Abstract
Various approaches have been described for metal-on-metal hip resurfacing. We compared the posterolateral and direct lateral approaches for complications, pain, function, and implant survival in the short and medium term for two surgeons in a consecutive series of 790 patients (909 hips; July 1997 to July 2004) followed until July 2007. The direct lateral approach group included 135 resurfacing procedures and the posterolateral group included 774 procedures. There was no difference between the two groups for age or gender. The minimum followup for the anterolateral group was 2 years (mean, 5.1 years; range, 2.0–9.4 years) and for the posterolateral group 2 years (mean, 5.5 years; range, 2.0–9.6 years). There were no differences between the two approaches for complications, additional surgery, implant survival, or Oxford hip scores. The 8-year survival rate was 97.9% (95% confidence interval, 89.9–100) for the direct lateral approach and 97.2% (95% confidence interval, 93.9–99.3) for the posterolateral approach. This study indicates both approaches offer excellent pain reduction and return to function after Birmingham hip resurfacing with no difference in survival or in the incidence of complications. An 8-year survival rate of 97% can be achieved using either the posterolateral approach or the direct lateral approach.
Level of Evidence: Level III, therapeutic study. See the Guidelines for authors for a complete description of levels of evidence.
Introduction
Since the reemergence of hip resurfacing using metal-on-metal bearings, the majority of surgeons have preferred the posterolateral approach, which is favored by its developers [18]. Many surgeons who do THA use a direct lateral approach such as that of Hardinge [9, 16] or Stephenson and Freeman [26] and would have a preference to apply a similar approach in hip resurfacing. In deciding which approach to use, consideration of survival and complications using one approach or another must be assessed. Hip resurfacing presents one major technical difficulty that conventional THA does not – that of preparing the acetabulum while the femoral head remains. The view of the acetabulum may be impaired because of the presence of this retained femoral head. A particular approach may make the operation more technically difficult and this may lead to poor component positioning, increased surgical complications, and higher revision rates. Operations that are complex or technically demanding can have a higher complication rate [10, 13]. For hip resurfacing, a current central issue is whether the use of a posterolateral approach may potentially jeopardize the femoral blood supply leading to femoral head collapse [19, 27, 29]. Direct studies of blood supply have been suggestive but not conclusive [25]. The crucial issue is whether any such theoretical insult might lead to implant failures on the femoral side and subsequent revision. Thus, implant survival is the preferred outcome measure for indicating any real effect on femoral head viability.
Surgical complications may vary between approaches or show a marked difference between different surgeons or different centers [13]. In THA, for example, debate has centered on dislocation rates when comparing direct lateral and posterolateral approaches [13]. When deciding on which approach to use, the type and frequency of the complications associated with each approach must be considered. Any clinically important difference in complication rates between approaches would have a bearing on which approach would be most suitable for hip resurfacing.
This study seeks to address whether there is any difference in the survival, functional outcome, mortality, or complications between the direct lateral and the posterior approach when used for hip resurfacing in two groups of comparable patients with osteoarthritis of the hip.
Materials and Methods
The study design was a case-control (two cohorts) study. A MySQL database was used for storing data on all patients treated by hip resurfacing at our institution, which was initiated in July 1997. A search of the database was performed in July 2007 to select two cohorts. At that stage, the database had details of 2765 hip resurfacing procedures. The preoperative details in the database include age, gender, primary diagnosis, address, and operating surgeon. Two groups were selected from the database in July 2007 by selecting procedures performed by one of the authors (AMT or RBT) from July 1997 through July 2004. The work of these two surgeons was chosen because the direct lateral approach is used by one of the surgeons (AMT) and the posterolateral approach by the other (RBT). Both surgeons have established practices and are experienced in hip arthroplasty. The surgeon who uses the direct lateral approach has a subspecialist interest in surgery for patients with rheumatoid arthritis. The surgeon using the posterolateral approach has a more extensive resurfacing practice, including expanded indications for the procedure such as osteonecrosis and complex secondary arthritis. The study was limited to cases of osteoarthritis to minimize potential confounding resulting from different diagnosis profiles of the two surgeons’ practices. Patients without the diagnosis of osteoarthritis and not based in the UK were excluded. A comparison of the demographics (age and gender) of the two groups in the database was performed to identify if either of these could act as confounding variables. The inclusion and exclusion criteria provided a direct lateral approach group of 135 resurfacing procedures in 111 patients and a posterolateral group of 774 procedures in 679 patients.
There was no difference in mean age at the time of surgery between the two groups (53 years [range, 27–72 years] for the direct lateral group and 54 years [range, 17–78 years] for the posterolateral group). Overall, 581 (64.0%) resurfacings were performed on male patients and 326 (36.0%) on female patients. There was no difference in the gender distribution between the two groups: there were 88 (65%) male and 47 (35%) female patients in the direct lateral group and 497 (64%) male and 277 (36%) female patients in the posterolateral group.
The Birmingham Hip Resurfacing™ system (Smith and Nephew PLC, London, UK) was used in all patients. This is an as-cast cobalt-chrome metal-on-metal total hip resurfacing design for hybrid fixation (cemented femoral component and uncemented acetabular component). Surgery was performed in a laminar airflow operating room with patients receiving an antiseptic prepreparation in the anesthesia room. Patients in both groups were supported in a lateral position for the procedure. Antibiotic prophylaxis was routinely 1.5 g cefuroxime on induction and then three subsequent doses of 750 mg cefuroxime. One of the surgeons (AMT) uses exhaust suits as an additional aseptic measure. Thromboprophylaxis was postoperative warfarin aiming to keep the international normalized ratio between 1.5 and 2.0.
The posterolateral approach has been described [28]. After division of the fascia lata, the gluteus maximus is split in the line of its fibers. Sharp dissection allows reflection of the external rotators from piriformis to gluteus maximus. A posterior capsulectomy is performed and the femoral head dislocated. It is delivered into the wound by a further anterior capsular release. The femoral neck is measured to determine the femoral component size. The acetabulum is reamed with hemispheric reamers, usually to within 2 mm of the desired size. Trialing is with a 1-mm undersized component. After placement of the acetabular component, the femur is prepared using a jig to define alignment in slight valgus. Drilling, sleeve reaming, and chamfering prepare the head to accept the definitive femoral component, which is fixed using Simplex® low-viscosity cement (Howmedica International, Limerick, UK). After reduction, the posterior repair is performed using a continuous looped polydiaxone suture.
The direct lateral approach is based on the technique described by Stephenson and Freeman [26]. The skin incision is centered on the greater trochanter and curves gently proximally and posteriorly. A generous length of incision is used to minimize muscle injury resulting from retraction [11]. The tensor fascia lata is divided in the line of the skin incision. The anterior fibers of the gluteus medius are reflected superoanteriorly from the greater trochanter without splitting proximally and without disruption to the vastus lateralis muscle distally. The gluteus minimus tendon is divided leaving a stump for suturing. The joint capsule is incised and a total capsulectomy is performed. The femoral neck is measured to determine the femoral component size. Femoral component alignment is assessed visually off the neck and 5° intentional valgus is introduced. The alignment and pin entry level are marked using a diathermy pencil. The pin entry level is normally at the upper margin of the gluteus maximus insertion onto the femur. The pin is placed slightly posterior to allow for neck anteversion, which is checked using the manufacturer’s femoral jig and a guidewire. The femur is drilled over the guidewire. During this maneuver, the leg is internally rotated to eliminate the risk of the guidewire being driven into the sciatic nerve. The femur then is prepared. This reduces the femoral head size, which facilitates observation of the acetabulum. It is important that the posterior capsule be completely incised at this stage to allow the femoral neck to be depressed inferiorly. The femoral head is protected while the acetabulum is reamed. The acetabular reamer acts as a retractor and minimal additional retraction is used on the anterior lip of the acetabulum. While reaming, if the femoral neck is not depressed adequately, the inferior osteophyte may be missed and risks placement of the cup in excessive abduction. After reaming, the acetabular component is impacted and checked. Before cementing the femoral component, the pin is removed from the lateral femur to allow venting. The femur is lavaged and secondary suction started. The femoral component is cemented using Simplex® low-viscosity cement (Howmedica International). The gluteus minimus and medius tendons are repaired using a continuous polydiaxone interlocking darn based on Kessler tendon repairs.
The postoperative regimen was the same for both groups. Full weightbearing was allowed routinely from the first day with walking aids as necessary. No restriction was placed on activities, including contact sports, after 6 months.
Details of postoperative early and late complications and any revision procedure performed at this institution or elsewhere were recorded in the database. The cause of any death during followup was recorded. The Oxford hip score (OHS) [4] was used to assess pain and function. Scores were recorded preoperatively and at each postoperative outpatient visit. Typical followup of patients included a review three times during the first year. Thereafter, visits were usually on an annual or biannual basis to 5 years with a planned review every 5 years. Patients were considered lost to followup if there had been no clinical review of the patient in the 2 years before July 2007. This ensured a minimal followup of 2 years. When necessary, data were supplemented by a mailed questionnaire and review of the patient notes to reduce the number of patients who were lost to followup.
There was complete followup data available for 130 (96.3%) patients from the anterolateral group and 705 (91.1%) patients from the posterolateral group. The minimum followup for the anterolateral group was 2 years (mean, 5.1 years; range, 2.0–9.4 years) and for the posterolateral group 2 years (mean, 5.5 years; range, 2.0–9.6 years). All patients, including those without complete followup, are included in the analysis up to the point of last followup of the patient.
Our institution is a large elective orthopaedic hospital and some patients may have attended an acute local hospital for treatment of a medical complication after discharge from this institution. The medical records of all patients in the anterolateral group were reviewed and contact made with the patient’s primary care physician when necessary to ensure accuracy of the reporting of the number of complications in the two groups. It was deemed infeasible to review all the medical records from the large posterolateral group. For this reason, it was decided to produce a subgroup of the posterolateral group by performing computer-based matching by age and gender with the anterolateral group [23]. This subgroup of the posterior group was used only for reporting complications. The whole of the posterolateral group (774 cases) was used in the reporting of mortality, implant survival, and functional scores.
The “R” statistical package was used for statistical analysis [23] with confidence intervals derived from the two-sample t test for continuous variables, the chi square test for dichotomous variables, and the Mann-Whitney test when appropriate. Functional scores were assessed using the median and interquartile ranges. For the OHS, only questionnaires with more than 10 (of a possible 12) questions answered were considered valid and the percentage of questions answered was taken as the final value [22]. The Kaplan-Meier method was used for survival analysis. The Peto method for calculating the lower confidence limit for survival was used. The level of significance applied was 95.0%. Power calculations assumed β = 0.2 and in the case of survival analysis that there were exponential distributions for both treatment groups, thus allowing estimation of the expected number of events in the two groups.
Results
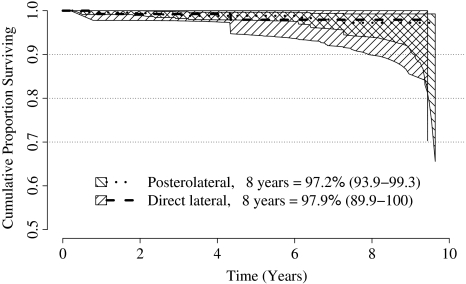
A log rank test did not show a difference in survival between the two groups for the duration of the study. Fourteen resurfacings had been revised: two (1.5%) in the direct lateral group and 11 (1.4%) in the posterolateral group (Table 1). The 8-year survival rate was 97.9% (95% confidence interval, 89.9%–100%) for the direct lateral approach and 97.2% (95% confidence interval, 93.9%–99.3%) for the posterolateral approach (Fig. 1; Tables 2, 3). If a difference in survival of 0.5% at 8 years was considered clinically significant, power analysis shows 25,000 cases would need to be recruited to both treatment arms to show this.
Table 1.
Details of revisions in the two groups
| Approach | Gender | Age (years) | Time to revision (years) | Reason for revision |
|---|---|---|---|---|
| Direct lateral | Male | 61 | 0.8 | Acetabular component moved into vertical position |
| Direct lateral | Female | 55 | 4.3 | Aseptic loosening acetabular component |
| Posterolateral | Female | 51 | 0.3 | Fracture neck of femur |
| Posterolateral | Female | 56 | 6.1 | Femoral head collapse; osteonecrosis |
| Posterolateral | Male | 54 | 6.4 | Femoral head collapse; osteonecrosis |
| Posterolateral | Male | 44 | 0.5 | Deep infection |
| Posterolateral | Female | 52 | 1.8 | Deep infection |
| Posterolateral | Female | 64 | 2.4 | Deep infection |
| Posterolateral | Female | 50 | 0.5 | Acetabular component moved into vertical position |
| Posterolateral | Female | 53 | 3.2 | Aseptic loosening acetabular component |
| Posterolateral | Female | 54 | 7.3 | Aseptic loosening acetabular component |
| Posterolateral | Female | 53 | 2.2 | Persistent pain; local inflammatory response; metal allergy? |
| Posterolateral | Female | 53 | 3.3 | Persistent pain; local inflammatory response; metal allergy? |
Fig. 1.
The graph depicts Kaplan-Meier survival curves for two surgical approaches using the Birmingham Hip Resurfacing™ system (survival to revision for any reason). The hatched areas represent the 95% confidence intervals.
Table 2.
Life table for the direct lateral approach group
| Year (x) | Number at risk in year x | Number of revisions in year x | Probability of revision in year x | Cumulative proportion surviving | 95% confidence interval |
|---|---|---|---|---|---|
| 0 | 135 | 0 | 0 | 1.000 | 1–1 |
| 1 | 131 | 1 | 0.008 | 0.992 | 0.978–1 |
| 2 | 128 | 0 | 0 | 0.992 | 0.978–1 |
| 3 | 113 | 0 | 0 | 0.992 | 0.977–1 |
| 4 | 85 | 0 | 0 | 0.992 | 0.974–1 |
| 5 | 60 | 1 | 0.017 | 0.979 | 0.944–1 |
| 6 | 41 | 0 | 0 | 0.979 | 0.937–1 |
| 7 | 21 | 0 | 0 | 0.979 | 0.920–1 |
| 8 | 11 | 0 | 0 | 0.979 | 0.899–1 |
| 9 | 4 | 0 | 0 | 0.979 | 0.856–1 |
Table 3.
Life table for the posterolateral approach group
| Year (x) | Number at risk in year x | Number of revisions in year x | Probability of revision in year x | Cumulative proportion surviving | 95% confidence interval |
|---|---|---|---|---|---|
| 0 | 774 | 0 | 0 | 1.000 | 1–1 |
| 1 | 709 | 3 | 0.004 | 0.996 | 0.991–1 |
| 2 | 686 | 1 | 0.001 | 0.994 | 0.989–1 |
| 3 | 630 | 3 | 0.005 | 0.990 | 0.982–0.0997 |
| 4 | 526 | 1 | 0.002 | 0.988 | 0.979–0.996 |
| 5 | 407 | 0 | 0 | 0.988 | 0.978–0.996 |
| 6 | 273 | 0 | 0 | 0.988 | 0.976–0.996 |
| 7 | 153 | 2 | 0.013 | 0.980 | 0.958–0.994 |
| 8 | 91 | 1 | 0.011 | 0.972 | 0.939–0.993 |
| 9 | 24 | 0 | 0 | 0.972 | 0.909–0.993 |
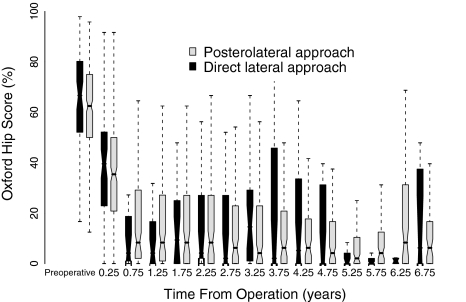
There was no difference in the OHS between the medians of the two groups at any time during the study as judged by the confidence intervals (Fig. 2). A total of 2530 OHS questionnaires were completed by 700 patients during the study period. One hundred eight (80.0%) patients from the anterolateral group and 592 (76.5%) patients from the posterolateral group completed preoperative and postoperative OHS questionnaires. Analysis of the outcome scores showed an improvement in function and pain at the same rate in the two groups. Preoperative median OHS was 65.6% (interquartile range [IQR], 52.0%–80.2%) for the direct lateral group and 62.5% (IQR, 50.0%–75.0%) for the posterolateral group. Maximum improvement was achieved by 12 months and was maintained for the duration of followup. Median OHS was 4.2% (IQR, 0.0%–16.7%) for the direct lateral group and 8.3% (IQR, 1.0%–27.2%) for the posterolateral group at 12 months.
Fig. 2.
This figure portrays the box and whisker plots of the Oxford hip scores for the two surgical approaches using the Birmingham Hip Resurfacing™ system, preoperatively and at 6-month intervals during followup. The notches represent the approximate 95% confidence intervals for the median.
Mortality rate was not different between the two groups using the proportion test. There was one death (one hip) in the direct lateral group and 17 deaths (20 hips) in the posterolateral group. Of the 18 deaths, one at 3.6 years was related to sepsis; the primary source of infection was a deep infection of the hip. Nine additional deaths had a cause that was unrelated to the hip resurfacing and the remaining eight had no cause available from the relevant hospital or general practitioner records. If there really was a difference in these mortality proportions, a power analysis shows 4100 cases in each group would be required to show this was not a false-negative.
There was no difference in the rate of early postoperative (less than 1 year) complications between the two groups. In the direct lateral group, there were two deep vein thromboses, one superficial wound infection, one sciatic nerve palsy, and one return to the operating room for washout of suspected early deep infection. The sciatic nerve palsy occurred when the guidewire for the femur was drilled through the lateral cortex into the nerve. Partial recovery occurred. The patient who had a washout 6 weeks postoperatively had negative cultures and no additional problems occurred with this patient. Another case from the direct lateral group had acetabular failure at 10 months. The acetabular component loosened and moved into a vertical position and this was revised with retention of the femoral component. In the posterolateral group, there were two deep vein thromboses, one superficial wound infection, and one large wound hematoma requiring drainage in the operating room. One fracture of the neck of the femur occurred at 3 months and was revised to a THA. Another patient from the posterolateral group had acetabular failure at 6 months. The acetabular component loosened and moved into a vertical position; this was revised with retention of the femoral component. One patient in the posterolateral group had an early deep infection at 6 months requiring two-stage revision to a THA.
Discussion
Hip resurfacing has regained prominence as a technique for primary hip arthroplasty during the last decade. Bone preservation on the femoral side presents some technical issues to the surgeon when preparing the acetabulum. The choice of surgical approach therefore is important to allow adequate exposure. The results of different approaches must be reproducible without any increase in complications. We addressed whether there was any difference in the survival, functional outcome, mortality, or complications between the direct lateral and the posterior approaches when used for hip resurfacing in two groups of comparable patients with osteoarthritis of the hip.
One limitation of our study is complete data were not available in 8% of the study group despite efforts to reestablish contact with patients lost to followup. However, we report on 835 hip resurfacing procedures that have complete followup between 2 and 9 years. If a difference in survival of 0.5% at 8 years was considered clinically significant, power analysis shows 25,000 cases would need to be recruited to both treatment arms. These large numbers are unlikely to be feasible in any setting other than a large prospective multicenter study.
The posterolateral approach to the hip may be extended if necessary. It is straightforward for accessing the hip between neuromuscular planes and conserving the abductor group of muscles. It is not proven to have an excessive risk of dislocation in THA, provided adequate posterior repair is performed [14]. In modern hip resurfacing, dislocations seem to be less common than in conventional hip arthroplasty [3, 28]. Concerns have been raised regarding a potential insult to the terminal subcapsular branches of the medial circumflex femoral artery [8, 12, 25]. However, it has been suggested, in established osteoarthritis, there are protective pathophysiologic changes to the blood supply of the femoral head [5, 29]. Beaulé et al. regard the extraosseous blood supply as being at further risk at the stage of femoral neck reaming [2]. It also was shown by using surrogate markers that bone perfusion is reduced when a posterolateral approach has been used compared with a direct lateral approach [6, 25]. It seems likely there is at least some temporary disruption of blood flow, but compensatory flow from inferior retinacular vessels is feasible [27]. It remains to be shown whether any disruption is irreversible [20]. A detrimental clinical effect has not been observed in other published series [21, 24, 28]. In our study, there were two revisions (0.3%) for femoral head collapse and osteonecrosis in 774 hip resurfacings with a posterolateral approach. In comparison, when a direct lateral approach was used, none of the 135 collapsed. If damage to vascularity of the femoral head as a result of the procedure does occur, clinical sequelae remain rare, at least during the first 8 years postsurgery. It also was suggested damage to the vascularity when adopting a posterolateral approach is a contributory factor in early femoral neck fracture [25]. The one femoral neck fracture that occurred in the posterolateral group at 4 months had superior femoral neck notching on the initial postoperative radiograph. At revision, there was no evidence of osteonecrosis of the femoral head beyond that expected with a femoral neck fracture. In this large series, femoral neck fracture seems to be associated with neck notching rather than as a result of vascular injury. Furthermore, the low number of femoral failures using either approach suggests stress shielding does not play an important role in implant failure in the medium term. An additional two revisions in one patient were for persistent pain. This patient had no history of metal allergy, but at surgery was found to have a marked inflammatory response consistent with an allergic response. Continued monitoring is required to identify all modes of failure in this group of patients undergoing metal-on-metal resurfacing.
Advocates of the direct lateral approach such as that of Freeman [6] point out the terminal ascending branches of the medial circumflex femoral artery are not disrupted [25]. In addition, during acetabular preparation, the femoral head is retracted out of the wound rather than toward the bulk of the rectus and iliopsoas muscles, as in the posterolateral approach.
Other approaches have been advocated, including that of Ganz et al. [7] and as described by Mont et al. [17] involving a trochanteric osteotomy. Inevitably, an osteotomy requires repair with a risk of nonunion or of the subsequent need to remove metalwork. Minimally invasive approaches have been reported but have not shown any benefit over traditional approaches and are not recommended [15, 17, 26].
Both approaches we described offer excellent return to function and pain reduction after Birmingham Hip Resurfacing™. The rate of improvement is not considerably different and there is no major difference in survival or in the incidence of complications. The designers of the current generation of hip resurfacings continue to advocate a posterolateral approach; for surgeons experienced with a direct lateral approach, this can be used safely with comparable results.
Acknowledgments
We thank the staff of the Research and Teaching Centre at the Royal Orthopaedic Hospital, Birmingham, for support in the production of this research.
Footnotes
The institution of the authors has received funding from Smith and Nephew PLC, London, UK.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed]
- 2.Beaulé PE, Campbell P, Shim P. Femoral head blood flow during hip resurfacing. Clin Orthop Relat Res. 2007;456:148–152. [DOI] [PubMed]
- 3.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. [DOI] [PubMed]
- 4.Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br. 1996;78:185–190. [PubMed]
- 5.Forrest N, Welch A, Murray AD, Schweiger L, Hutchison J, Ashcroft GP. Femoral head viability after Birmingham resurfacing hip arthroplasty: assessment with use of [18F] fluoride positron emission tomography. J Bone Joint Surg Am. 2006;88(suppl 3):84–89. [DOI] [PubMed]
- 6.Freeman MA. Some anatomical and mechanical considerations relevant to the surface replacement of the femoral head. Clin Orthop Relat Res. 1978;134:19–24. [PubMed]
- 7.Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83:1119–1124. [DOI] [PubMed]
- 8.Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br. 2000;82:679–683. [DOI] [PubMed]
- 9.Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg Br. 1982;64:17–19. [DOI] [PubMed]
- 10.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83:1622–1629. [DOI] [PubMed]
- 11.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: a histologic and enzymatic analysis. Spine. 1996;21:941–944. [DOI] [PubMed]
- 12.Khan A, Yates P, Lovering A, Bannister GC, Spencer RF. The effect of surgical approach on blood flow to the femoral head during resurfacing. J Bone Joint Surg Br. 2007;89:21–25. [DOI] [PubMed]
- 13.Kim WY, Muddu BN. Eleven-year results of the ABG I hip replacement. Int Orthop. 30:182–184. [DOI] [PMC free article] [PubMed]
- 14.Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res. 2006;447:34–38. [DOI] [PubMed]
- 15.McMinn DJ, Daniel J, Pynsent PB, Pradhan C. Mini-incision resurfacing arthroplasty of hip through the posterior approach. Clin Orthop Relat Res. 2005;441:91–98. [DOI] [PubMed]
- 16.Minns RJ, Crawford RJ, Porter ML, Hardinge K. Muscle strength following total hip arthroplasty: a comparison of trochanteric osteotomy and the direct lateral approach. J Arthroplasty. 1993;8:625–627. [DOI] [PubMed]
- 17.Mont MA, Ragland PS, Marker D. Resurfacing hip arthroplasty: comparison of a minimally invasive versus standard approach. Clin Orthop Relat Res. 2005;441:125–131. [DOI] [PubMed]
- 18.NJR Steering Committee. National Joint Registry for England and Wales 3rd Annual Clinical Report. The NJR Centre, Hemel Hempstead, UK, February 2006. Available at: http://www.njrcentre.org.uk/documents/reports/annual/3rd/NJR_AR2_LR.pdf. Accessed July 2007.
- 19.Nork SE, Schär M, Pfander G, Beck M, Djonov V, Ganz R, Leunig M. Anatomic considerations for the choice of surgical approach for hip resurfacing arthroplasty. Orthop Clin North Am. 2005;36:163–170, viii. [DOI] [PubMed]
- 20.Nötzli HP, Siebenrock KA, Hempfing A, Ramseier LE, Ganz R. Perfusion of the femoral head during surgical dislocation of the hip: monitoring by laser Doppler flowmetry. J Bone Joint Surg Br. 2002;84:300–304. [DOI] [PubMed]
- 21.Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip: a five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88:592–600. [DOI] [PubMed]
- 22.Pynsent PB, Adams DJ, Disney SP. The Oxford hip and knee outcome questionnaires for arthroplasty. J Bone Joint Surg Br. 2005;87:241–248. Errata. J Bone Joint Surg Br. 2005;87:1166. [DOI] [PubMed]
- 23.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006.
- 24.Revell MP, McBryde CW, Bhatnagar S, Pynsent PB, Treacy RBC. Metal-on-metal hip resurfacing in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(suppl 3):98–103. [DOI] [PubMed]
- 25.Steffen RT, Smith SR, Urban JPG, McLardy-Smith P, Beard DJ, Gill HS, Murray DW. The effect of hip resurfacing on oxygen concentration in the femoral head. J Bone Joint Surg Br. 2005;87:1468–1474. [DOI] [PubMed]
- 26.Stephenson PK, Freeman MA. Exposure of the hip using a modified anterolateral approach. J Arthroplasty. 1991;6:137–145. [DOI] [PubMed]
- 27.Sugamoto K, Ochi T, Takahashi Y, Tamura T, Matsuoka T. Hemodynamic measurement in the femoral head using laser Doppler. Clin Orthop Relat Res. 1998;353:138–147. [DOI] [PubMed]
- 28.Treacy RBC, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty: a minimum follow-up of five years. J Bone Joint Surg Br. 2005;87:167–170. [DOI] [PubMed]
- 29.Whiteside LA, Lange DR, Capello WR, Fraser B. The effects of surgical procedures on the blood supply to the femoral head. J Bone Joint Surg Am. 1983;65:1127–1133. [PubMed]