Abstract
Although the use of ultrasound in the diagnosis and early treatment of developmental dysplasia of the hip (DDH) has reduced the number of patients diagnosed late and decreased the number of operative procedures, surgical treatment is still needed in some patients. Late cases continue to occur as a result of missing the screening examination, being normal at initial screening and missing followup. Dysplasia may persist despite appropriate nonoperative or operative treatment. Many of these patients subsequently undergo closed or open reduction and femoral or acetabular reconstruction. Ultrasound of the hips is generally used up to 6 or 8 months of age, during which time the hips are largely cartilaginous, and radiographs after that time when bony development is more complete. Options to supplement ultrasound and radiography include arthrography, computed tomography, and magnetic resonance imaging. Several advances have been made in the imaging of DDH and its complications including acetabular labral pathology and of femoroacetabular impingement (FAI). We review imaging techniques other than ultrasound used in the management of DDH.
Level of Evidence: Level V, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Ultrasound of the hips for developmental dysplasia of the hip (DDH) was introduced at least 25 years ago [11] and has had a tremendous impact on the early detection of DDH [14]. Although there are continuing controversies regarding what type of scan to perform, when to perform the scan, and which infants should have a scan, DDH is being diagnosed at an earlier age, leading to more successful and less invasive treatment [12, 15]. However, there continue to be “missed” or late cases, even in countries that have instituted screening programs for all infants [4]. Some infants miss the initial screening examination; some infants who at first appear to have normal hips later develop acetabular dysplasia. Even when treatment is instituted at an early age and is appropriate, acetabular dysplasia may persist and require acetabular reconstruction [3]. Ultrasound has limited application in the imaging of late DDH, possibly only for measurement of anteversion of the femur [28]. Plain films, computed tomography (CT), or MRI are used in the evaluation of late or persistent hip dysplasia.
We review the role of imaging in the late diagnosis of dysplasia, as well as decision-making for surgical treatment of patients with DDH and complications such as osteonecrosis. We examine some of the classic articles that describe imaging in DDH as well as recent literature primarily focusing on newer imaging techniques (CT and MRI). Finally, owing to the considerable recent interest in the role of labral pathology and femoroacetabular impingement (FAI) in patients with DDH and imaging in these conditions, we review the crucial concepts.
Plain Radiographs
The goals of imaging in late DDH are to first make the diagnosis, then to quantitate the abnormality, and finally to assist in surgical planning. The anteroposterior radiograph is the primary tool for making the initial diagnosis of missed or late DDH beyond 6 to 9 months. This condition usually occurs in young patients, who may not have had neonatal hip examination and present with limping or a waddling gait or in older children with hip pain who either may have been normal at birth but developed dysplasia later or who were treated as a neonate and have residual dysplasia.
The anteroposterior (AP) radiograph is usually obtained with a pelvic shield to minimize ovarian radiation. This may be done supine or erect, however, the importance of having a well-centered radiograph with the elimination of excessive pelvic tilt cannot be overemphasized [7, 42]. The standing AP radiograph also provides an estimate of the limb length discrepancy.
In late DDH, radiographic findings include variable displacement of the femoral head(s), deformity of the femoral head from osteonecrosis or abnormal version, and an abnormal acetabulum, which is enlarged and angled, retroverted, or shallow with lateralization of the femoral head resulting from thickening of the medial acetabular structures (Fig. 1). If degenerative disease has developed, there may also be narrowing of the hip joint, eburnation, and cyst formation. Advanced arthritic change predicts poor response to a hip preservation procedure. A more subtle abnormality is prominence of the lateral aspect of the femur at the head-neck junction indicating an aspherical femoral head and possible impingement (Fig. 2) [5].
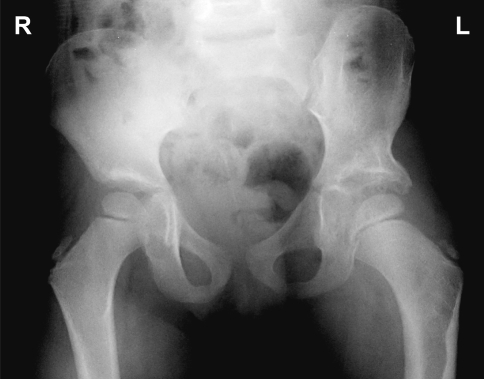
Fig. 1.
A 5-year-old child was diagnosed at 15 months after open reduction and Pemberton osteotomy; the child is now asymptomatic. The figure shows postoperative deformity of the ilium and acetabular roof with an enlarged acetabulum and normally contained femoral head.
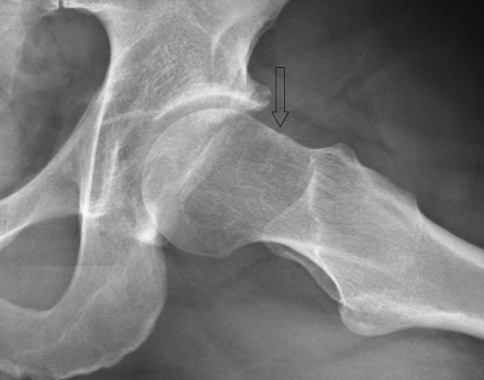
Fig. 2.
A lateral view of the proximal femur in a patient with decreased head neck offset demonstrating a “bump” at the head-neck junction (arrow) where the femur is impinging against the acetabular rim is shown.
Acetabular coverage is best measured on radiographs, lateral coverage on the AP radiograph using the acetabular index as well as the center-edge angle of Wiberg, and anterior coverage using the ventral center-edge angle on the false profile view of Lequesne [26] (ie, 65° internal rotation) [2, 7, 30, 34, 51]. Values less than 15° are associated with considerable dysplasia or subluxation and often suggest the need for operative intervention.
Acetabular version is assessed by looking at the anterior and posterior margins of the acetabulum and determining where they meet. They normally meet at the superolateral margin of the acetabulum. In a retroverted acetabulum they meet in the mid-portion of the acetabulum. This is called the “crossover sign” (Fig. 3) [34]. The midportion of the posterior acetabulum is lateral to that of the anterior acetabulum by at least 1.5 cm. If retroverted, they cross over more medially, and there is less distance between the anterior and posterior margins. Although the acetabulum is typically anteverted in DDH, approximately one in six cases will have a retroverted acetabulum. This is extremely important to recognize when planning a pelvic osteotomy to be able to rotate the acetabular fragment in the appropriate direction and minimize instability or impingement. CT is now used to measure acetabular version directly.
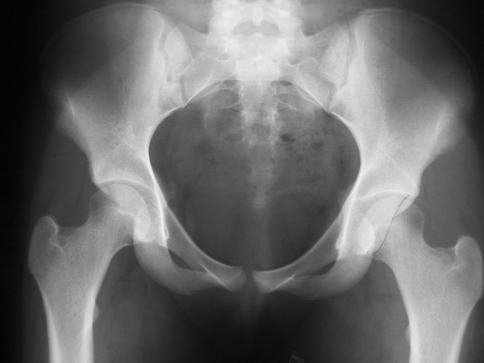
Fig. 3.
A well centered anteroposterior radiograph of the pelvis is shown, demonstrating a crossover sign with the anterior margin of the acetabulum (dotted line) meeting the posterior margin (solid line) in the middle of the acetabulum instead of meeting at the superolateral margin of the acetabulum. This is suggestive of acetabular retroversion.
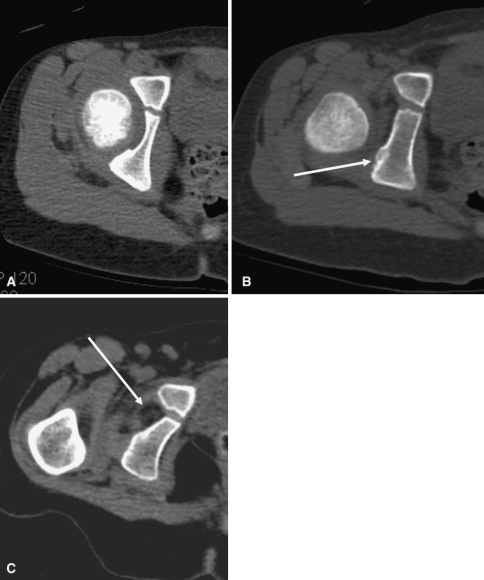
Hip joint width, measured from the femoral head to the acetabulum superiorly, medially, and at the teardrop, may also be helpful because it reflects the amount of cartilage present and, if narrowed symmetrically, suggests chondrolysis or osteoarthritis [37]. This can also be measured by CT or MRI and would then include the thickness of the cartilage in the axial plane as well as the coronal plane (Fig. 4) [40]. The observation of chondrolysis is probably more important than any measurement. Femoral chondrolysis was at one time measured using plain films, but the method was technically difficult, and it is now measured with CT. Abnormal femoral chondrolysis can be suspected when the greater trochanter is fully visualized on an anteroposterior radiograph of the pelvis with the knees in neutral position [48].
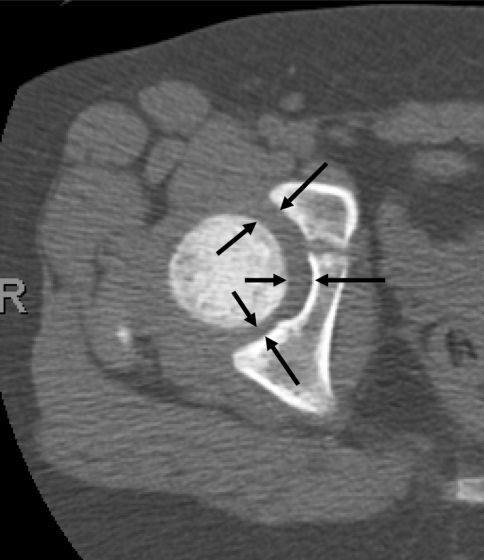
Fig. 4.
The figure shows normal hip joint width measurement demonstrated on axial computed tomography (arrows). Note wider joint anteriorly than posteriorly.
Femoral neck-shaft angle measurement may be needed when planning reconstruction that calls for femoral osteotomy. The measurement should be obtained on a supine radiograph with the toes pointed medially [22]. It is important to remember external rotation increases the measurement and should be avoided. The measurement is typically increased in DDH [34].
The abduction, internal rotation view of the hip is important in confirming the femoral head can be concentrically reduced in the acetabulum. Lack of appropriate centering of the femoral head within the acetabulum is a contraindication to performing a reconstructive hip osteotomy.
Arthrography
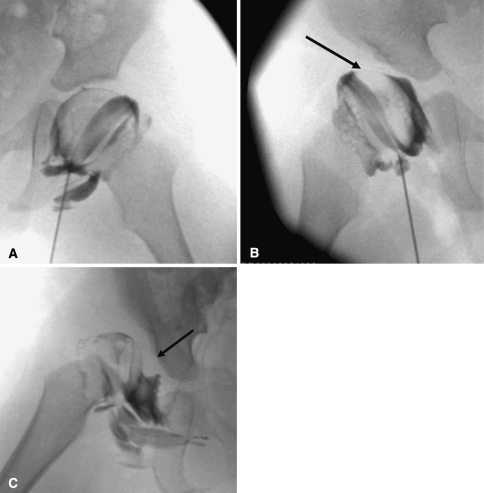
In infants or young patients with missed DDH, arthrography is typically performed intraoperatively at the time of reduction to assess the anatomy of the hip and impediments to reduction. While performing a reconstructive osteotomy, the arthrogram helps to demonstrate the best position of the femur to obtain concentric reduction of the hip and thus helps in planning correction. When performing an arthrogram, it is important to use the correct concentration and amount of contrast. The contrast should be diluted with saline to a ratio of 1:1. Using less diluted contrast will obscure the anatomy. Several approaches to the joint can be used, depending on the operator’s experience [2]. It is important to avoid the femoral artery and nerve during placement of the needle. Once the needle is inserted, a small amount of contrast (1 cc) is injected under fluoroscopic guidance to confirm needle placement in the hip. Further contrast is injected to opacify the joint (2–3 cc), after which the needle is removed. If there is extravasation but there is still contrast in the hip, satisfactory images can usually be obtained [2, 47]. One of the pitfalls of arthrography is injection of too much contrast into the joint, which simulates capsular laxity. This can be differentiated from incongruity because it will surround the femoral head rather than pool focally as it does when there is incongruity (Fig. 5A-C).
Fig. 5A–C.
(A) A normal hip arthrogram demonstrates thin rim of contrast surrounding the femoral head and labrum covering the femoral head laterally. (B) A subluxated hip with acetabular dysplasia, pooling of contrast medially, and deflected labrum (arrow) is shown. (C) A dislocated hip with inverted labrum (arrow), narrowing of the joint capsule, and acetabular dysplasia is shown.
Computed Tomography (CT)
Before ordering a CT, it is important to realize the high radiation burden [9]. It is important to limit the number of CT scans to what is really necessary and to reduce the dosage as much as possible by using a low radiation technique [8, 21, 38, 39]. The technique (KVP, mA, time) is usually determined by the radiologist or the technologist, but concern from the practitioner will help reinforce a safe standard of practice.
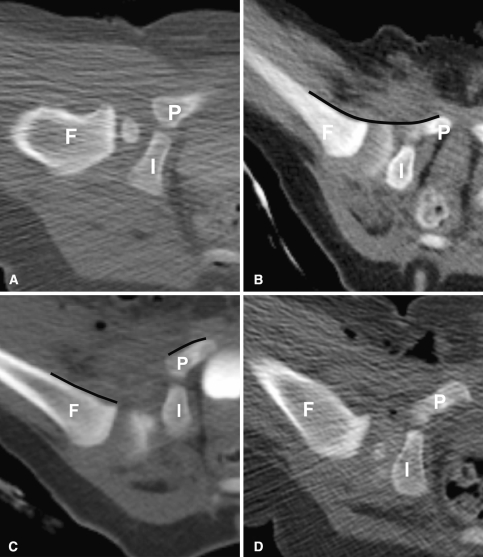
In infants, plain films in cast have been used to evaluate the postreduction hip, but CT provides superior information because of the ability to look at the positioning in the axial plane (Fig. 6A), and because of the difficulty visualizing the hip on radiographs as a result of the cast material. This type of CT scan is performed with the patient in a spica cast, either in the frog leg position after closed reduction or with the femora partially flexed and abducted after open reduction with contrast from an intraoperative arthrogram if performed immediately after the procedure (Fig. 6B). Typically a pilot view is obtained, which the technologist uses to plan the study either by performing limited cuts through the hips or by performing a limited spiral scan with 5-mm cuts. In interpreting the scan, it is important to be sure the landmarks of the hip joint are present, namely the pubic bone, the ischium, the intervening triradiate cartilage, and the femoral head or metaphysis. Substantial tilting of the pelvis makes the images more difficult to interpret and should be avoided. In the normally positioned hip, when casted in the frog leg position, there should be a smooth arc formed by the anterior aspect of the femoral neck and the anterior aspect of the pubic bone (the CT equivalent of Shenton’s line) [27, 44]. In a dislocated hip, the line is interrupted (Fig. 6C). If this is seen following a closed or open reduction, the patient should be brought back to the operating room for repositioning of the hip. Sometimes the hip is displaced laterally or posteriorly but not completely dislocated (Fig. 6D), and then a followup CT is generally performed in 1 or 2 weeks to determine if there has been interval correction, or at least improvement. After open reduction, only a small part of the metaphysis is seen, and the position of the femoral head is extrapolated from the position of the metaphysis. This is easier if the femoral head is ossified or if there is contrast in the hip, but often by the time the patient arrives in the CT suite, the contrast has partially resorbed.
Fig. 6A–D.
(A) A computed tomography scan shows a normally positioned hip after closed reduction with contrast surrounding the femoral head. (B) Arthrographic contrast surrounding the femoral head after successful closed reduction of the hip is demonstrated. Note the smooth arc (line) formed by the femoral metaphysis and the pubic bone anteriorly. (C) A dislocated hip is shown. Note discontinuity of the normal arc (discontinuous line) formed by the femoral metaphysis and pubic bone. Air is seen in the soft tissues from the procedure. (D) This is a mildly posteriorly displaced hip after closed reduction. F = femur; I = ischium; P = pubis.
In addition to assessing the quality of reduction, the degree of dysplasia can also be ascertained, in particular any deformity of the posterior aspect of the acetabulum. The normal acetabulum has a smoothly rounded shape with a well-defined posterior lip (Fig. 7A). In the dysplastic hip, the posterior acetabulum is often straightened without a well-defined posterior margin (Fig. 7B). It can also be smaller than the normal acetabulum. Thickening of the fat in the medial acetabulum (pulvinar) is often seen in dysplastic hips (Fig. 7C). Two- and three-dimensional reconstructions are not applicable to postreduction CT because of the thickness and the limited number of cuts.
Fig. 7A–C.
(A) The figure shows a normal acetabulum with wide radius of curvature and a smooth articular surface. (B) An example of a dysplastic acetabulum is shown: straightening and irregularity of the ischium (arrow). (C) Another example of a dysplastic acetabulum is shown: dislocated hip and fatty pulvinar centrally (arrow).
In patients where pelvic osteotomy is planned, CT scan aids in planning reconstruction [1]. In particular the surgeon can localize the location and magnitude of acetabular deficiency and plan placement of the bone graft at an appropriate location. Most patients with DDH have an anterosuperior deficiency and therefore, a Pemberton or Salter osteotomy is the preferred acetabular procedure as these both improve anterior coverage. If the CT reveals deficiency that is superior or posterosuperior, a Dega type osteotomy may be used with the largest bone graft being added at the site of maximal deficiency. In a capacious acetabulum also a Dega type of osteotomy is indicated.
We recommend obtaining axial cuts, usually with thin collimation (2.5-mm thickness or less). We reprocess the data at thinner intervals, and create two- and three-dimensional “reformatted” or “reconstructed” images. Three-dimensional images can be reconstructed with the femoral head in the acetabulum, or the acetabulum and femoral head can be viewed independently depending on the information the clinician needs. Although the three-dimensional images provide an overall picture of the hips and pelvis, the axial and two-dimensional reformatted images are more helpful in obtaining measurements of the acetabulum roof, cartilage thickness, acetabular rotation, and acetabular anteversion (see below) [50]. In postoperative patients, metal streak artifact can limit the value of the scan, particularly the three-dimensional images.
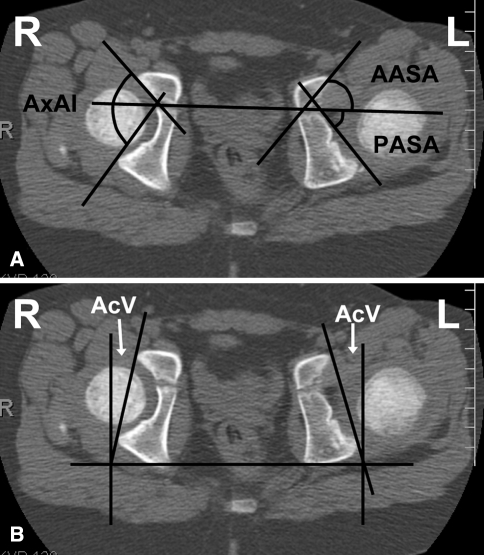
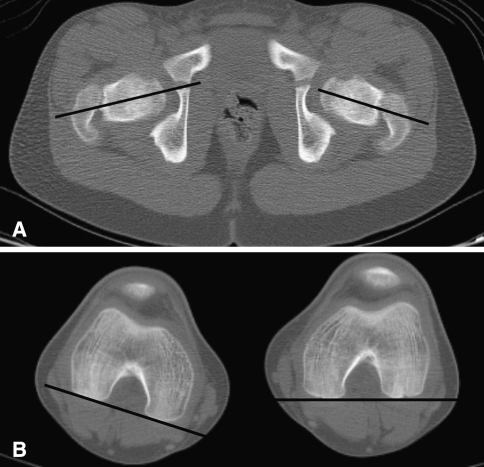
When making measurements of the acetabulum, it is important to select the correct image. If the pelvis is rotated or tilted, the reformatted axial image, rather than the initially scanned image, should be used. On the CT workstation, the user first rotates and tilts the images so that the hips are symmetric and, after selecting the best image and plane that demonstrates the desired anatomic feature, makes the measurements. For the axial measurements of the acetabulum the image that shows both femoral heads should be used, and the appropriate landmarks should be present (ie, the center of the femoral head, the largest pubic and ischial components, and the triradiate cartilage). The anterior and posterior sector acetabular angles (AASA, PASA) measure anterior and posterior acetabular development, respectively, and the axial acetabular index (AxAI) is the sum of these two angles, indicating the depth of the acetabulum (Fig. 8A) [13].
Fig. 8A–B.
(A) A computed tomography scan for measurement of acetabular angles is shown (anterior and posterior sector angles [AASA, PASA]). The axial acetabular index (AxAI) is the sum of the two angles. (B) Acetabular anteversion (AcV) measurement is demonstrated.
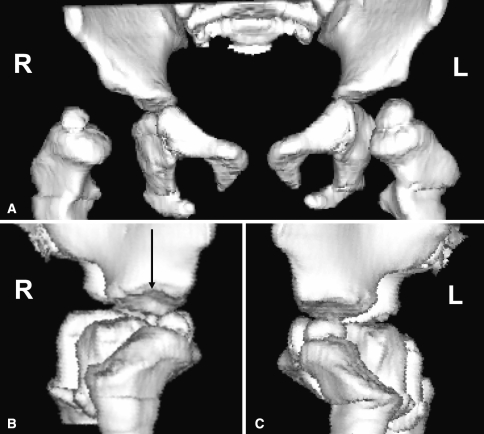
The most important angle is probably the angle of version of the acetabulum. This angle is formed by the intersection of a line connecting the anterior and posterior rim of the acetabulum and a line perpendicular to the line between the posterior pelvic margins (Fig. 8B) at the center of the acetabulum [19]. The angle of version of the acetabulum determines the direction and magnitude of rotation of the acetabular fragment when performing a pelvic osteotomy because of the potential for impingement and early degenerative change if the angle of version is abnormal. Anterior and posterior center-edge angles can be obtained from the axial images and indicate coverage in the axial plane. Three-dimensional CT scans may also be used to assess acetabular morphology (Fig. 9A-C) [23].
Fig. 9A–C.
(A) A three-dimensional computed tomography scan in anterior and bilateral lateral views demonstrates anterior dislocation on the right. (B) Abnormal tilting of the right acetabular roof on the lateral view (arrow) is shown. (C) Normal tilting on contralateral side.
CT of femoral version is measured by performing limited cuts through the femoral head and neck and through the distal femur and calculating the rotation. The femoral neck/head anteversion or retroversion is estimated by superimposing the femoral head center image on the distal transcondylar knee image. Version of the proximal femur is the angle between the posterior condylar tangent and the transverse longitudinal femoral neck and head axis (Fig. 10A–B) [18, 31, 49]. Tibial torsion is sometimes measured at the same time by obtaining cuts through the proximal (posterior tangential line at the tibial plateau) and distal tibia (transmalleolar axis) [20, 41]. Normal femoral and acetabular version measures 15° to 20° and this value may be normal or increased in DDH. This increased anteversion as well as proximal femoral deformity has implications for stem selection during total hip arthroplasty in patients with DDH [32]. The McKibbin instability index is the sum of the angles of femoral and acetabular anteversion, normally 30° to 60°. Abnormal McKibbin index is not uncommon in patients with late DDH and can be increased, indicating instability, or more commonly decreased [48]. Both increased and decreased measurements are associated with pain.
Fig. 10A–B.
A computed tomography scan shows the femur for femoral anteversion with cuts through the hips (A) and distal femora (B) with angle formed by lines drawn through the axis of the femoral neck and femoral condyles. There was internal rotation of the right knee but satisfactory anteversion bilaterally.
Image-guided navigation, a technique based on CT, can be used intraoperatively to assist the surgeon in selecting the best sites for osteotomies as well as to position the fragments accurately while minimizing the risk of joint compromise during the operative procedure [24]. The scan is typically obtained at a prescribed thickness, typically 1 mm thick sections although this may vary by system used, without angulation of the CT gantry and may require a separate CT from the one used for original measurements [24].
Magnetic Resonance Imaging (MRI)
MRI has not been routinely used in DDH, but indications are increasing. MRI is attractive because of the lack of ionizing radiation, the visualization of soft tissue structures (especially the acetabular rim, the acetabular cartilage, and the labrum which is best seen with MR arthrography), and the multiplanar capability. The primary disadvantages are the cost and the need for sedation [17]. At our institution, the global charges for MRI of the pelvis is $2087 without IV contrast and $2443 with IV gadolinium. When MR arthrogram is obtained, there is an extra charge for injection. Arthrography global charge is $1365 (plus anesthesia charge if needed). The global charge for AP and frogleg views of the pelvis is $270. Sedation is typically needed in children under 7 years of age and is usually achieved with chloral hydrate or pentobarbital [10]. This requires monitoring and generates nursing and medication charges ($700 hospital charge, $1000-2,000 physician fee). CT of the pelvis without IV contrast charge is $1625, and with contrast, $1877.
MRI can be used to confirm placement of the hip after closed reduction in the infant. Most studies use sedation or anesthesia to prevent motion degradation of the images [9, 29]. An abbreviated (average scan time 3 minutes for 2 sequences) scan not requiring sedation in children in a spica cast has been described by Laor et al. [25]. They did have image degradation in 3 of their 10 patients but did not need to rescan them. The scan included a coronal T1 weighted localizer (TR 300, TE 17) and an FSE proton density weighted axial sequence with fat suppression (TR 3000, TE 17), echo-train length = 8, 256–192 matrix, two signal averages, slice thickness 3–5 mm with 0–1 mm gap. These sequences take very little time and can be repeated if the child moves, providing the anatomical information without radiation.
Better definition of the capital femoral epiphysis in infants is an advantage, but neither CT nor MR imaging seems to predict subsequent development of the hip or the need for future surgery.
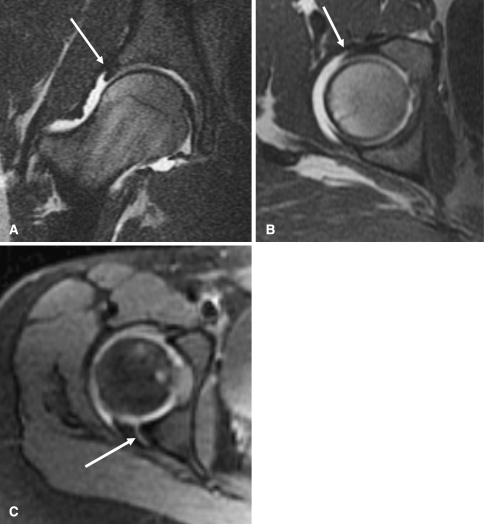
Femoroacetabular impingement and labral tears are increasingly recognized as a cause of hip pain or disability in adolescents and young adults, and they are fairly common in late DDH because of the acetabular and femoral head deformity. MR arthrography is the best way to diagnose labral tears and impingement. It is performed by injecting diluted gadolinium mixed with saline (1:100), lidocaine, and iodinated contrast into the hip joint [35]. If too much gadolinium is used, the hip will be obscured because of the paramagnetic effect of gadolinium. MR arthrography with sagittal oblique or radial sequence imaging is the best way to diagnose impingement. Sagittal oblique imaging is preferred as it demonstrates proximal femoral anatomy as well as acetabular anatomy. MRI findings include edema and cyst formation in the acetabular rim and cartilage or labral degeneration or tear (Fig. 11A-C) [16, 36]. Like in CT, postoperative metal causes artifact (signal dropout) limiting diagnostic value.
Fig. 11A–C.
The figure shows T1-weighted magnetic resonance arthrogram images of the hip. (A) A normal hip and intact labrum (arrow) is shown. (B) A normal hip and intact labrum (arrow) is shown. (C) A torn posterior labrum in a different patient (arrow) is shown.
The labrum is typically hypertrophic in DDH and may have associated tears or develop paralabral cysts. The recess superficial and cranial to the superior labrum and also the cleft between the anteroinferior and posteroinferior labrum near the transverse acetabular ligament are normal findings, and should not be mistaken for labral tears.
There are two types of impingement, cam and pincer impingement. Cam impingement is seen due to lack of femoral head neck offset and damage to the acetabular labrum with a contrecoup injury to the acetabular cartilage. This can result in extensive cartilage delamination on the acetabular side. Femoral head sphericity and femoral head neck offset are measured by epiphyseal extension, the amount of femoral head-neck offset, and the (alpha) angle [33, 43]. The alpha angle is best measured on an oblique image through the center of the femoral neck. A circle outlining the femoral head is drawn. Next, a line along the long axis of the femoral neck bisecting the circle is drawn. Another line is then drawn from the center of the circle to the point at which the femoral head or neck protrudes beyond the confines of the circle anteriorly. The alpha angle is the angle between these 2 lines. Notzli et al. [33] noted that an alpha angle of greater than 55° was likely to be associated with symptomatic impingement.
Cam impingement is best seen on a true lateral view of the hip, and is associated with an increased alpha angle (> 55 degrees), labral tear and cartilage delamination on MRI.
Pincer impingement is the result of abutment from the abnormal acetabulum (retroverted acetabulum, coxa profunda) with a primary labral tear [5, 46]. Radiographic signs suggestive of pincer impingement include evidence of acetabular retroversion (crossover sign), increased acetabular depth (coxa profunda - femoral head center less than 15 mm lateral to the ilioischial line), negative sourcil angle, blunted labrum on radial sequence MRI and corresponding or kissing lesion on the femoral neck. Most hips will show some evidence of pincer as well as cam impingement, but one usually predominates.
The femoral head can also be assessed for acute osteonecrosis and complications of surgery, including infection [45]. The measurements of the acetabulum could potentially be obtained on MRI as well as on CT, but the landmarks are not as well seen on MRI, particularly in the dysplastic acetabulum.
Recent advances in MR imaging, such as the use of 3 Tesla MR imaging may obviate the need for intraarticular contrast due to superior imaging quality. Biochemical MR imaging (dGEMRIC- dynamic Gadolinium Enhanced Magnetic Resonance Imaging of Cartilage) is being investigated as a potential guide to help select patients for hip preservation surgery. One study demonstrated a greater degree of preoperative changes of arthritis detected by MRI predicted worse short-term outcomes for periacetabular osteotomies (PAO) [6].
Discussion
Developmental dysplasia of the hip is a common condition, and we have more information and technology to diagnose it early and treat it. Despite these advances, some patients are still diagnosed at a late stage and others do not respond to treatment as anticipated or have complications of treatment. Although radiography is the mainstay of diagnosis in late DDH, CT and MRI have increasing roles in assessing and treating late DDH and its complications. Imaging in the older child or young adult with DDH helps define the underlying pathoanatomy as well plan appropriate treatment. The treatment of the asymptomatic adolescent or young adult with DDH remains controversial. However, when surgery is anticipated, appropriate imaging, often with CT, aids in defining the dysplasia and selecting the appropriate procedures, including pelvic and/or femoral osteotomies.
MR imaging has become invaluable in assessing labral abnormalities. In the absence of underlying osseous abnormalities, most surgeons believe isolated labral tears are best treated with arthroscopic techniques. However, when patients with labral tears also have underlying osseous deformities that create cam or pincer impingement some surgeons believe these deformities require additional surgery.
CT scans and MR images are useful in assessing acetabular version and therefore helpful in preoperative planning of pelvic osteotomies. MR imaging may allow surgeons to detect which patients will likely do poorly after a hip osteotomy and thus help in selecting which patients may benefit from hip preservation procedures.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Argenson JN, Ryembault E, Fletcher X, Brassart N, Parratte S, Aubaniac JM. Three-dimensional anatomy of the hip in osteoarthritis after developmental dysplasia. J Bone Joint Surg Br. 2005;87:1192–1196. [DOI] [PubMed]
- 2.Bowen JR, Kotzias-Neto A. Developmental Dysplasia of the Hip. Brooklandville MD. Data Trace Publishing Co 2006;31–32:70–77.
- 3.Bowen JR, Kruse R. Complications in the treatment of developmental dysplasia of the hip. In: Epps CH Jr, Bowen JR, eds. Complications in Pediatric Orthopaedic Surgery, 3rd ed. Philadelphia, PA: JB Lippincott; 1994.
- 4.Clarke NM, Clegg J, Al-Chalabi AN. Ultrasound screening of hips at risk for CDH. Failure to reduce the incidence of late cases. J Bone Joint Surg Br. 1989;71:9–12. [DOI] [PubMed]
- 5.Clohisy MD, Keeney JA, Schoenecker PL. Preliminary assessment and treatment guidelines for hip disorders in young adults. Clin Orthop Relat Res. 2005;441:163–179. [DOI] [PubMed]
- 6.Cunningham T, Jessel R, Zurakowski D, Millis MB, Kim YJ. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage to predict early failure of Bernese periacetabular osteotomy for hip dysplasia. J Bone Joint Surg Am. 2006;88:1540–1548. [DOI] [PubMed]
- 7.Delaunay S, Dussault RG, Kaplan PA, Alford BA. Radiographic measurements of dysplastic adult hips. Skeletal Radiol. 1997;26:75–81. [DOI] [PubMed]
- 8.Donnelly LF, Emery KH, Brody AS, Laor T, Gylys-Morin VM, Anton CG, Thomas SR, Frush DP. Minimizing radiation doses for pediatric body applications of single detector helical CT: strategies at a large pediatric hospital. AJR Am J Roentgenol. 2001;176:303–306. [DOI] [PubMed]
- 9.Duffy CM, Taylor FN, Coleman L, Graham HK, Nattrass GR. Magnetic resonance imaging evaluation of surgical management in developmental dysplasia of the hip childhood. J Pediatr Orthop. 2002;22:92–100. [DOI] [PubMed]
- 10.Egelhoff JC, Ball WS Jr, Koch BL, Parks TD. Safety and efficacy of sedation in children using a structured sedation program. AJR Am J Roentgenol. 1976;168:1259–1262. [DOI] [PubMed]
- 11.Graf R. The diagnosis of congenital hip-joint dislocation by the ultrasonic Combound treatment. Arch Orthop Trauma Surg. 1980;97:117–133. [DOI] [PubMed]
- 12.Graf R. Fundamentals of sonographic diagnosis of infant hip dysplasia. J Pediatr Orthop. 1984;4:735–740. [DOI] [PubMed]
- 13.Gugenheim JJ, Gerson LP, Sadler C, Tullos HS. Pathologic morphology of the acetabulum in paralytic and congenital hip instability. J Pediatr Orthop. 1982;2:397–400. [DOI] [PubMed]
- 14.Harcke HT. Screening newborns for developmental dysplasia of the hip: the role of sonography. AJR Am J Roentgenol. 1994;162:395–397. [DOI] [PubMed]
- 15.Harcke HT, Grissom LE. Performing dynamic sonography of the infant hip. AJR Am J Roentgenol. 1990;155:837–844. [DOI] [PubMed]
- 16.Hodler J, Uy JS, Goodwin D, Trudell D, Resnick D. MR arthrography of the hip: improved imaging of the acetabular labrum with histologic correlation in cadavers. AJR Am J Roentgenol. 1995;165:887–891. [DOI] [PubMed]
- 17.Hubbard AM, Dormans JP. Evaluation of developmental dysplasia, Perthes disease, and neuromuscular dysplasia of the hip in children before and after surgery: an imaging update. AJR Am J Roentgenol. 1995;164:1067–1073. [DOI] [PubMed]
- 18.Hubbard DD, Staheli LT. The direct radiographic measurement of femoral torsion using axial tomography: technique comparison with an indirect radiographic method. Clin Orthop Relat Res. 1972;86:16–20. [DOI] [PubMed]
- 19.Jacquemier M, Jouve JL, Bollini G, Panuel M, Migliani R. Acetabular anteversion in children. J Pediatr Orthop. 1992;12:373–375. [DOI] [PubMed]
- 20.Jakob RP, Haertel M, Stussi E. Tibial torsion calculated by computerized tomography and compared to other methods of measurement. J Bone Joint Surg Br. 1980;62:238–242. [DOI] [PubMed]
- 21.Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard JA, Saini S. Strategies for CT radiation dose optimization. Radiology. 2004;230:619–628. [DOI] [PubMed]
- 22.Keats TE, Teeslink R, Diamond AE, Williams JH. Normal axial relationships of the major joints. Radiology. 1966;87:904–907. [DOI] [PubMed]
- 23.Kim HT, Wenger DR. The morphology of residual acetabular deficiency in childhood hip dysplasia: three-dimensional computed tomographic analysis. J Pediatr Orthop. 1997;17:637–647. [DOI] [PubMed]
- 24.Langlotz F, Bachler R, Berlemann U, Nolte LP, Ganz R. Computer assistance for pelvic osteotomies. Clin Orthop Relat Res. 1998;354:92–102. [DOI] [PubMed]
- 25.Laor T, Roy DR, Mehlman CT. Limited magnetic resonance imaging examination after surgical reduction of developmental dysplasia of the hip. J Pediatr Orthop. 2000;20:572–574. [DOI] [PubMed]
- 26.Lequesne M. The false profile view of the hip: role, interest, economic considerations. Joint Bone Spine. 2002;69:109–113. [DOI] [PubMed]
- 27.Mandell DM, Loder RT, Hensinger RN. The predictive value of computed tomography in the treatment of developmental dysplasia of the hip. J Pediatr Orthop. 1998;18:794–798. [DOI] [PubMed]
- 28.Miller F, Liang Y, Merlo M, Harcke HT. Measuring anteversion and femoral neck-shaft angle in cerebral palsy. Dev Med Child Neurol. 1997;39:113–118. [DOI] [PubMed]
- 29.Mitchell PD, Chew NS, Goutos I, Healy JC, Lee JC, Evans S, Hulme A. The value of MRI undertaken immediately after reduction of the hip as a predictor of long-term acetabular dysplasia. J Bone Joint Surg. 2007;89:948–952. [DOI] [PubMed]
- 30.Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77:985–989. [DOI] [PubMed]
- 31.Murphy SB, Simon SR, Kijewski PK, Wilkinson RH, Griscom NT. Femoral anteversion. J Bone Joint Surg Am. 1987;69:1169–1176. [PubMed]
- 32.Noble PC, Kamaric E, Sugano N, Matsubara M, Harada Y, Ohzono K, Paravic V. Three-dimensional shape of the dysplastic femur: implications for THR. Clin Orthop Relat Res. 2003;417:27–40. [PubMed]
- 33.Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84:556–560. [DOI] [PubMed]
- 34.Peelle MW, Della Rossa GJ, Maloney WJ, Curry MC, Clohisy JC. Acetabular and femoral radiographic abnormalities associated with labral tears. Clin Orthop Relat Res. 2005;441:327–333. [DOI] [PubMed]
- 35.Petersilge CA. Chronic adult hip pain: MR arthrography of the hip. Radiographics. 2000;20:S43–S52. [DOI] [PubMed]
- 36.Petersilge CA, Haque MA, Petersilge WJ, Lewin JS, Lieberman JM, Buly R. Acetabular labral tears: evaluation with MR arthrography. Radiology. 1996;200:231–235. [DOI] [PubMed]
- 37.Pogrund H, Bloom R, Mogle P. The normal width of the adult hip joint: the relationship to age, sex and obesity. Skeletal Radiol. 1983;10:10–12. [DOI] [PubMed]
- 38.Radiation Risks and Pediatric Computed Tomography (CT): A Guide for Health Care Providers. National Cancer Institute. Available at: http://www.cancer.gov/cancertopics/causes/radiation-risks-pediatrics-CTj. Accessed: February 5, 2008.
- 39.RadiologyInfo Web site. Radiological Society of North America. Available at: http://www.radiologyinfo.org/en/safety/index.cfm?pg=sfty.xray. Accessed: November 20, 2007.
- 40.Rush BH, Branson RT, Ogden JA. Legg-Calve-Perthes disease: detection of cartilaginous and synovial changes with MR imaging. Radiology. 1988;167:473–476. [DOI] [PubMed]
- 41.Schneider B, Laubenberger J, Jemlich S, Groene K, Weber HM, Langer M. Measurement of femoral antetorsion and tibial torsion by magnetic resonance imaging. Br J Radiol. 1997;70:575–579. [DOI] [PubMed]
- 42.Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;407:241–248. [DOI] [PubMed]
- 43.Siebenrock KA, Wahab KH, Werlen S, Kalhor M, Leunig M, Ganz R. Abnormal extension of the femoral head epiphysis as a cause of cam impingement. Clin Ortho Relat Res. 2004;418:54–60. [DOI] [PubMed]
- 44.Smith BG, Kasser JR, Hey LA, Jaramillo D, Millis MB. Postreduction computed tomography in developmental dislocation of the hip: part I: analysis of measurement reliability. J Pediatr Orthop. 1997;17:626–630. [DOI] [PubMed]
- 45.Stoller D. Magnetic Resonance Imaging in Orthopaedics, Sports Medicine. Baltimore, MD: Lippincott Williams and Wilkins; 2005:150–156.
- 46.Stoller D, Tirman P, Bredella M, eds. Diagnostic Imaging: Orthopaedics. New York, NY: Elsevier; 2004:82–86.
- 47.Tönnis MD. Congenital Dysplasia, Dislocation of the Hip in Children and Adults. New York, NY: Springer Verlag; 1984:143–155.
- 48.Tönnis D, Heinecke A. Current concepts review: acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81:1747–1770. [DOI] [PubMed]
- 49.Weiner DS, Cook AJ, Hoyt WA Jr, Oravec CE. Computed tomography in the measurement of femoral anteversion. Orthopedics. 1978;1:299–306. [DOI] [PubMed]
- 50.Weiner LS, Kelley MA, Ulin RI, Wallach D. Development of the acetabulum and hip: computed tomography analysis of the axial plan. J Pediatr Orthop. 1993;13:421–442. [DOI] [PubMed]
- 51.Wiberg B. Studies on dysplastic acetabular and congenital subluxation of the hip joint, with special reference to the complication of osteo-arthritis. Acta Chir Scand. 1939;83:1–135.