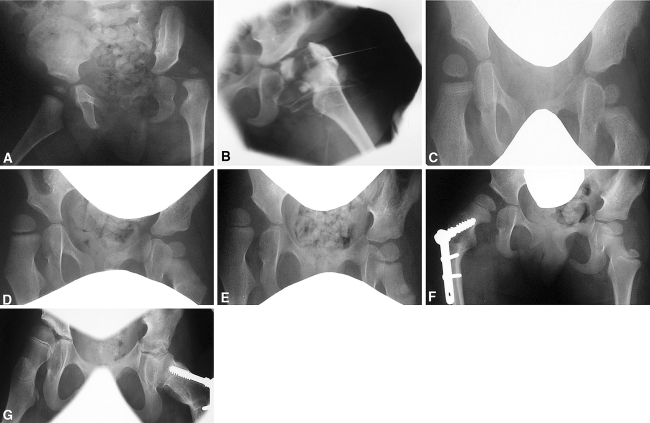
The six hips that radiographically showed persistent severe dysplasia were all cases that presented after 3 months of age. It is therefore likely all of these cases (patients 3, 7, 9, 12, and 15) demonstrated NDH, as the picture of persistent dysplasia which does not resolve despite appropriate treatment, combined with clearly abnormal migration percentage, is particularly typical of this condition [7, 12, 17]. This is demonstrated in patient 7, who was at high risk of developing NDH due to the diagnosis of spastic quadriplegia cerebral palsy with poor gross motor function [16]. The patient presented with DDH at age 8 months, and had closed reductions on both sides, which were unsuccessful in preventing further subluxation. The severe bilateral dysplasia and progressive migration, primarily of the right hip, can be clearly seen in the radiographic evidence (Fig. 4A–E). Further bilateral osteotomies were required (Fig. 4F–G), but both hips had loss of function and demonstrated arthritic change at age 12 years. Due to the nonresolution of the migration percentage especially in the right hip (Fig. 5) and the minimal effect of the operations, it is very likely this case represents true NDH without any coexisting DDH.
Fig. 4A–G.
Radiographic evidence charting acetabular dysplasia in patient 7 at (A) age 0.67 years shows the dislocation of the left hip. (B) At age 0.67 years, arthrogram confirms dislocation of the left hip. (C) Reduction of the left hip and early migration of the right hip is visible at age 1.34 years. (D) At age 1.97 years, there is progressive migration of the right hip. (E) Subluxation of the right hip is apparent at age 2.81 years. (F) At age 3.61 years the right hip is pictured following varus osteotomy, and (G) the left hip at age 9.76 years following varus osteotomy.
Fig. 5.
The migration percentage of both hips of patient 7 is shown to show variable natural history (see Fig. 4).
Unlike Wilkinson [21, 22] suggested, we did not observe cases of DDH which appeared directly caused by a neuromuscular condition but we did find the natural history and progression of persistence and progression of acetabular dysplasia in NDH was as one would intuitively expect, different from DDH.
The incidence in this series of neurological abnormalities being directly associated with hip DDH is extremely low and we believe rules out direct etiological association.
Acknowledgments
We thank Mrs. D. J. Collins for secretarial assistance.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aronsson DD, Goldberg MJ, Kling TF, Roy DR. Developmental dysplasia of the hip. Pediatrics. 1994;94:201–208. [PubMed]
- 2.Barlow TG. Early diagnosis and treatment of congenital dislocation of the hip. Proc R Soc Med. 1963;56:804–806. [DOI] [PMC free article] [PubMed]
- 3.Boniforti FG, Fujii G, Angliss RD, Benson MKD. The reliability of measurements in pelvic radiographs in infants. J Bone Joint Surg Br. 1997;79:570. [DOI] [PubMed]
- 4.Cashman JP, Round J, Taylor G, Clarke NMP. The natural history of developmental dysplasia of the hip after early supervised treatment in the Pavlik harness. J Bone Joint Surg Br. 2002;84:418–425. [DOI] [PubMed]
- 5.Clarke NMP. Developmental dysplasia of the hip. In: Bulstrode C, ed. Oxford Textbook of Orthopedics, Trauma. Oxford, UK: Oxford University Press; 2002:2543–2548.
- 6.Cooke PH, Cole WG, Carey RP. Dislocation of the hip in cerebral palsy: natural history and predictability. J Bone Joint Surg Br. 1989;71:441–446. [DOI] [PubMed]
- 7.Cornell MS. The hip in cerebral palsy. Dev Med Child Neurol. 1995;37:3–18. [DOI] [PubMed]
- 8.Elbourne D, Dezateux C, Arthur R, Clarke NMP, Gray A, King A, Quinn A, Gardner F, Russell G. Ultrasonography in the diagnosis and management of developmental hip dysplasia (UK Hip Trial): clinical and economic results of a multicentre randomised controlled trial. Lancet. 2002;360:2009–2017. [DOI] [PubMed]
- 9.Faraj S, Atherton WG, Stott NS. Inter- and intra-measurer error in the measurement of Reimers’ hip migration percentage. J Bone Joint Surg Br. 2004;86:434–437. [DOI] [PubMed]
- 10.Gul R, Coffey JC, Khayyat G, McGuinness AJ. Late presentation of developmental dysplasia of the hip. Ir J Med Sci. 2002;171:139–140. [DOI] [PubMed]
- 11.Kim HT, Wenger DR. Location of acetabular deficiency and associated hip dislocation in neuromuscular hip dysplasia: three dimensional computed topographic analysis. J Pediatr Orthop. 1997;17:143–151. [DOI] [PubMed]
- 12.Reimers J. The stability of the hip in children: a radiological study of the results of muscle surgery in cerebral palsy. Acta Orthop Scand Suppl. 1980;184:1–100. [DOI] [PubMed]
- 13.Rueda J, Carroll NC. Hip instability in patients with myelomeningocele. J Bone Joint Surg Br. 1972;54:422–431. [PubMed]
- 14.Samilson RL, Tsou P, Aamoth G, Green WM. Dislocation and subluxation of the hip in cerebral palsy. Pathogenesis, natural history and management. J Bone Joint Surg Am. 1972;54:863–873. [PubMed]
- 15.Sharpe P, Mulpuri K, Chan A, Cundy PJ. Differences in risk factors between early and late diagnosed developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed. 2006;91:158–162. [DOI] [PMC free article] [PubMed]
- 16.Soo B, Howard JJ, Boyd RN, Reid SM, Lanigan A, Wolfe R, Reddihough D, Grahm HK. Hip displacement in cerebral palsy. J Bone Joint Surg Am. 2006;88:121–129. [DOI] [PubMed]
- 17.Speigel DA, Flynn JM. Evaluation and treatment of hip dysplasia in cerebral palsy. Orthop Clin North Am. 2006;37:185–196. [DOI] [PubMed]
- 18.Tönnis D. Normal values of the hip joint for the evaluation of x-rays in children and adults. Clin Orthop Relat Res. 1976;119:39–47. [PubMed]
- 19.Weinstein SL, Mubarak SJ, Wenger DR. Developmental Hip Dysplasia and Dislocation: Part I. Instr Course Lect. 2004;53:523–530. [PubMed]
- 20.Weinstein SL, Mubarak SJ, Wenger DR. Developmental Hip Dysplasia and Dislocation: Part II. Instr Course Lect. 2004;53:531–542. [PubMed]
- 21.Wilkinson JA. Congenital Displacement of the Hip. Berlin, Germany: Springer Verlag; 1985:44–45.
- 22.Wilkinson JA, Sedgwick EM. Occult spinal dysraphism in established congenital dislocation of the hip. J Bone Joint Surg Br. 1988;70:744–749. [DOI] [PubMed]
- 23.Wirth T, Stratmann L, Hinrichs F. Evolution of late presenting developmental dysplasia and associated surgical procedures after 14 years of neonatal ultrasound screening. J Bone Joint Surg Br. 2004;86:585–589. [PubMed]
- 24.Wynne-Davies R. Acetabular dysplasia and familial joint laxity: two etiological factors in congenital dislocation of the hip. A review of 589 patients and their families. J Bone Joint Surg Br. 1970;52:704–716. [PubMed]