Abstract
This study tested the hypothesis that glutamatergic ionotropic (AMPA/kainate) receptors and neurokinin receptors (NKR) are important in the regulation of respiratory motor output during development in the bullfrog. The roles of these receptors were studied with in vitro brainstem preparations from pre-metamorphic tadpoles and post-metamorphic frogs. Brainstems were superfused with an artificial cerebrospinal fluid at 20–22°C containing CNQX, a selective non-NMDA antagonist, or with substance P (SP), an agonist of NKR. Blockade of glutamate receptors with CNQX in both groups caused a reduction of lung burst frequency that was reversibly abolished at 5 μM (P<0.01). CNQX, but not SP, application produced a significant increase (P<0.05) in gill and buccal frequency in tadpoles and frogs, respectively. SP caused a significant increase (P<0.05) in lung burst frequency at 5 μM in both groups. These results suggest that glutamatergic activation of AMPA/kainate receptors is necessary for generation of lung burst activity and that SP is an excitatory neurotransmitter for lung burst frequency generation. Both glutamate and SP provide excitatory input for lung burst generation throughout the aquatic to terrestrial developmental transition in bullfrogs.
Keywords: CNQX, AMPA/kainate receptors, Neurokinin receptors, amphibian, Lithobates catesbeiana
1. Introduction
Glutamate and Substance P (SP) have been shown to be critical neurotransmitters in the regulation of respiratory rhythm in the mammalian brainstem (Smith et al. 1991; Yamamoto et al. 1992; McCrimmon et al. 1995; Gray et al. 1999, 2001; Wenninger et al. 2004); however, there is little known of the role of these neurotransmitters in the regulation of respiratory rhythm in other vertebrates. In the lamprey brainstem in vitro, both ionotropic and metabotropic glutamate receptors contribute to neurotransmission within the respiratory network; however, non-NMDA AMPA/kainate receptors are necessary for respiratory rhythm generation (Bongianni et al. 1999). In turtles, NMDA receptors play a small but significant role in bulbospinal respiratory synaptic transmission (Johnson and Mitchell, 1998), but the role of non-NMDA receptors and SP in respiratory rhythm generation in turtles has not been investigated.
Superfusion of glutamate or SP in the adult frog brainstem produces a biphasic effect: a transient inhibition followed by a significant increase in fictive breathing (Perry et al. 1995). Microinjection of glutamate into the adult brainstem (McLean et al. 1995) or of the non-NMDA glutamate receptor agonist, AMPA, into discrete locations in the post-metamorphic frog brainstem, has been shown to differentially affect buccal and lung bursts in different regions of the brainstem (Wilson et al. 2002). These data suggest that, in the post-metamorphic frog, the areas of the brainstem responsible for generating buccal burst activity may be anatomically distinct from those areas that generate lung burst activity. Although these studies have led to important insights into the location and regulation of the respiratory rhythm generating regions in mature air-breathing anurans, it is unclear how glutamate and SP modulate respiratory-related motor output at the brainstem level and throughout development.
The bullfrog brainstem in vitro has proven to be an excellent model for studying the development of respiratory rhythm generation, thus providing direct developmental comparisons that are not yet possible with other vertebrate models (Hedrick, 2005). Several studies have demonstrated developmentally-associated changes in the modulation of respiratory-related neural output associated with the transition from aquatic to terrestrial lifestyle. For example, blocking synaptic inhibition in the pre-metamorphic tadpole brainstem in vitro abolishes gill activity, but not lung activity (Galante et al., 1996; Broch et al., 2002) whereas in adult brainstems lung activity is abolished (Broch et al. 2002). Nitric oxide inhibits lung respiratory rhythm generation in pre-metamorphic tadpoles, but is excitatory in post-metamorphic frog and adult brainstems (Hedrick et al., 1998; Hedrick et al., 2005). Neuromodulators such as serotonin (5-HT) and norepineprhine regulate respiratory rhythm differentially in pre-metamorphic and post-metamorphic amphibians (Belzile et al. 2002; Kinkead et al. 2002; Fournier and Kinkead, 2006; Fournier et al. 2007). These studies indicate that a considerable degree of neuromodulation of the respiratory rhythm and pattern circuits occurs during ontogenetic maturation of the amphibian brainstem. However, the role of excitatory amino acids and peptides that regulate the respiratory network during amphibian development is largely unknown.
Given the importance of glutmatergic and peptidergic excitatory mechanisms for respiratory rhythm generation in mammals, turtles and lamprey, and the limited data from post-metamorphic and adult amphibians, the present study sought to determine the involvement of non-NMDA glutamate receptors, and SP, in the regulation of respiratory motor output during development in the North American bullfrog (Lithobates catesbeiana). We hypothesized that glutamate and SP are important, excitatory neurotransmitters for respiratory burst generation in the amphibian brainstem.
2. Materials and Methods
2.1. Animals
Experiments were performed on total of 13 pre-metamorphic and 11 post-metamorphic North American Bullfrogs, Lithobates (formerly Rana) catesbeiana (see Frost et al. 2006). Tadpoles were classified according to the staging criteria of Taylor and Köllros (T-K, 1946). Pre-metamorphic tadpoles ranged from T-K stages VI–XVII (paddle and foot stages), and post-metamorphic frogs ranged from T-K stages XXIII–XXV. Animals were acquired from a commercial supplier (Charles D. Sullivan Co., Inc.; Nashville, TN, USA). Pre-metamorphic tadpoles were kept in plastic tank aquaria with oxygenated, dechlorinated tap water and were fed boiled spinach twice per week. Frogs were kept in plastic aquaria that provided dechlorinated water and a dry area. These animals were fed small crickets twice per week. All animals were maintained at room temperature (20–22°C). All experimental procedures were approved by the CSUEB Institutional Animal Care and Use Committee.
2.2. In vitro brainstem preparation
Animals were anesthetized prior to surgery with a dilute (0.5%) solution of ethyl-m-aminobenzoate (MS-222) buffered to pH 7.8 with sodium bicarbonate. Once the breathing movements ceased (2–5 min for pre-metamorphic tadpoles; and 5–10 min for frogs) and withdrawal and eye blink reflexes were abolished, the animals were removed from anesthetic. Tadpoles were weighed, and then placed under ice for 20–30 minutes to reduce metabolism and maintain anesthesia for subsequent dissection.
A small opening was made in the cranium using iris scissors for the transection of brainstem at rostral to the optic lobes to remove the forebrain. The brainstem was exposed and all nerves anterior to the brachial nerves were carefully cut at their exit from the skull. During decerebration and dissection, the brainstem was supplied with constant perfusion of cold (5–10 °C), oxygenated (98% O2 and 2% CO2) artificial cerebrospinal fluid (aCSF) with following composition (mmol 1−1): NaCl 104.0, KCl 4.0, MgCl2 1.4, NaHCO3 25.0, CaCl2 2.4, glucose 10.0. The entire dissection was completed in approximately 5–10 minutes.
Once the brainstem was removed, it was placed in the recording chamber (7 ml). The brainstem was pinned ventral side up and the dura gently removed. Throughout this process, and during all subsequent experiments, the recording chamber was continuously perfused with oxygenated (98% O2, 2% CO2) aCSF equilibrated to room temperature (20–22 °C) from a reservoir at rate of 5–10 ml min−1 at a pH of 7.8 to 7.9.
Nerve roots of cranial nerves (CN) V (trigeminal), VII (facial), X (vagus), and XII (hypoglossal) from the brainstem that normally innervate respiratory muscles were attached to suction electrodes fabricated from 1 mm diameter thin-walled capillary glass tubing (A-M Systems, Carlsborg, WA, USA). Previous studies with amphibian brainstem preparations have verified that the neural activities in CN V, VII, X and XII are correlated with breathing in intact animals (Gdovin et al., 1998; Sakakibara, 1984). Neural signals were amplified 10,000 times with an AC amplifier (A-M systems model 1700), filtered (10 Hz – 2 kHz) and saved to PC-type computer (Dell, Pentium 4) that interfaced with data acquisition system sampling at 2 kHz (Powerlab 8/SP; AD Instruments, Milford, MA, USA).
2.3. Experimental protocol
The selective non-NMDA glutamate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Sigma, St. Louis, MO, USA), was used to block AMPA/kainate receptors. Substance P (SP, Sigma) was used as an agonist for neurokinin receptors (NKR). Each brainstem preparation was used once for a single drug (CNQX, N=11 or SP, N=13).
CNQX (0.1, 0.5, 1.0, 5.0 μM) was dissolved in 100% dimethylsulfoxide (DMSO) and diluted with aCSF to achieve the desired concentrations. The highest concentration of DMSO used was 0.1%, which was the highest concentration needed to dissolve the maximum concentration of CNQX used (5 μM). Previous studies from our laboratory have shown that this concentration of DMSO has no effect on respiratory motor output in the bullfrog brainstem. SP (0.1, 1.0, 5.0 μM) was dissolved in aCSF to achieve the desired concentrations. For each experiment, the brainstem preparation was superfused with oxygenated aCSF for 1 h, or until the signal of fictive respiration was stable, before a 30 min control recording was obtained. After the initial control recording was taken, each brainstem was applied with superfusate of aCSF containing a drug (CNQX or SP). Each concentration of the drug was superfused for 35 min before increasing the concentration. All brainstem preparations were returned to the control aCSF (washout) for up to 2 h after drug exposure. Respiratory-related motor output was recorded throughout the experiment and the last 10 min of the data collected during drug exposure was used for analysis.
Fictive gill activity in the pre-metamorphic tadpole and buccal activity in the post-metamorphic frog are characterized as high frequency, low amplitude bursts occurring in CN V, VII and X. Fictive lung ventilation was characterized as neural bursts occurring singly or in clusters and appeared as low frequency and high amplitude neural bursts. Fictive lung bursts occur simultaneously in CN V, VII, and XII in pre-metamorphic and post-metamorphic frogs and have burst durations of about 1 s (Gdovin et al., 1998). Discharges of motor output not meeting criteria were classified as non-respiratory activity and were excluded from analysis (e.g. Hedrick and Winmill, 2003). Absolute burst frequency is defined as neural bursts per minute, regardless of the pattern of lung burst events (i.e. single breaths or episodes). Lung burst frequency (min−1) was measured as the number of bursts in a 10 min period. Burst duration was measured from the onset deviation from the baseline to the return to baseline in the integrated neural trace. Burst amplitude was analyzed as a percentage of control from the integrated neural trace. Gill or buccal bursts were analyzed during the last 10 min of recorded data and frequency (min−1) was measured from a continuous 1–2 min portion of that data in which there were no lung burst events.
2.4. Statistical analysis
A one-way analysis of variance with repeated measures (ANOVA) followed by Dunnett’s multiple-comparison test (Zar, 1974) was used for evaluation of statistical significance between fictive breaths during drug administration compared with that in control period, within each experimental group. Data are reported as mean ± s.e.m. Statistical significance was assumed if P< 0.05. All statistical and graphical analyses were carried out using a commercially available software program (Graphpad Prism, v. 5.0 (PC version), San Diego, CA, USA).
3. Results
3.1 Effects of non-NMDA receptor blockade with CNQX
Blockade of non-NMDA glutamatergic receptors with CNQX produced similar effects in both pre-metamorphic tadpole and frog brainstems. There was a significant reduction of lung burst frequency at 1 μM CNQX (P<0.05) and lung burst activity was completely abolished at 5 μM CNQX (P<0.01) in both groups (Fig. 1). The abolition of lung burst activity was completely reversible in all preparations with frequency returning to control levels during washout with aCSF (P>0.05; Fig. 2). In contrast to the cessation of lung burst activity, CNQX applied to both pre-metamorphic and frog brainstems significantly increased the gill burst frequency in tadpoles and buccal burst frequency in frog brainstems (Fig. 1).
Fig. 1.
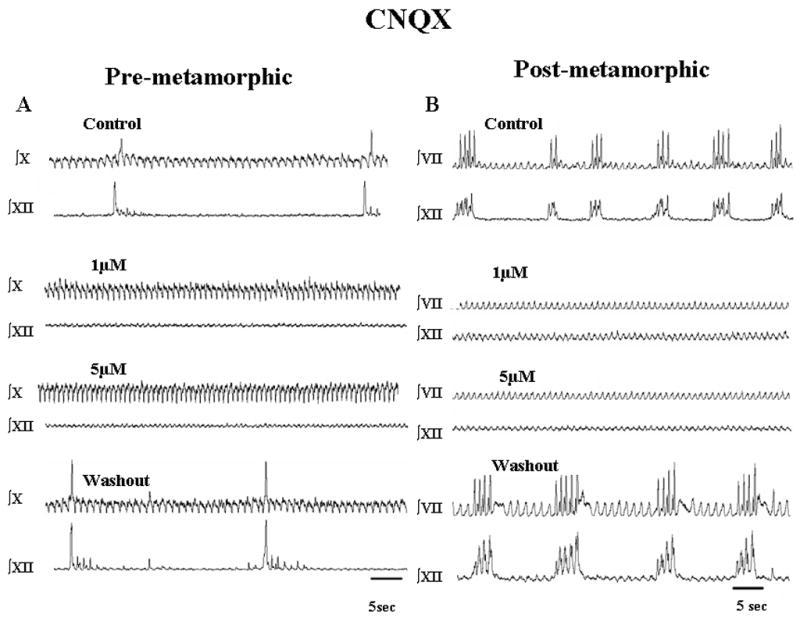
Respiratory-related neural activity recorded from tadpole brainstem preparations. Effects of CNQX (control, 1, 5 μM) followed by washout with aCSF are shown as integrated activity from CN X and XII (A; pre-metamorphic brainstems) and CN VII and CN XII (B; post-metamorphic brainstems). In both preparations, lung burst activity (high amplitude, infrequent bursts) was abolished with CNQX and resumed upon washout. The low amplitude, more frequent gill bursts (pre-metamorphic) and buccal bursts (post-metamorphic) were unaffected by CNQX.
Fig. 2.
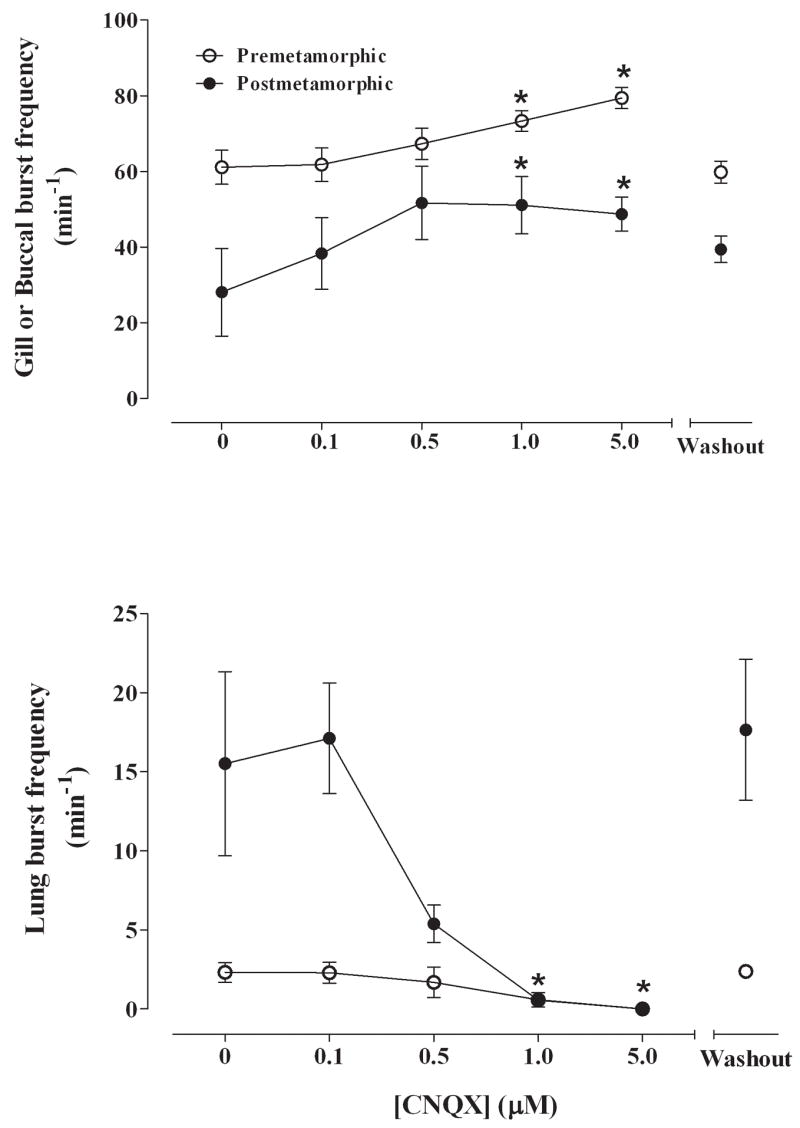
Summary of effects of CNQX (0, 0.1, 0.5, 1.0, 5.0 μM) on pre-metamorphic (unfilled circles) and post-metamorphic (filled circles) brainstems measured as gill or buccal burst frequency (min−1) (A) and lung burst frequency (B). There were significant increases in gill and buccal burst frequency and a complete abolition of lung bursts in both groups. *P<0.01 compared with control (Dunnett’s test).
Gill burst frequency in pre-metamorphic tadpoles was 61.1 ± 4.5 min−1 in control conditions. Application of CNQX significantly increased gill burst frequency to 73.4 ± 2.8 min−1 with 1 μM CNQX (P<0.01) and to 79.4 ± 2.9 min−1 with 5 μM CNQX (Fig. 2A; P<0.001). Gill activity returned to control levels upon washout with control aCSF. Gill burst duration was 0.94 ± 0.10 s in control and decreased significantly (P<0.01) to 0.69 ± 0.02 s at the highest concentration of CNQX (5 μM). Gill burst amplitude was unaffected by CNQX (P>0.05; Fig. 3A).
Fig. 3.
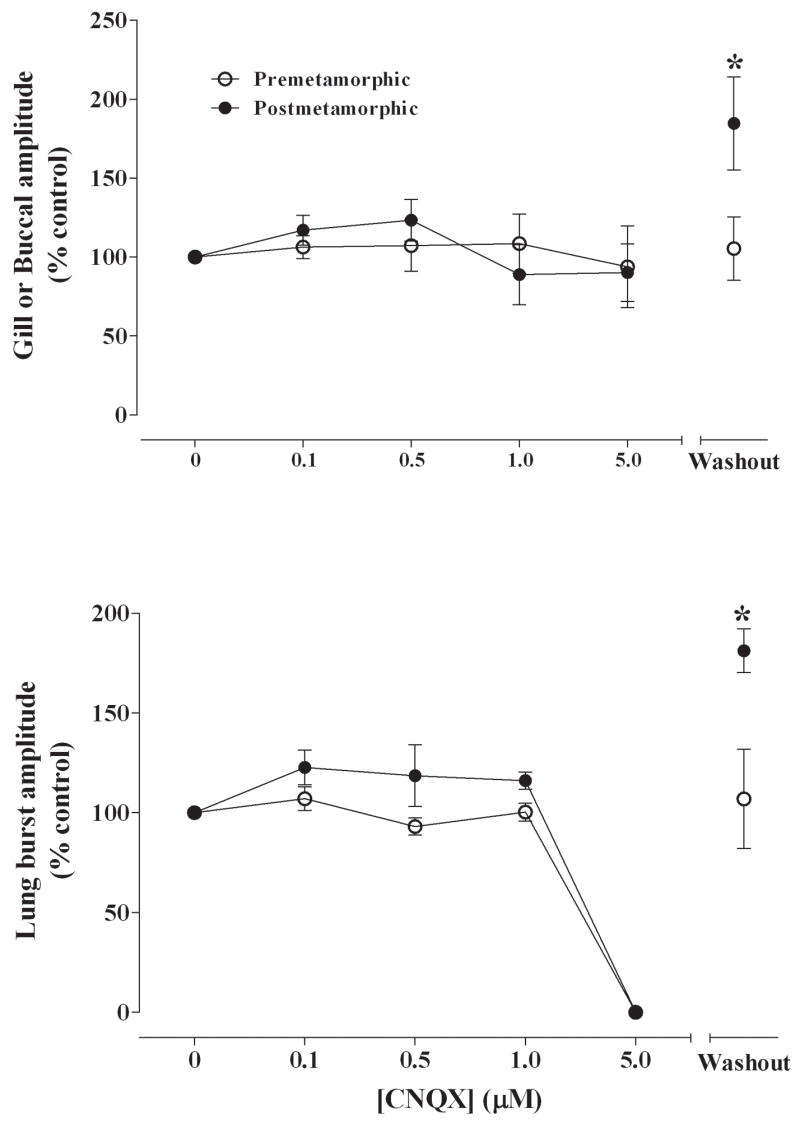
Summary of effects of CNQX (0, 0.1, 0.5, 1.0, 5.0 μM) on gill or buccal burst amplitude (% control) (A) and lung burst amplitude (B) for pre-metamorphic (unfilled circles) and post-metamorphic (filled circles) brainstems. There were significant increases of buccal and lung burst amplitudes compared with control for post-metamorphic brainstems. *P<0.01 compared with control (Dunnett’s test).
Buccal bursts were present in 5 of 6 post-metamorphic frog brainstems. Buccal burst frequency was 28.1 ± 11.6 min−1 under control conditions and blockade with 0.5 μM to 5 μM CNQX significantly increased buccal burst frequency to approximately 50 bursts min−1 compared with control (Fig. 2A; P<0.05). Buccal burst frequency returned to control levels following washout with aCSF. Although buccal burst amplitude was unaltered during superfusion with CNQX, there was a significant increase in burst amplitude to 185 ± 29% of control during the washout period (P<0.01; Fig. 3A). Buccal burst duration was 0.75 ± 0.07 s in control and remained constant throughout the exposure to CNQX.
Lung burst frequency in pre-metamorphic animals was 2.3 ± 0.6 min−1 with control aCSF superfusion. Blocking non-NMDA receptors with CNQX in pre-metamorphic brainstems significantly decreased lung burst frequency to 0.6 ± 0.5 min−1 with 1 μM CNQX (Fig. 2B; P<0.05) and lung activity was reversibly abolished with 5 μM CNQX (P<0.01). Lung burst amplitude remained constant at concentrations up to 1 μM CNQX before bursts were abolished and then returned to control levels during washout (Fig. 3B). Lung burst duration was 0.67 ± 0.06 s was not affected by CNQX.
In post-metamorphic frog brainstem preparations, lung burst frequency was 15.5 ± 5.8 min−1 with control superfusion and significantly decreased to 0.6 ± 0.3 min−1 with 1 μM CNQX (Fig. 2B; P<0.05) and was completely abolished with 5 μM CNQX (P<0.01). Lung burst duration was 0.60 ± 0.04 s and was not affected by CNQX exposure. Lung burst amplitude was also unaffected during drug application; however, lung burst amplitude was 181 ± 11% of control during the washout period (P<0.01; Fig. 3B).
3.2 Effects of substance P
Stimulation of NKR with SP at concentrations up to 5 μM increased lung burst frequency in both pre-metamorphic and post-metamorphic frog brainstems (Fig. 4). Gill burst frequency in pre-metamorphic tadpole brainstems was 65.5 ± 4.4 min−1 in control conditions and application of SP at concentrations up to 5 μM had no significant effects on gill burst frequency (P>0.05; Fig. 5A). Substance P also had no effect on gill burst amplitude. Gill burst duration was 0.90 ± 0.05 s and was unaffected by SP (P>0.05; Fig. 6A).
Fig. 4.
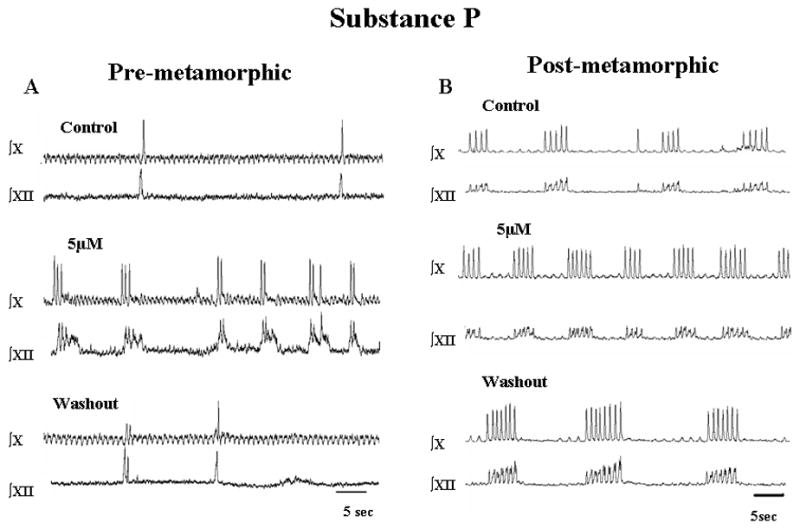
Effects of Substance P on respiratory-related neural activity from tadpole brainstem preparations. Responses of pre-metamorphic (A) and post-metamorphic (B) brainstems are shown for the highest concentration of SP used (5 μM) followed by washout with aCSF. Note the increase in lung burst frequency with SP.
Fig. 5.
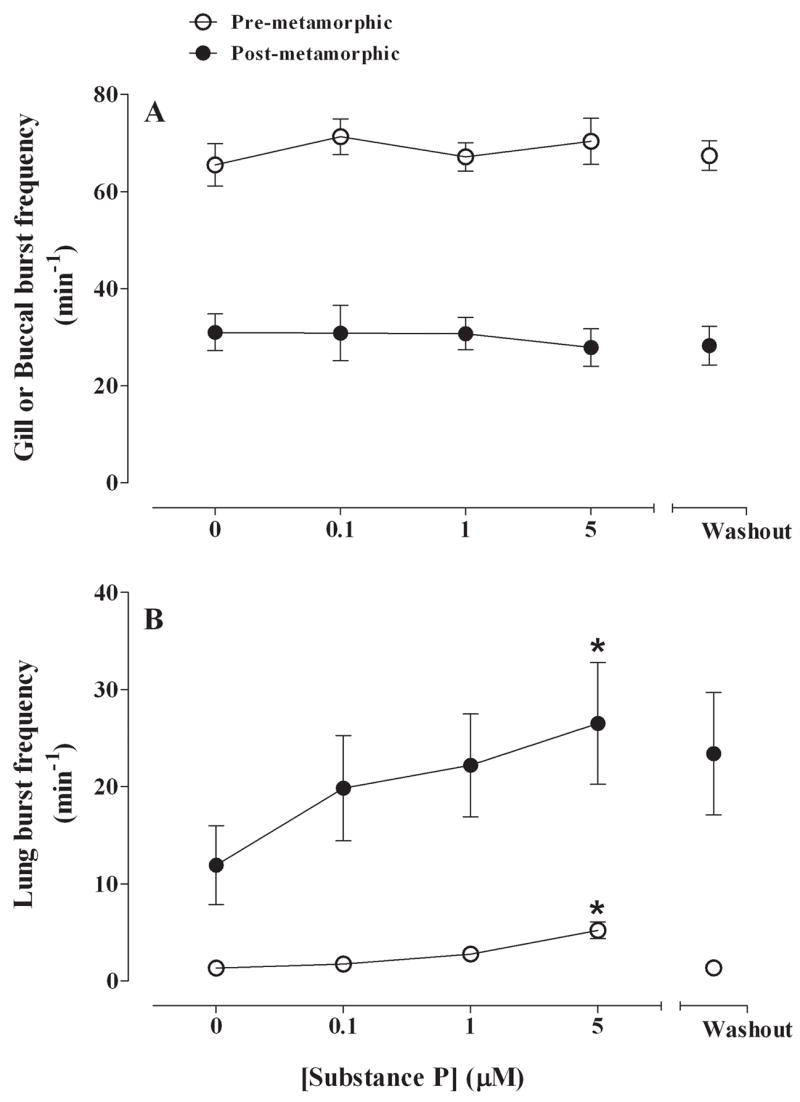
Summary of effects of SP (control, 0.1, 1, 5 μM) on gill and buccal burst frequency (min−1) (A) and lung burst frequency (B) for pre-metamorphic (unfilled circles) and post-metamorphic (filled circles) brainstems. Gill and buccal burst frequency was unaffected by SP whereas lung burst frequency increased significantly. *P<0.05 compared with control (Dunnett’s test).
Fig. 6.
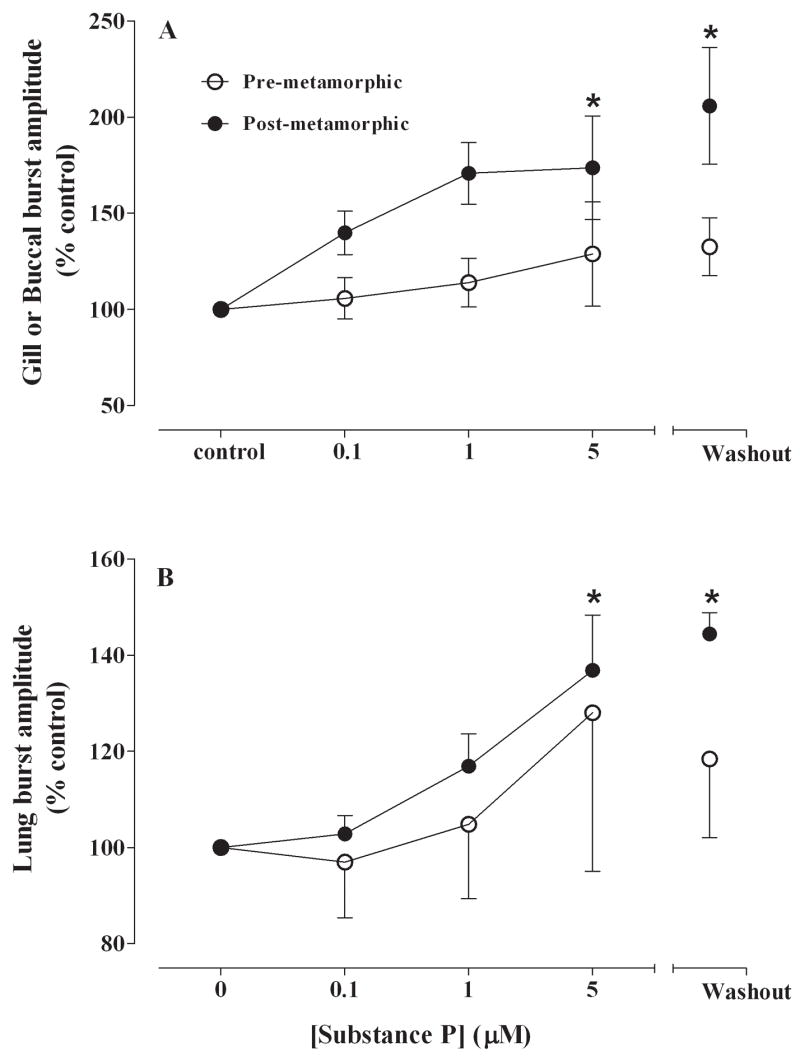
Summary of effects of SP (control, 0.1, 1, 5 μM) on gill and buccal burst amplitude (% control) (A) and lung burst amplitude (B) for pre-metamorphic (unfilled circles) and post-metamorphic (filled circles) brainstems. There were significant increases in buccal and lung burst amplitudes for post-metamorphic brainstems at the highest SP concentration (5 μM) which persisted throughout the washout period. *P<0.05 compared with control (Dunnett’s test).
Buccal frequency was 32.7 ± 3.4 min−1 in post-metamorphic frog brainstems, and SP at concentrations up to 5 μM had no effect on buccal burst frequency (Fig. 5A). Although SP had no effect on buccal burst amplitude during drug application, there was a significant increase of amplitude to 185 ± 32% of control during the washout period (P<0.05; Fig. 6A). Buccal burst duration was 0.88 ± 0.07 s and did not change with SP exposure.
Superfusion of SP significantly increased lung burst frequency from 1.3 ± 0.4 min−1 in pre-metamorphic control brainstems to 5.2 ± 0.9 min−1 at a concentration of 5 μM (Fig. 5B; P<0.001) and returned to control levels upon washout. SP had no effect on lung burst amplitude (Fig. 6B) and burst duration was 0.63 ± 0.08 s in control and was unaffected by SP.
Application of SP to post-metamorphic frog brainstem preparations produced similar results to that of pre-metamorphic animals with a significant increase of lung burst activity from 11.9 ± 4.1 min−1 to 26.5 ± 6.3 min−1 at 5 μM SP (P<0.05; Fig. 5B) which also returned to control levels during washout. Substance P significantly increased lung burst amplitude to 138 ± 14% of control at 5 μM and this increase in amplitude was maintained throughout a 2 h washout period (Fig. 6B; P<0.05). Lung burst duration was 0.64 ± 0.05 s in control and did not change during SP exposure or during washout.
4. Discussion
The results from this study revealed that glutamate, acting via non-NMDA (AMPA/kainate) receptors, is necessary for the expression of lung burst activity in brainstems from both pre-metamorphic and post-metamorphic frogs. The reversible cessation of lung burst activity, without a corresponding decrease in gill or buccal activity, also suggests that lung burst activity and gill or buccal burst activity are regulated independently. The results with SP indicate that stimulation of NKR also provides an excitatory input for generation of lung bursts, but not gill or buccal respiratory bursts. These data indicate that glutamate and SP provide excitatory drives for lung burst generation throughout the aquatic to terrestrial developmental period in the bullfrog brainstem.
Although the in vitro amphibian model has provided useful insights into developmental regulation of respiratory-related motor output, there are some important caveats regarding the limitations of interpreting results from experiments with bath-applied drugs. First, the precise locations of the respiratory rhythm and pattern-forming circuits are unknown and bath-application of agonists and antagonists could be acting anywhere throughout the brainstem. These drugs could have both direct and indirect effects on the respiratory rhythm and pattern-forming circuits. In addition, the specificities of the pharmacological agents are generally determined in mammalian systems and so the affinities of these agents for amphibian receptor sites are unknown. The application of drugs in these experiments occurred over a relatively long period of time before being washed out, thus long-lasting effects such as increased metabolites or receptor dynamics might be a confounding factor. Despite these limitations, the results presented here are consistent with a body of literature indicating that endogenous glutamatergic stimulation of non-NMDA receptors is necessary for the expression of lung ventilation and that SP is an excitatory neurotransmitter for lung ventilation.
4.1. Role of non-NMDA receptors in respiratory rhythm generation
This study has shown clearly that glutamatergic stimulation of non-NMDA receptors is necessary for expression of lung bursts in the developing amphibian brainstem. This is, to our knowledge, the first demonstration of a necessary role for non-NMDA receptors for the generation of lung bursts in the amphibian brainstem, but is consistent with studies from other vertebrates (see below). The major effects of CNQX were on lung burst generation, with inconsistent effects on respiratory burst pattern formation that were limited to unexplained increases in buccal and lung burst amplitude in post-metamorphic brainstems. Perry et al. (1995) previously showed that glutamate application in the adult brainstem produces a transient inhibition, followed by a sustained excitatory response for lung burst output. Glutamate superfusion would, of course, stimulate all ionotropic and metabotropic receptors, thus a complex response might be expected from glutamate application. Other studies that have investigated the role of glutamate in the amphibian brainstem have been limited to microinjection experiments designed to map the extent of the respiratory rhythm generator (McLean et al. 1995; Wilson et al. 2002). Moreover, these studies have used adult or post-metamorphic frogs and did not examine earlier developmental stages. Microinjection of glutamate (McLean et al. 1995) or the non-NMDA agonist, AMPA (Wilson et al. 2002), reveal an area of the rostral brainstem that appears to be responsible for modulating lung burst frequency. Wilson et al. (2002) also identified a more caudal region of the brainstem that appears to be responsible for modulating buccal burst frequency in post-metamorphic animals. Thus, microinjection data indicate that the generation of lung and buccal bursts are localized to different brain regions in the post-metamorphic frog.
An essential role for non-NMDA receptors in the expression of respiratory rhythm generation has been demonstrated using in vitro preparations from mammals and the lamprey. In the neonatal rat brainstem in vitro or the rhythmically-active slice preparation containing the pre-Bötzinger complex (pre-BötC), CNQX causes a dose-dependent reduction of respiratory rhythm and a reversible cessation of respiratory activity (Greer et al. 1991; Smith et al. 1991; Funk et al. 1993). The lamprey brainstem respiratory rhythm is also dependent on non-NMDA receptors since CNQX also abolishes respiratory-related bursting in vitro (Bongianni et al. 1999). The concentrations of CNQX necessary to abolish respiratory rhythm in the neonatal rat brainstem and in the lamprey brainstem (1–10 μM) are very similar to the concentrations needed to abolish lung burst activity the bullfrog brainstem.
Previous studies have shown that modulation of lung burst activity in the anuran brainstem by specific neurotransmitters and/or neuromodulators changes during development. For example, fast inhibitory neurotransmission by GABAA and glycine receptors appears to be relatively unimportant in pre-metamorphic tadpoles (Galante et al. 1996; Broch et al. 2002), but is essential for expression of lung burst activity in the adult brainstem (Broch et al. 2002). Low concentrations of 5-HT have little effect on lung burst activity in pre-metamorphic tadpoles, but increase lung burst frequency in post-metamorphic tadpoles (Kinkead et al. 2002). Nitric oxide (NO) is inhibitory to lung burst frequency in pre-metamorphic tadpoles, but excitatory to lung burst frequency in post-metamorphic and adult frogs (Hedrick et al. 1998, 2005). The changing role of NO in the amphibian respiratory network is similar to the developmental effects of NO in modulating the spinal rhythm generator for swimming in the amphibian spinal cord (McLean and Sillar, 2004). Gap junction regulation of lung burst activity is important in larval brainstems, but not in the adult brainstem (Winmill and Hedrick, 2003) similar to that shown in developing mammals (Bou-Flores and Berger, 2001; Solomon et al. 2003). These studies suggest that a number of different neuromodulatory receptor systems increase their influence over lung burst activity throughout development. By contrast, the effects of non-NMDA receptor modulation and SP appear to be excitatory throughout the developmental period. Thus, we would hypothesize that the role of glutamate, and perhaps SP, is to provide consistent excitatory input to neural circuits that produce lung burst activity throughout development while other neuromodulatory systems develop more slowly to shape the final respiratory patterns seen in the post-metamorphic brainstem. Clearly, more work will need to be done to test this hypothesis.
In amphibians, gill and buccal burst activity appears to be a network-driven process because it is abolished by chloride-free solutions or by antagonists to chloride channels (Galante et al. 1996; Broch et al. 2002). Lung burst activity, however, appears to be dependent upon a pacemaker-like mechanism during the pre-metamorphic stages (Broch et al. 2002), but ‘switches’ to a network-based mechanism after metamorphosis (Broch et al. 2002; Hedrick, 2005). This conclusion is based on the finding that lung burst activity in the pre-metamorphic brainstem continues in the presence of chloride channel antagonists, or with chloride-free aCSF, but these manipulations abolish lung burst activity in the adult brainstem (Broch et al. 2002). The hypothesis of a pacemaker-to-network transition in the amphibian brainstem is similar to the maturational hypothesis for the development of respiratory rhythm generation in the mammalian brainstem (cf. Richter and Spyer, 2001). Regardless of the mechanisms that generate lung burst activity, the data from this study suggest that endogenous glutamatergic activation of non-NMDA receptors is a key source of excitation for lung burst activity throughout development.
In contrast to the effects on fictive lung bursts, blockade of non-NMDA receptors unexpectedly increased the frequency of gill and buccal bursts. Although the mechanism underlying this response is unclear, one possibility is that glutamate, acting via non-NMDA receptors, has a direct inhibitory effect on the gill/buccal rhythm generators which is revealed by non-NMDA receptor blockade with CNQX. However, this seems unlikely since non-NMDA-mediated inhibition of respiratory neurons has not been previously demonstrated. A more likely explanation is that the gill and buccal rhythm generators are under tonic inhibition and that blockade of non-NMDA receptors disinhibits this tonic input. One possible source of inhibition is from the lung rhythm generator. It has been hypothesized that the lung and buccal rhythms in the post-metamorphic frog brainstem are produced by separate, but coupled, oscillators (Wilson et al. 2002). Their model attempts to account for the initiation and facilitation of lung burst events (episodes) that are characteristic of the post-metamorphic frog brainstem, but are generally not observed in pre-metamorphic tadpole brainstems (see Fig. 1). Clearly more work is necessary to create testable models that explain respiratory rhythm generation in amphibians throughout development.
4.2. Role of Substance P in respiratory rhythm generation
Substance P is a neuropeptide of the tachykinin family that mediates its effects via neurokinin (NK) receptors NK-1, NK-2 and NK-3 in mammals (Maggi, 1995). In the bullfrog, Rana (=Lithobates) catesbeiana, 3 additional peptides have been identified: ranatachykinins (RTK) A, B, and C (Kangawa et al. 1993). These RTKs and SP have been shown to activate signal transduction pathways that produce Ca2+ elevation and desensitization of the bullfrog SP receptor, an orthologue of the mammalian NK-1 receptor (Perrine et al. 2000; Simmons, 2006). SP has the highest affinity for NK-1 receptors and stimulation of NK-1 receptors has been shown to increase respiratory activity in mammals both in vitro and in vivo (Hedner et al. 1984; Yamamoto et al. 1990; Ptak et al. 1999, 2000). More recent studies have shown that NK-1 receptors are critical for the expression of normal respiratory rhythm in mammals. Destruction of NK-1 immunoreactive neurons in the preBötC produces abnormal breathing patterns in adult rats (Gray et al. 2001) and adult goats (Wenninger et al. 2004). These studies point to a critical role for NK-1 receptors within the pre-Bötzinger complex in the expression of normal breathing in mammals. In this study, SP stimulated respiratory frequency in both groups, but also resulted in a longer term increase in buccal and lung burst amplitude in the frog brainstem. The increase in respiratory frequency and amplitude is consistent with the actions of SP in mammals. Although it is unclear how SP increased burst amplitude only in the post-metamorphic animals, the increase of intracellular Ca2+ by NKR stimulation may produce a type of long-lasting ‘plasticity’ response similar to that seen in turtles with 5-HT stimulation (Johnson et al. 2002). SP-positive staining neurons are found throughout the amphibian brainstem, but particularly in the midbrain region and reticular formation (Stuesse et al. 2001). However, it remains to be determined if there are specific brainstem sites that regulate respiratory rhythm generation by NKR in amphibians.
Our data corroborate an earlier report that SP produces an excitatory respiratory response in the adult amphibian brainstem (Perry et al. 1995). The excitatory effects of SP in that study were abolished by brainstem exposure to kynurenate suggesting that the effects of SP may be mediated through a glutamatergic pathway (Perry et al. 1995). This is consistent with a study in rats showing that NK-1-immunoreactive neurons of the pre-BötC are also glutamatergic (Guyenet et al. 2002). Although we did not investigate the possible interaction between glutamate and SP in this study, our data indicate that both neurotransmitters provide an excitatory drive for lung ventilation throughout development.
4.3. Evolutionary perspectives
In mammals there is considerable evidence to suggest that the preBötC is a critical region for respiratory rhythm generation; data from in vitro and in vivo models support this hypothesis (Smith et al. 1991; Gray et al., 2001; Wenninger et al., 2004). Recent data also suggest that the preBötC and the more rostral para-facial respiratory group (pFRG; Onimaru and Homma, 2003) represent synaptically-coupled networks that interact to produce a rhythmic respiratory motor output (Janczewski and Felman, 2006). This dual network hypothesis for respiratory rhythm generation proposes that the pFRG generates expiratory rhythm whereas the preBötC generates inspiratory rhythm (Onimaru et al. 2006). A key evolutionary link for this hypothesis is thought to arise from the conservation of dual buccal and lung oscillators in frogs that respond similarly to opiates as the pFRG and preBötC (Vasilakos et al. 2005; Wilson et al. 2006; Feldman and Del Negro, 2006). Although this is an intriguing hypothesis, similar responses to neurotransmitters do not necessarily suggest structural or functional homologies of vertebrate respiratory oscillators. The evolution of neural circuits that subserve a particular motor behavior, such as breathing, is difficult to determine, even in simple nervous systems (Katz and Harris-Warrick, 1999).
In this study, we demonstrated that SP provides an excitatory drive for lung burst activity, but had no effect on gill or buccal rhythms. In mammals, SP is excitatory to preI neurons in the pFRG region (Yamamoto et al. 1992) and the preBötC (Gray et al. 1999). If, as postulated, the buccal rhythm in amphibians is homologous with the pFRG oscillator and lung rhythm is homolgous with the preBötC oscillator, then the responses to SP should be similar in both amphibians and mammals. Because SP stimulated only lung burst activity, and not gill or buccal activity, our results with SP do not support the hypothesis of homologous dual respiratory oscillators in amphibians and mammals. Although it is difficult to extrapolate findings from amphibians to mammals, future studies of non-mammalian vertebrates that incorporate a phylogenetic analysis may reveal important insights into the evolution of vertebrate respiratory oscillators (Striedter, 1998).
Acknowledgments
The comments of two anonymous reviewers are gratefully acknowledged. This study was supported by National Institutes of Health, MBRS/SCORE grant SO6 48135 to MSH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belzile O, Gulemetova R, Kinkead R. Role of 5-HT2A/C receptors in serotonergic modulation of respiratory motor output during tadpole development. Respir Physiol Neurobiol. 2002;133:277–282. doi: 10.1016/s1569-9048(02)00169-6. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Deliagina TG, Grillner S. Role of glutamate receptor subtypes in the lamprey respiratory network. Brain Res. 1999;826:298–302. doi: 10.1016/s0006-8993(99)01251-2. [DOI] [PubMed] [Google Scholar]
- Broch L, Sandoval AV, Morales RD, Hedrick MS. Regulation of the respiratory central pattern generator by chloride-dependent inhibition during development in the bullfrog (Rana catesbeiana) J Exp Biol. 2002;205:1161–1169. doi: 10.1242/jeb.205.8.1161. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol. 2001;85:1543–1551. doi: 10.1152/jn.2001.85.4.1543. [DOI] [PubMed] [Google Scholar]
- Frost D, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, De Sá RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC. The amphibian tree of life. Bull Am Mus Nat Hist. 2006;297:1–370. [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;26:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier S, Kinkead R. Noradrenergic modulation of respiratory motor output during tadpole development: role of α-adrenoceptors. J Exp Biol. 2006;209:3685–3694. doi: 10.1242/jeb.02418. [DOI] [PubMed] [Google Scholar]
- Fournier S, Allard M, Roussin S, Kinkead R. Developmental changes in central O2 chemoreflex in Rana catesbeiana: the role of noradrenergic modulation. J Exp Biol. 2007;210:3015–3026. doi: 10.1242/jeb.005983. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medually slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Galante RJ, Kubin L, Fishman AP, Pack AI. Role of chloride-mediated inhibition in respiratory rhythmogenesis in an in vitro brainstem of tadpole, Rana catesbeiana. J Physiol. 1996;492:545–558. doi: 10.1113/jphysiol.1996.sp021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdovin D, Torgerson CS, Remmers JE. Neurorespiratory pattern of gill and lung ventilation in the decerebrate spontaneously breathing tadpole. Respir Physiol. 1998;113:61–63. doi: 10.1016/s0034-5687(98)00061-9. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the PreBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldmean JL. Normal breathing requires pre-Bötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generations and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functional heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–16. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick MS. Development of respiratory rhythm generation in ectothermic vertebrates. Respir Physiol Neurobiol. 2005;149:29–41. doi: 10.1016/j.resp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Hedrick MS, Winmill RE. Excitatory and inhibitory effects of tricaine (MS-222) on fictive breathing in the bullfrog brainstem. Am J Physiol. 2003;284:R405–R412. doi: 10.1152/ajpregu.00418.2002. [DOI] [PubMed] [Google Scholar]
- Hedrick MS, Chen AK, Jessop KL. Nitric oxide changes its role as a modulator of respiratory motor activity during development in the bullfrog (Rana catesbeiana) Comp Biochem Physiol. 2005;142A:231–240. doi: 10.1016/j.cbpb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Hedrick MS, Morales RD, Parker JM, Pacheco JLH. Nitric oxide modulates respiratory-related neural activity in the isolated brainstem of the bullfrog. Neurosci Lett. 1998;251:81–84. doi: 10.1016/s0304-3940(98)00485-6. [DOI] [PubMed] [Google Scholar]
- Hedner J, Hedner T, Wessberg P, Jonason J. Interaction of substance P with the respiratory control system in the rat. J Pharmacol Exp Ther. 1984;228:196–201. [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. N-methyl-D-aspartate-mediated bulbospinal respiratory drive is pH/PCO2-insentitive in turtle brainstem-spinal cord. Respir Physiology. 1998;113:201–212. doi: 10.1016/s0034-5687(98)00079-6. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JER, Henderson DR, Wenninger MR, Mitchell GS. Serotonin elicits long-lasting enhancement of rhythm respiratory activity in turtle brain stems in vitro. J Appl Physiol. 2001;91:2703–2712. doi: 10.1152/jappl.2001.91.6.2703. [DOI] [PubMed] [Google Scholar]
- Kangawa K, Kozawa H, Hino J, Minamino N, Matsuo H. Four novel tachykinins in frog (Rana catesbeiana) brain and intestine. Regul Pept. 1993;46:81–88. doi: 10.1016/0167-0115(93)90016-2. [DOI] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM. The evolution of neuronal circuits underlying species-specific behavior. Curr Opin Neurobiol. 1999;9:628–633. doi: 10.1016/S0959-4388(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Belzile O, Gulemetova R. Serotonergic modulation of respiratory motor output during tadpole development. J Appl Physiol. 2002;93:936–946. doi: 10.1152/japplphysiol.00104.2002. [DOI] [PubMed] [Google Scholar]
- Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Mitchell GS, Dekin MS. Glutamate, GABA and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. 2. Vol. 79. New York: Dekker; 1995. pp. 151–218. (Lung Biol Health Dis. Ser.). [Google Scholar]
- McLean DL, Sillar KT. Metamodulation of a spinal locomotor network by nitric oxide. J Neurosci. 2004;24:9561–9571. doi: 10.1523/JNEUROSCI.1817-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean HA, Perry SF, Remmers JE. Two regions in the isolated brainstem of the frog that modulate respiratory-related activity. J Comp Physiol. 1995;177:135–144. doi: 10.1007/BF00225094. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I, Feldman JL, Janczewski WA. Point: Counterpoint: The parafacial respiratory group (pFRG)/pre-Bötzinger complex (preBötC) is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100:2094–2098. doi: 10.1152/japplphysiol.00119.2006. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Whitehead TL, Hicks RP, Szarek JL, Krause JE, Simmons MA. Solution structures in SDS micelles and functional activity at the bullfrog substance P receptor of ranatachykinin peptides. J Med Chem. 2000;43:1741–1753. doi: 10.1021/jm000093v. [DOI] [PubMed] [Google Scholar]
- Perry SF, McLean HA, Kogo N, Kimura N, Kawasaki H, Sakurai M, Kabotyanski EA, Remmers JE. The frog brainstem preparation as a model for studying the central control of breathing in tetrapods. Braz J Med Biol Sci. 1995;28:1339–1346. [PubMed] [Google Scholar]
- Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. NMDA and non-NMDA receptors may play distinct roles in timing mechanisms and transmission in the feline respiratory network. J Physiol. 1994;474:509–523. doi: 10.1113/jphysiol.1994.sp020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, DiPasquale E, Monteau R. Substance P and central respiratory activity: a comparative in vitro study on fetal and newborn rat. Brain Res Dev Brain Res. 1999;114:217–227. doi: 10.1016/s0165-3806(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Ptak K, Konrad M, DiPasquale E, Tell F, Hilaire G, Monteau R. Cellular and synaptic effect of substance P on neonatal phrenic motoneurons. Eur J Neurosci. 2000;12:126–138. doi: 10.1046/j.1460-9568.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y. The pattern of respiratory nerve activity in the bullfrog. Jpn J Physiol. 1984;34:827–838. doi: 10.2170/jjphysiol.34.269. [DOI] [PubMed] [Google Scholar]
- Simmons MA. Functional selectivity of NK1 receptor signaling: Peptide agonists can preferentially produce receptor activation or densensitization. J Pharmacol Exp Therap. 2006:907–913. doi: 10.1124/jpet.106.109512. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger Complex: A Brainstem Region That May Generate Respiratory Rhythm in Mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Chon KH, Rodriguez MN. Blockade of brainstem gap junctions increases phrenic burst frequency and reduces phrenic burst synchronization in adult rat. J Neurophysiol. 2003;89:135–149. doi: 10.1152/jn.00697.2002. [DOI] [PubMed] [Google Scholar]
- Strauss C, Wilson RJA, Remmers JE. Developmental disinhibtion: turning off inhibition turns on breathing in vertebrates. J Neurobiol. 2000;45:75–83. doi: 10.1002/1097-4695(20001105)45:2<75::aid-neu2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Striedter GF. Progress in the study of brain evolution: from speculative theories to testable hypotheses. Anat Rec. 1998;253:105–112. doi: 10.1002/(SICI)1097-0185(199808)253:4<105::AID-AR5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Adli DSH, Cruce WLR. Immunohistochemical distribution of enkephalin, substance P, and somatostatin in the brainstem of the leopard frog, Rana pipiens. Micr Res Tech. 2001;54:229–245. doi: 10.1002/jemt.1135. [DOI] [PubMed] [Google Scholar]
- Takeda M, Matsumoto S. Effects of NMDA and non-NMDA receptor antagonists on the medullary inspiratory neuronal activity during spontaneous augmented breaths in anesthetized rats. Brain Res. 1998;781:194–201. doi: 10.1016/s0006-8993(97)01249-3. [DOI] [PubMed] [Google Scholar]
- Taylor AC, Köllros JJ. Stages in the normal development of Rana pipiens larvae. Anat Rec. 1946;94:7–24. doi: 10.1002/ar.1090940103. [DOI] [PubMed] [Google Scholar]
- Vasilakos K, Wilson RJA, Kimura N, Remmers JE. Ancient gill and lung oscillators may generate the respiratory rhythm of frogs and rats. J Neurobiol. 2005;62:369–385. doi: 10.1002/neu.20102. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Bötzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol. 2004;97:1620–1628. doi: 10.1152/japplphysiol.00952.2003. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Vasilakos K, Harris MB, Straus C, Remmers JE. Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J Phyisol. 2002;540(2):557–570. doi: 10.1113/jphysiol.2001.013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJA, Vasilakos K, Remmers JE. Phylogeny of vertebrate rhythm generators: The oscillator homology hypothesis. Respir Physiol Neurobiol. 2006;154:47–60. doi: 10.1016/j.resp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Winmill RE, Hedrick MS. Gap junction blockade with carbenoxolone differentially affects fictive breathing in larval and adult bullfrogs. Respir Physiol Neurobiol. 2003;138:239–251. doi: 10.1016/j.resp.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Onimaru H, Homma I. Effects of substance P on respiratory rhythm and pre-inspiratory neurons in the ventrolateral structure of rostral medulla oblongata: an in vitro study. Brain Res. 1992;599:272–274. doi: 10.1016/0006-8993(92)90401-t. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Prentice-Hall; Englewood Cliffs, NJ: 1974. [Google Scholar]


