Abstract
Following chemotaxonomic evidence, the PE and CHCl3 extracts of the roots of the botanical Angelica sinensis (Oliv.) Diels (Dang Gui) were investigated for in vitro anti-TB activity, in parallel to studying their serotonergic and GABAergic potential. The activities were confirmed to chemically overlap with the neurotropic active principles present in medium lipophilic fractions. Phytochemical investigations led to the isolation of five polyynes: falcarindiol (1), 9Z,17-octadecadiene-12,14-diyne-1,11,16-triol,1-acetate (2), oplopandiol (3), heptadeca-1-ene-9,10-epoxy-4,6-diyne-3,8-diol (4), and the new polyyne 8-hydroxy-1-methoxy-(Z)-9-heptadecene-4,6-diyn-3-one (5), as characterized by spectroscopic techniques including 1D, 2D NMR and HR-MS. All compounds were tested against two pathogenic strains of Mycobacterium tuberculosis (H37Rv and Erdman) in vitro in a microplate Alamar Blue assay (MABA). The most potent anti-TB constituents were 1 and 2, exhibiting MIC values of 1.4-26.7 μg/ml; 3 showed moderate MICs (49.5 and 50.2 μg/ml, respectively) while 4 and 5 were weakly active (MIC> 60 μg/ml). Notably, none of the five compounds exhibited significant cytotoxicity against VERO cells. These findings not only reveal a new potential area of therapeutic value for A. sinensis, but also underline the role of polyynes as anti-TB active principles in ethnobotanical preparations, and as lead compounds.
Keywords: Angelica sinensis, botanicals, dong quai, dang gui, polyynes, anti-TB activity, tuberculosis
INTRODUCTION
Every year approximately 2 million people die from curable tuberculosis (TB). Herbal medicines and other alternative therapies are increasingly used for TB treatment, especially for drug-resistant TB and multidrug-resistant tuberculosis (MDR-TB). Angelica sinensis (Oliv.) Diels (commonly called Dang Gui or Dong Quai) is a perennial apiaceous herb indigenous to northwest China. Its roots have been used for many diseases in Asia, especially for women’s disorders, including anemia, dysmenorrhea, amenorrhea, premenstrual and menopausal syndromes (China Pharmacopoeia 2000). However, no previous study of A. sinensis for anti-TB activities has been reported.
Parallel to ongoing studies of the serotonergic and GABAergic potential of Dang Gui (Deng et al., 2006a and 2006b), the present work followed chemotaxonomic evidence linked to potential anti-TB activity. Polyynes are characteristic triple-unsaturated natural products that have been found in seven plant families, i.e. Araliaceae, Campanulaceae, Asteraceae, Pittisporaceae, Oleaceae, Santalaceae and Apiaceae (Hansen et. al., 1986), and are known to occur in A. species. These highly unsaturated natural products have previously been reported to exhibit general antifungal and antibacterial, but also anti-mycobacterial activities (Furumi et. al., 1998; Kemp, 1978; Kobaisy et. al., 1997). Preliminary investigation of the petroleum ether (PE) and chloroform (CHCl3) extracts of Dang Gui roots confirmed the presence of anti-TB effects in vitro. These activities phytochemically overlapped with the neurotropic activities and were also concentrated in medium lipophilic fractions. The detailed overall phytochemical investigation of the PE and CHCl3 Dang Gui extracts led to the isolation and identification of five polyynes (1-5). All polyynes 1-5 were tested for inhibitory activity toward the growth of M. tuberculosis H37Rv and Erdman in vitro by using the MABA assay. In addition, 1-5 were evaluated for cytotoxicity against VERO cells and selectivity indices (SI) were determined as a measure for the drug lead potential of the compounds.
MATERIALS AND METHODS
General Methods and Plant collection
General materials and instrumentation were as previously described (Deng et al., 2006b). The roots of A. sinensis were purchased in 2000 from Kiu Shun Trading Ltd., Vancouver, Canada and identified by the authors at UIC (Deng et al., 2003). A voucher specimen is deposited at the UIC/NIH Center for Botanical Dietary Supplements Research, Chicago, IL, USA.
Extraction and Isolation
The detailed isolation procedures were performed as previously described. (Deng et al., 2006b). Briefly, 8 kg of the pulverized roots of Angelica sinensis (Oliv.) Diels were extracted with methanol and the extract sequentially partitioned with PE, CHCl3, and BuOH. The PE and CHCl3, which contained the anti-TB activity, were combined and then fractionated by vacuum liquid chromatography (VLC). An aliquot (6.6 g) of subfraction A was further fractionated by repeated VLC now eluting with a gradient of PE-EtOAc-MeOH, and finally purified by reverse-phase (RP) preparative HPLC (75% MeOH in H2O, 6 mL/min). This resulted in the isolation of the five polyynes 1 (48.1 mg, tR = 73 min), 3 (1.6 mg, tR = 91 min), 4 (1.4 mg, tR = 46 min), and 5 (0.7 mg, tR = 94 min). Another subfraction B (4.5 g) was re- chromatographed by VLC over a silica gel column with a stepwise gradient of hexane-CHCl3-EtOAc (20:80:0 → 0:0:100), and then further purified by RP-C18 medium pressure liquid chromatography (MPLC) eluting with a MeOH-H2O gradient from 50% MeOH in H2O to 100% MeOH.). Finally, preparative thin-layer chromatography (TLC) (silica gel, 1 mm, 20 cm × 20 cm), developed with CH2Cl2-EtOAc (7:3), yielded compound 2 (8.7 mg, Rf = 0.6).
Anti-TB biological assays
Bacterial strains
For the preparation of the inocula of the two virulent strains of M. tuberculosis, H37Rv (ATCC 27294) and Erdman (ATCC 35801) bacteria were grown in 100 ml of Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with 0.2% (vol/vol) glycerol (Sigma Chemical Co., Saint Louis, Mo.), 10% (vol/vol) OADC (oleic acid, albumin, dextrose, catalase; Difco), and 0.05% (vol/vol) Tween 80 (Sigma), also referred to as 7H9GC-T80.
Anti-TB Microplate Alamar blue Assay (MABA)
Anti-TB susceptibility testing of extracts and isolates was determined in the fluorometric Microplate Alamar Blue Assay (MABA) assay as described previously (Collins et. al., 1997) in black, clear-bottomed, 96-well microplates (black view plates; Packard Instrument Company, Meriden, Conn.) in order to minimize background fluorescence. Initial drug dilutions were prepared in DMSO, and subsequent twofold dilutions were performed in 0.1 ml of 7H12 medium in the microplates. Rifampin (RMP, Sigma) was used as a positive control, the solvent (DMSO) as a negative control.
For inoculation, both M. tuberculosis strains were diluted in 7H12 media to reach approximately 2 × 10 5 CFU mL-1, and 0.1 ml was added to wells containing the test compounds in 100 μl of 7H12. Plates were incubated at 37 °C for 6 days. At day 7 of the incubation, 20 μl of Alamar Blue solution (Trek Diagnostic Systems, Cleveland, Ohio) and 12.5 μl of 20% Tween 80 were added to all of the wells, and the plates were re-incubated at 37 °C for 24 hours. Fluorescence was measured in a Victor II multilabel fluorometer (Perkin Elmer Life Sciences Inc., Boston, MA) at 530 nm and 590 nm. Thus, minimum inhibitory concentrations (MICs) were determined after incubation for 7 days at 37°C, defining percent inhibition as 1-(test well fluorescence units/mean FU fluorescence units of triplicate wells containing only bacteria) × 100. The MIC values refer to the concentration at which samples exhibited an inhibition of 90%.
Cytotoxicity assay
Evaluation of the cytotoxic activity of isolates in Vero cells (African green monkey kidney cells) was performed as described previously (Cantrell et. al., 1996) using the CellTiter 96 aqueous non-radioactive cell proliferation assay (Promega Corp., Madison, WI). The IC50 was defined as the reciprocal dilution resulting in 50% inhibition of the Vero cells. In addition, selectivity index (SI) values were determined as the ratio of cytotoxicity over the MIC. The cytotoxicity was determined by exposing the Vero cells to different concentrations of the samples. Stock solutions of the samples were prepared at 12.8 mg/mL-1, the positive control Rifampin (RMP) at 100 mg/mL-1 in DMSO. Geometric three-fold dilutions were performed in 96 well clear cell culture plates using the cell culture medium MEM (Gibco, Grand Island, NY) supplemented with 10% of fetal bovine serum (HyClone, Logan, UT). Final DMSO concentrations did not exceed 1% v/v.
Drug dilutions were distributed in duplicate in 96-well tissue culture plates (Becton Dickinson Labware, Lincoln Park, NJ) at a volume of 50 μl per well. An equal volume containing 5 × 105 Vero cells (CCL-81; American Type Culture Collection, Rockville, MD) was added to each well and incubated at 37°C in an atmosphere of 5% CO2 in air. After 72 hours, cell viability was measured using the CellTiter 96 aqueous non-radioactive cell proliferation assay (Promega Corp., Madison, WI) according to the manufacturer’s instructions. Absorbance 490 nm was read in a Victor II reader (Perkin Elmer Life Sciences Inc., Boston, MA). The IC50 was determined using a Curve-fitting program.
RESULTS AND DISCUSSION
The lipophilic PE and CHCl3 partitions of the total extract of the dried roots of Angelica sinensis (Oliv.) Diels contains most of the anti-TB activity of the botanical. In the MABA bioassay, two pathogenic strains of M. tuberculosis, H37Rv and Erdman, were used to evaluate the in vitro anti-TB activities of the extracts and the isolated compounds. The results (Table 1) indicate that the methanol crude extract of the roots of A. sinensis, together with PE and CHCl3 partitions, showed anti-TB activities with MIC values at 85.1, 64.0 and 63.1 μg/ml, respectively, while the BuOH partitions and the H2O phase of the extract displayed MIC values over 128 μg/ml against the H37Rv strain. For the Erdman strain, however, only the CHCl3 partition exhibited weak anti-TB activity with an MIC value at 124.6 μg/ml, while the other partitions were considered inactive (MIC values > 128 μg/ml).
Table 1.
Anti-TB activities, cytotoxicity (IC50) and selectivity indices (SIs) of extract partitions and the isolated polyynes 1-5 from A. sinensis
| Extract/compounds | MICa,b(μg/mL) | Cytotoxicity IC50 (μg/mL) | SIc | ||
|---|---|---|---|---|---|
| Erdman | H37Rv | Erdman | H37Rv | ||
| MeOH extract | >128 | 85.1 | |||
| PE extract | >128 | 64.0 | |||
| CHCl3 extract | 124.6 | 63.1 | |||
| BuOH extract | >128 | >128 | |||
| H2O extract | >128 | >128 | |||
| 1 | 6.0 | 26.7 | >120 | >19 | >4 |
| 2 | 1.4 | 25.3 | >120 | >117 | >4 |
| 3 | 49.5 | 50.2 | >120 | >2 | >2 |
| 4 | >60 | >60 | >120 | n/a | n/a |
| 5 | >60 | >60 | >120 | n/a | n/a |
| RMPd | 0.05 | 0.06 | 111.6 | 2232 | 1860 |
Minimum inhibitory concentration defined as the concentration at which the samples exhibit an inhibition of ≥ 90% in the MABA assay.
Average ± SD of triplicate determinations.
Selectivity index (SI = IC50/MIC).
Positive control (Rifampin); negative control was solvent (DMSO).
n/a = not applicable.
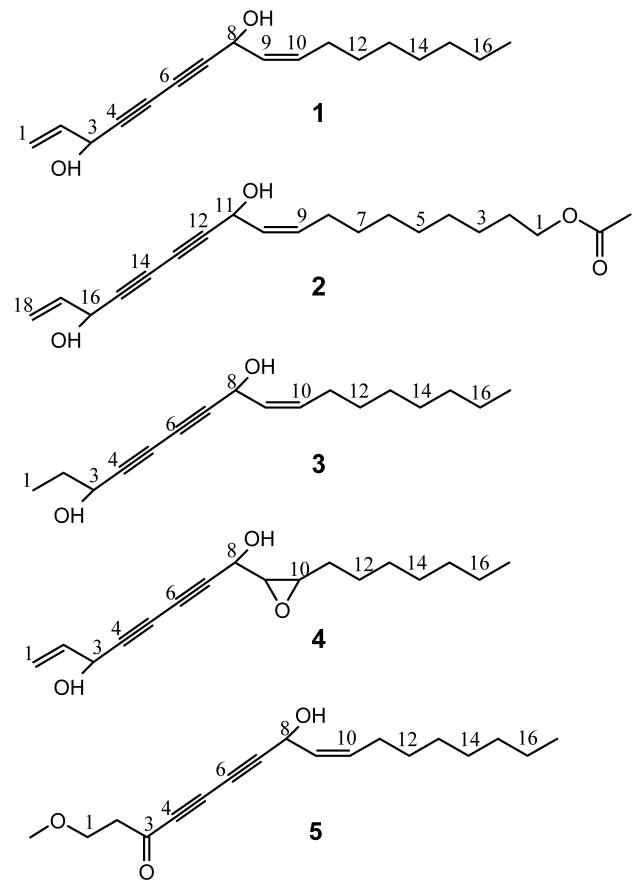
Extensive chromatographic fractionation of the combined extract led to the isolation of five polyynes. Their structures were elucidated/identified as falcarindiol (1) (Zheng et. al., 1999), 9Z,17-octadecadiene-12,14-diyne-1,11,16-triol,1-acetate (2) (Liu et. al., 1998), oplopandiol (3) (Kobaisy et. al., 1997), heptadeca-1-ene-9,10-epoxy-4,6-diyne-3,8-diol (4) (Fujimoto et. al., 1991), and the new 8-hydroxy-1-methoxy-(Z)-9-heptadecene-4,6-diyn-3-one (5). As only incomplete spectroscopic data are available in the literature, their 1H and 13C NMR spectral data are summarized for all compounds in Tables 2 and 3. The NMR spectral assignment incorporates results from a full-spin analysis of the 1H spectrum of polyyne 1, which will provide a template for other polyyne compounds.
Table 2.
1H-NMR data of polyyne 1-5 from A. sinensis [CDCl3, 400 MHz, δH (J Hz)]
| Position in compounds 1,3-5 [2] | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 [18] | Ha: 5.466 ddd (17.1, 1.2, 1.0); Hb: 5.254 dt (10.2, 1.2) | Ha: 5.263, dt (1.2, 10.0); Hb: 5.498, dt (1.2, 17.0) | 1.017, t (7.4) | Ha: 5.468, d (17.0); Hb: 5.286, d (10.2) | 3.719, t (6.0) |
| 2 [17] | 5.943, ddd (17.1, 10.2, 5.3) | 5.951, ddd (17.0, 10.2, 5.3) | 1.749, m | 5.954, ddd (17.0, 10.2, 5.2) | 2.821, t (6.0) |
| 3 [16] | 4.936, ddd (5.3, 1.0, 1.0) | 4.952, br d (5.3) | 4.385, t (6.4) | 4.960, d (5.2) | |
| 8 [11] | 5.213, dt (8.3, 1.0) | 5.212, d (8.1) | 5.204, d (8.2) | 4.296, d (7.4) | 5.265, dd (8.4, 1.0) |
| 9 [10] | 5.521, dd (10.7, 8.3) | 5.526, ddt (10.6, 8.1, 1.0) | 5.517, ddt (10.6, 8.2, 1.3) | 3.190, dd (7.4, 4.4) | 5.525, ddt (10.6, 8.4, 1.4) |
| 10 [9] | 5.606, dt (10.7, 7.4) | 5.768, ddt (10.6, 7.4, 1.0) | 5.613, ddt (10.6, 7.4, 0.9) | 3.067, dt (5.5, 4.4) | 5.670, ddt (10.6, 1.0, 7.6) |
| 11 [8] | 2.105, ddt (8.4, 7.4, 1.2) | 2.108, dt (1.3, 7.4) | 2.113, ddt (7.4, 7.4, 1.3) | 1.560, m | 2.212, dtd (7.6, 7.6, 1.4) |
| 12 [7] | 1.390, m | 1.391, m | 1.389, m | 1.215-1.405, m | 1.329, m |
| 13-16 [3-6] | 1.240-1.330, m | 1.259-1.310,m | 1.279-1.293, m | 1.215-1.405, m | 1.252-1.333, m |
| 17 [2] | 0.882, t (7.0) | 1.630, m | 0.885, t (7.0) | 0.890, t (7.0) | 0.884, t (7.5) |
| [1] | 4.062, t (6.8) | ||||
| [2′] | 2.051, s | ||||
| 1-OCH3 | 3.348, s |
Table 3.
The 13C-NMR spectral data of polyynes 1-5, isolated from A. sinensis (CDCl3, 100 MHz)
| Position compounds 1,3-5 [2] | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 [18] | 117.3 | 117.3 | 9.3 | 117.6 | 67.1 |
| 2 [17] | 135.7 | 135.8 | 30.7 | 135.6 | 45.7 |
| 3 [16] | 63.4 | 63.4 | 64.1 | 63.3 | 184.6 |
| 4 [15] | 78.2 | 78.4 | 80.7 | 76.3 | 75.7 |
| 5 [14] | 70.3 | 70.2 | 68.6 | 72.2 | 77.4 |
| 6 [13] | 68.7 | 68.7 | 68.9 | 70.7 | 67.9 |
| 7 [12] | 79.8 | 79.7 | 79.2 | 78.7 | 87.6 |
| 8 [11] | 58.5 | 58.6 | 58.7 | 62.2 | 59.1 |
| 9 [10] | 127.6 | 127.8 | 127.8 | 59.5 | 127.1 |
| 10 [9] | 134.6 | 134.4 | 134.6 | 57.7 | 135.8 |
| 11 [8] | 31.8 | 29.7 | 27.7 | 28.1 | 28.1 |
| 12 [7] | 29.3 | 29.1 | 29.3 | 31.7 | 27.3 |
| 13 [6] | 29.2 | 29.1 | 29.1 | 29.3 | 29.4 |
| 14 [5] | 29.1 | 28.9 | 29.2 | 29.1 | 29.4 |
| 15 [4] | 27.7 | 28.6 | 22.7 | 26.6 | 32.1 |
| 16 [3] | 22.6 | 27.6 | 31.8 | 22.8 | 22.9 |
| 17 [2] | 14.1 | 25.8 | 14.1 | 14.1 | 14.3 |
| [1] | 64.7 | ||||
| [1′] | 171.6 | ||||
| [2′] | 21.1 | ||||
| 1-OCH3 | 58.9 |
Evaluation of the in vitro anti-TB activities of the isolates 1-5 indicated that compounds 1 and 2 exhibited the most potent anti-TB activities with MIC values of 6.0 μg/ml and 1.4 μg/ml vs. the Erdman strain, as well as 26.7 μg/ml and 25.3 μg/ml vs. the H37Rv strain, respectively. Compound 3 displayed moderate activity with MIC values of 49.5 and 50.2 against the two strains, respectively, while the MICs of 4 and 5 were above 60 μg/ml in both strains and, thus, were considered inactive isolates.
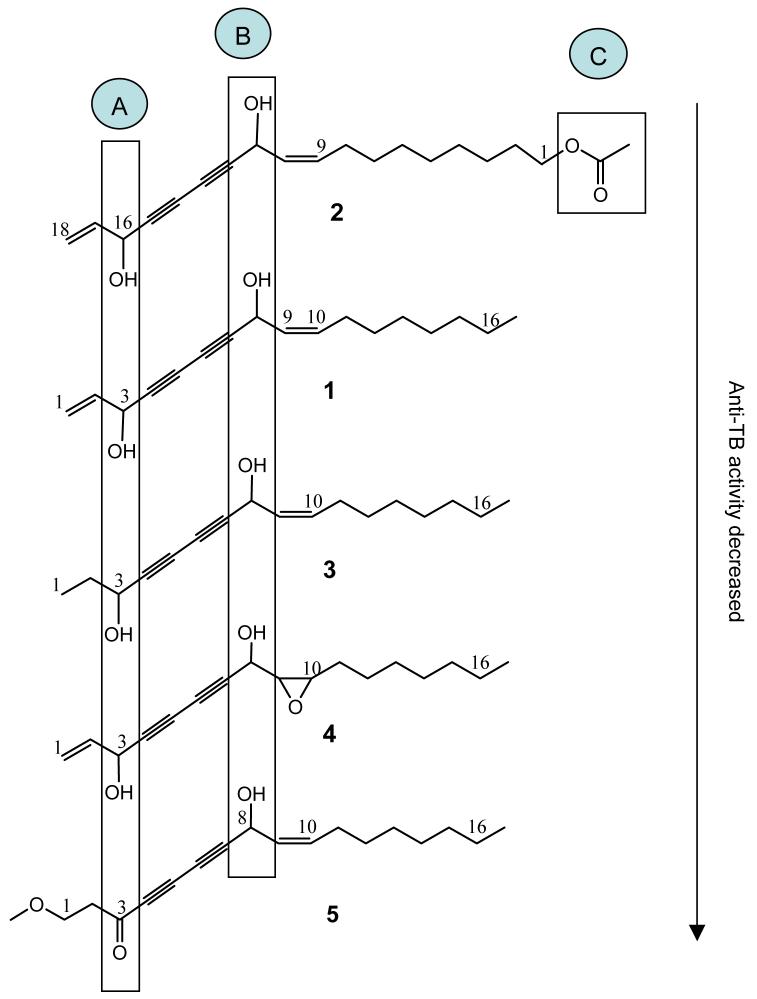
Despite the limitations of the dataset, figure 1 provides a preliminary summary of the anti-TB structure-activity relationships of the polyynes, comparing the structures of the isolates 1-5 and sorting them according to their overall in vitro anti-TB potency. This comparison reveals that both functional groups in area A (hydroxyl group in position 16 of polyyne 2, and in position 3 of polyynes 1 and 3) and B (9,10 olefinic bond) are likely to be essential for the anti-TB activity of polyynes, and is consistent with a recent SAR study reporting a relationship between activity and predicted lipophilicity parameters (Zlohet. al., 2007). Comparing the differences in activity of the pairs 1/3 and 1/4, the A-terminal unsaturation might contribute to the anti-TB activity, but does not appear to be essential. In addition, the presence of functional groups in area C (an acetate group attached to a saturated aliphatic terminal position) might enhance this activity significantly, as observed in the case of 2.
Figure 1.
Preliminary structure-activity relationships of anti-TB polyynes isolated from A. sinensis.
Another important biological observation is that, despite the fact that polyynes are widely considered to be cytotoxic, none of the five isolates exhibited measurable cytotoxicity against VERO cells, defining an IC50 value of more than 120 μg/ml as a cutoff point. Accordingly, the polyynes 1-3 were found to be rather selective for anti-TB activity, with a selectivity index (SI=IC50/MIC) being as high as >100 for polyyne 2 (precise SI determination limited by the 120 μg/ml cytotoxicity cutoff). Finally, the biological observations summarized in Table 1 represent an interesting reversal in the trend of the activities versus the two tested strains starting out with the extracts: while the crude partitions were more active against H37Rv, the isolated polyynes exhibited stronger activities against the Erdman strain. Because the anti-TB activity of the extract can be more broadly ascribed to non-polar components, the isolated polyynes represent an important, but not the only part of the active principle. Further research is required to influence the synergy (Inui et al., 2006; Inui et al., 2007) and the presence of other classes of anti-TB active compounds in Dang Gui botanical preparations. At this point, however, the biological potency of polyyne 2 already warrants its further evaluation using in vivo models of tuberculosis infection in mice. The findings also underline the role of polyynes as anti-TB active principles in ethnobotanical preparations, and establish an interesting new biological target for A. sinensis.
Acknowledgements
This work was supported in part by grant P50 AT00155 through the Office of Dietary Supplements (ODS), the National Center for Complementary and Alternative Medicine (NCCAM), and the Office for Research on Women’s Health (ORWH). The authors have no conflict of interest. This study was performed according to the academic code of ethics. The contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
Contract/grant sponsor: NCCAM/ODS/NIH
REFERENCES
- Cantrell CL, Lu T, Fronczek FR, Fischer NH, Adams LB, Franzblau SG. Antimycobacterial cycloartanes from Borrichia frutescens. J Nat Prod. 1996;59:1131–1136. doi: 10.1021/np960551w. [DOI] [PubMed] [Google Scholar]
- Collins L, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicr Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Pharmacopoeia Committee . Pharmacopoeia of the People’s Republic of China. Radix Angelica sinensis; Beijing, P.R. China: 2000. p. 101. [Google Scholar]
- Deng S. Ph.D. Dissertation. University of Illinois at Chicago; Chicago (IL): 2005. Phytochemical Investigation of Bioactive Constituents from Angelica sinensis. [Google Scholar]
- Deng S, Chen S, Liu J, Wang Z, Nikolic D, van Breeman RB, Santarsiero BD, Mesecar AD, Fong HHS, Farnsworth NR, Pauli GF. A Novel GABAergic Phthalide Dimer from Angelica sinensis (Oliv.) Diels. Phytochem Anal. 2006a;17:398–405. doi: 10.1002/pca.937. [DOI] [PubMed] [Google Scholar]
- Deng S, Chen S, Yao P, Nikolic D, van Breeman RB, Bolton JL, Fong HHS, Farnsworth NR, Pauli GF. Serotonergic Activity-guided phytochemical investigation of the roots of Angelica sinensis. J. Nat Prod. 2006b;69:536–541. doi: 10.1021/np050301s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Satoh M, Takeuchi N, Kirisawa M. Cytotoxic acetylenes from Panax quinquefolium. Chem Pharm Bull. 1991;39:521–523. doi: 10.1248/cpb.39.521. [DOI] [PubMed] [Google Scholar]
- Furumi K, Fujioka T, Fujii H, Okabe H, Nakano Y, Matsunaga H, Katano M, Mori M, Mihashi K. Novel antiproliferative falcarindiol furanocoumarin ethers from the root of Angelica japonica. Bioorg Med Chem Lett. 1998;8:93–96. doi: 10.1016/s0960-894x(97)10193-7. [DOI] [PubMed] [Google Scholar]
- Hansen L, Boll PM. Polyacetylenes in Araliaceae: their chemistry, biosynthesis and biological significance. Phytochemistry. 1986;25:285–293. [Google Scholar]
- Inui T, Wang Y, Cho S, Wan B, Smith D, Franzblau S, Pauli GF. 47th Annual Meeting of the American Society of Pharmacognosy (ASP); Arlington (VA). 2006.p. 136. [Google Scholar]
- Inui T, Wang Y, Smith D, Franzblau S, Pauli GF. Counter-current chromatography- based analysis of synergy in an anti-tuberculosis ethnobotanical. J Chromatogr A. 2007 doi: 10.1016/j.chroma.2007.01.127. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp MS. Falcarindiol: an antifungal polyacetylene from Aegopodium podagraria. Phytochemistry. 1978;17:1002. [Google Scholar]
- Kobaisy M, Abramowski Z, Lermer L, Saxena G, Hancock REW, Towers GHN, Doxsee D, Stokes RW. Antimycobacterial polyynes of Devil’s Club (Oplopanax horridus), a North American native medicinal plant. J Nat Prod. 1997;60:1210–1213. doi: 10.1021/np970182j. [DOI] [PubMed] [Google Scholar]
- Liu J-H, Zschocke S, Bauer R. A polyacetylenic acetate and a coumarin from Angelica pubescens f. biserrata. Phytochemistry. 1998;49:211–213. [Google Scholar]
- Zheng G, Lu W, Cai J. Stereoselective total synthesis of (3R,8S)-falcarindiol, a common polyacetylenic compound from umbellifers. J Nat Prod. 1999;62:626–628. doi: 10.1021/np980418z. [DOI] [PubMed] [Google Scholar]
- Zloh M, Bucar F, Gibbons S. Quantum chemical studies on structure activity relationship of natural product polyacetylenes. Theor. Chem. Acc. 2007;117:247–252. [Google Scholar]