Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 induces filamentous actin-rich “pedestals” on intestinal epithelial cells. Pedestal formation in vitro requires translocation of bacterial effectors into the host cell, including Tir, an EHEC receptor, and EspFU, which increases the efficiency of actin assembly initiated by Tir. While inactivation of espFU does not alter colonization in two reservoir hosts, we utilized two disease models to explore the significance of EspFU-promoted actin pedestal formation. EHEC ΔespFU efficiently colonized the rabbit intestine during co-infection with wild type EHEC, but co-infection studies on cultured cells suggested that EspFU produced by wild type bacteria might have rescued the mutant. Significantly, EHECΔespFU by itself was fully capable of establishing colonization at 2 days post-inoculation but unlike wild type, failed to expand in numbers in the cecum and colon by 7 days. In the gnotobiotic piglet model, an espFU deletion mutant appeared to generate actin pedestals with lower efficiency than wild type. Furthermore, aggregates of the mutant occupied a significantly smaller area of the intestinal epithelial surface than those of the wild type. Together, these findings suggest that, after initial EHEC colonization of the intestinal surface, EspFU, may stabilize bacterial association with the epithelial cytoskeleton and promote expansion beyond initial sites of infection.
Keywords: EHEC, EspFU, filamentous actin, intestinal colonization
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) is a frequent cause of food-related outbreaks of diarrhea and hemorrhagic colitis in developed nations world-wide (reviewed in (Kaper et al., 2004)). Although these pathogenic E. coli do not invade beyond the gastrointestinal tract, the intestinal absorption of Shiga toxin, an EHEC-produced AB5-type toxin can result in serious systemic disease including the hemolytic uremic syndrome (Nataro and Kaper, 1998; Karmali et al., 1983; Riley et al., 1983). E. coli O157:H7 is the most common EHEC serotype associated with both sporadic infection and outbreaks in the United States, but other serogroups have been increasingly associated with human disease (Johnson et al., 2006; Karch et al., 2005).
EHEC, together with enteropathogenic E. coli (EPEC) and Citrobacter rodentium, are often referred to as “A/E pathogens” because all three of these enteric bacteria are able to induce distinctive ultrastructural changes, known as attaching and effacing (A/E) lesions, on intestinal epithelial cells (Moon et al., 1983). The formation of A/E lesions by this group of extracellular pathogens is thought to promote colonization and damage of the intestinal epithelium because mutants that are incapable of generating A/E lesions are diminished in their capacity to colonize the intestine and cause disease in experimental animals and human volunteers (Deng et al., 2003; Ritchie et al., 2003; Marches et al., 2000; Tacket et al., 2000; Abe et al., 1998; Tzipori et al., 1995; Donnenberg et al., 1993b; Donnenberg et al., 1993a). Moon and co-workers first described the morphological features of these lesions, which include the localized effacement of the brush border microvilli, intimate bacterial attachment to the host epithelium and the assembly of electron-dense fibrillar structures underneath attached bacteria producing a “pedestal-like” protrusion from the cell (Moon et al., 1983).
EHEC, EPEC and C. rodentium all share a homologous ~35 kb DNA region called the locus of enterocyte effacement (LEE) pathogenicity island that contains most or all of the genes necessary for A/E lesion formation (Elliott et al., 1999; McDaniel et al., 1995). The LEE encodes transcriptional regulators, the components of a type III secretion system, the outer membrane adhesin, intimin, and several effector proteins that are translocated directly into host cells by the type III apparatus (reviewed in (Garmendia et al., 2005)). One of the translocated effectors essential for pedestal formation is Tir, which inserts into the host plasma membrane with a hairpin loop topology in which the central extracellular portion of Tir serves as a receptor for intimin (de Grado et al., 1999; Hartland et al., 1999; Kenny et al., 1997). Tir-intimin interaction mediates the intimate attachment of bacteria to the epithelial cell surface, and mutants incapable of producing Tir or intimin are also incapable of colonization in experimental infections (Sheng et al., 2006; Deng et al., 2003; Ritchie et al., 2003; Marches et al., 2000).
Whereas the central region of Tir binds intimin, the amino and carboxyl termini are located in the host cytoplasm, where they interact with host proteins and induce remodelling of the cytoskeleton and pedestal formation (Hayward et al., 2006; Goosney et al., 2001). Studies of pedestal formation in cultured mammalian cells indicate that A/E pathogens can employ several pathways to induce actin assembly. In EPEC, at least three pathways have been distinguished, one mediated by phosphorylated Tir tyrosine 474 (Y474) and the host adaptor Nck, a second mediated by phosphorylated Y474 that functions independently of Nck, and a third mediated by Tir Y454, which also functions independently of Nck. In contrast to these EPEC pathways, canonical EHEC strains encode a Tir that lacks a Nck-binding sequence (Campellone et al., 2002; Gruenheid et al., 2001; DeVinney et al., 1999), but contains Y458, the equivalent of EPEC Tir Y454 (Campellone et al., 2006). Whereas this Nck-independent pathway leads to inefficient actin assembly in EPEC, EHEC encodes an additional type III-translocated effector, EspFU (also known as TccP) that binds and activates the host protein N-WASP (Campellone et al., 2004; Garmendia et al., 2004) to increase the efficiency of this pathway (Brady et al., 2007). In the absence of EspFU, EHEC binds to host cells at wild type efficiency, but induces only low (but detectable) levels of actin polymerization in cultured cells (Campellone et al., 2004).
Recent studies have shown that mutations in one or more of these actin assembly pathways does not necessarily diminish the ability of these mutants to colonize and form A/E lesions in vivo. For example, a C. rodentium Tir mutant lacking the equivalent of Y474 did not undergo efficient tyrosine phosphorylation or induce actin assembly in vitro, yet still colonized mice, forming A/E lesions detectable by electron microscopy (Deng et al., 2003). Similarly, an EPEC strain expressing a Tir derivative lacking both Y474 and Y454 was unable to recruit actin in vitro but was still capable of generating A/E lesions on human intestinal explants (Schuller et al., 2007). Finally, an EHEC espFU mutant, which inefficiently triggers actin assembly on cultured monolayers, colonized the intestine of infected calves and lambs indistinguishably from wild type and formed A/E lesions in bovine ligated loops (Vlisidou et al., 2006).
Whereas calves and lambs serve as models to investigate factors that promote EHEC persistence in reservoir hosts, in the current study, we investigated the role of EspFU in colonization of the intestines of infant rabbits and gnotobiotic piglets, two disease models that might provide insight into EHEC colonization of the human intestine. We found that an espFU mutant colonized rabbits with wild type efficiency at an early time point, but unlike the wild type, failed to increase in numbers in the cecum and large intestine as infection progressed. By adapting a high resolution, in situ fluorescence-based imaging technique to directly visualize EHEC cells bound to the luminal surfaces of the piglet intestinal epithelium, we found that the espFU mutant formed smaller bacterial aggregates on the mucosal surface than the wild type. Taken together our observations suggest that following initial EHEC colonization, EspFU may stabilize bacterial association with the epithelial cytoskeleton and promote expansion beyond initial sites of infection.
RESULTS
An EHEC espFU mutant shows no colonization defect in co-infection experiments
EHEC colonizes the infant rabbit ileum, cecum and large bowel and causes diarrhea and intestinal inflammation in an intimin- and Tir-dependent manner (Ritchie et al., 2003). To examine the potential role of EspFU during colonization in this disease model, we generated an espFU deletion mutant in EHEC strain 905, a clinical E. coli O157:H7 isolate that has been used previously in the rabbit model (Ritchie and Waldor, 2005; Ritchie et al., 2003). We initially carried out co-infection (competition) experiments between the wild type strain and this isogenic espFU mutant. The readout for co-infection studies is the competitive index (C.I.), i.e., the ratio of mutant to wild type CFU in infected tissues divided by the ratio of mutant to wild type CFU in the inoculum. This experimental design limits the effects of animal-to-animal variation since the wild type strain serves as an internal control in every animal and often provides a more sensitive way to detect colonization defects than single infection experiments (Logsdon and Mecsas, 2003). As a control to validate the competition format for these experiments, we performed a competition experiment between isogenic kanamycin-resistant and kanamycin-sensitive EHEC strains. In the kanamycin-resistant strain, we replaced lacZ (a gene not required for EHEC intestinal colonization in single infection rabbit experiments; J.M.R. and M.K.W. unpublished observations) with a kanamycin-resistance gene. At day 7 after oro-gastric inoculation with 5x108 bacteria per 90 gram rabbit, we found approximately equal numbers of 905 and 905ΔlacZ::kan in homogenates of different regions of the intestine, yielding a C.I. of approximately 1.0 (Table 1), validating the competition format in this animal model. Co-infection experiments using 905 and 905Δtir provided additional validation for the competition format. The 905Δtir mutant, which is highly attenuated in single infection experiments (Ritchie et al., 2003), was also markedly attenuated in co-infection experiments, where C.I. values ≤ 0.0003 were found in all sampled regions (Table 1).
Table 1.
Co-infection of an espFU mutant with wild type EHEC does not reveal a role for espFU in intestinal colonization.
| Expt | Post- infection day | Dosea | strain | C.I. valuesb in intestinal homogenates or stool samples: | |||
|---|---|---|---|---|---|---|---|
| ileum | cecum | mid-colon | stool | ||||
| I c | 7 | 5x108 | ΔlacZ | 1.13 (0.56 – 2.30) | 0.99 (0.55 – 1.76) | 1.34 (0.65 – 2.74) | 1.10 (0.57 – 2.14) |
| Δtir | 2 x 10−4 c,* (7x10−5–6x10−4) | 3 x 10−5 * (1x10−5–7x10−5) | 3x 10−4 * (1x10−4–9x10−4) | 1x10−6 * (6x10−7–2x10−6) | |||
| ΔespFU | 1.48 (0.97 – 2.26) | 1.41 (1.08 – 1.86) | 1.17 (0.70 – 1.96) | 1.38 (1.05 – 1.82) | |||
| II d | 2 | 5 x 106 | ΔespFU | 1.20 (0.95 – 1.53) | 1.25 (0.99 – 1.58) | 1.25 (0.99 – 1.57) | 1.27 (0.94 – 1.70) |
| 5 x 104 | ΔespFU | 0.64 (0.35 – 1.16) | 0.54 (0.28 – 1.06) | 0.59 (0.35 – 1.00) | 0.76 (0.33 – 1.76) | ||
Total dose of 905 and its derivatives (i.e. 2.5 x 108 CFU per strain), adjusted to 90g rabbit weight.
C.I. (competitive index) is the ratio of mutant to wild type CFU recovered from indicated intestinal site following infection divided by the ratio of mutant to wild type CFU in the inoculum. Values marked with “*” were significantly (P≤0.05) different from those obtained from ΔlacZ co-infection in corresponding tissue segments, calculated using the Student’s t-test (two-way).
In experiment I, values are geometric mean (95% confidence interval) of 6 rabbits for ΔlacZ, 11 rabbits for Δtir and 11 rabbits for ΔespFU co-infection experiments.
In experiment II, 7 rabbits were tested at a dose of 5 x 106. 31 rabbits were tested at a dose of 5 x 104; of these, 12 yielded neither wild type nor mutant, 5 yielded almost exclusively wild type, and 4 yielded almost exclusively ΔespFU. In rabbits that yielded almost exclusively either one or the other strain, the very small number of colonies from the less frequently detected strain precluded an accurate calculation of the C.I., so the C.I.s in the table were calculated from the remaining 10 rabbits that showed significant colonization by both strains.
When we co-inoculated 3-day old infant rabbits with approximately equal numbers of 905 and 905ΔespFU, the espFU mutant did not exhibit a colonization defect. Approximately equal numbers of 905 and 905ΔespFU CFU were recovered in samples from the ileum, cecum, mid-colon and stool at 7 days post-inoculation resulting in a C.I. of ~1 (Table 1, experiment I). Similar experiments examining colonization 2 days after inocula of 5x106 or 5x104 per 90 g rabbit weight yielded similar findings, as all C.I.s did not significantly differ from 1 (Table 1, experiment II). The latter dose is apparently near the ID50 for EHEC in this model, because 12 out of 31 (39%) rabbits did not become infected and 9 of the 31 (29%) became infected almost exclusively with one strain (Table 1 legend). In these 9 rabbits, 5 became predominantly infected with wild type 905 and 4 with the espFU mutant, indicating equal propensity for establishing infection. Thus, co-infection experiments did not provide evidence for a role for espFU in initiating intestinal colonization.
The F-actin assembly defect of an espFU mutant can be trans-complemented by a co-infecting bacterium
The lack of a discernible colonization defect by the espFU mutant in these competition assays might result from the ability of 905ΔespFU to take advantage of EspFU injected into epithelial cells by wild type 905. In fact, before the identification of EspFU, DeVinney and coworkers showed that whereas an EPEC strain expressing EHEC Tir could not generate actin pedestals in vitro, co-infection with an EHEC strain competent for type III secretion “trans-complemented” this defect (DeVinney et al., 2001). To investigate whether such trans-complementation is due to EspFU, we co-infected HeLa cells with strains that were competent or incompetent for EspFU translocation. In these experiments and in the piglet infection studies described below, we utilized TUV93-0, a Shiga toxin-deficient derivative of the prototype sequenced O157:H7 strain EDL933, in order to analyze EHEC association with intestinal epithelia while avoiding the neurological complications that result from Shiga toxin expression (Campellone et al., 2007). To allow the two strains to be distinguished using anti-O157 antiserum, we infected cells with TUV93-0, an (O157-positive) EHEC strain competent for EspFU delivery, and KC12, an EPEC strain (O157-negative) that does not express EspFU and in which the endogeneous EPEC tir is replaced with EHEC tir (Campellone et al., 2002). KC12 harboring pespFU, an EspFU–encoding plasmid, generates pedestals in a fashion mechanistically identical to canonical EHEC strains ((Campellone et al., 2002); Figure 1, row 2), but in the absence of EspFU, KC12 is incapable of efficient pedestal formation ((Campellone et al., 2004); Figure 1, row 3). However, KC12 was able to form pedestals when co-infected with TUV93-0 (Figure 1, row 4). Interestingly, pedestal formation by TUV93-0 appeared diminished upon co-infection with KC12, suggesting that the co-infecting KC12 might be effectively sequestering translocated EspFU in this in vitro system (Figure 1, row 4 arrowhead). Trans-complementation was dependent upon co-infection of the same cell by both KC12 and TUV93-0 (data not shown), and on EspFU, because no KC12-associated pedestals were observed upon co-infection with TUV93-0ΔespFU (Figure 1, row 5). These observations strongly suggest that EspFU, translocated into HeLa cells by TUV93-0, can promote pedestal formation by EspFU-deficient KC12.
Figure 1. Efficient trans-complementation by EspFU but not Tir during mixed infection on HeLa cells.

Infected monolayers were examined microscopically after staining with DAPI to visualize bacteria, anti-O157 antiserum to specifically detect EHEC strains and fluorescent phalloidin to detect F-actin. For merged images, DAPI staining is shown in blue, bacteria in green, F-actin in red, and foci of co-localized bacteria and F-actin in yellow. HeLa cells were infected singly or in combination with the indicated strains. In rows 4 or 7, large arrowheads on merged images indicate TUV93-0 or its derivatives; arrows indicate efficient (row 4) or inefficient (row 7) trans-complementation, i.e., sites of actin assembly associated with KC12 (anti-O157 negative bacteria).
In contrast to the EHEC espFU mutant, the EHEC tir mutant demonstrated a severe colonization defect in the competition assay in rabbits, suggesting that a tir mutant cannot be efficiently trans-complemented by Tir translocated by a co-infecting strain. To test this, we utilized KC12Δtir/pespFU, a strain that cannot generate pedestals due to the lack of Tir (Figure 1, row 6). Although co-infection with TUV93-0 resulted in detectable Tir-dependent localized actin assembly beneath KC12Δtir/pespFU (Figure 1, rows 7 and 8), indicating some degree of Tir trans-complementation, the intensity of F-actin assembly was significantly lower than that observed with trans-complementation by EspFU (compare Figure 1, rows 4 and 7, arrows). Whereas co-infection of TUV93-0 with KC12 appeared to diminish pedestal formation by TUV93-0, co-infection with KC12Δtir/pespFU did not, suggesting that KC12Δtir/pespFU did not sequester Tir away from TUV93-0 to an extent sufficient to inhibit pedestal formation (Figure 1, row 7, arrowhead). These results are consistent with a hypothesis that the absence of a colonization defect of the espFU mutant in the in vivo competition experiments described above results from efficient in vivo trans-complementation of this espFU mutant by wild type bacteria also present in the rabbit intestine. In contrast, the very poor trans-complementation of tir in vitro is consistent with the severe colonization defect of the tir mutant observed in the in vivocompetition experiments.
In single infection experiments the espFU mutant is defective in intestinal colonization
To assess the role of espFU during animal infection without the potentially confounding effect of trans-complementation, we performed single infection experiments with the 905ΔespFU mutant. In these experiments, 3 day-old rabbits were orogastrically inoculated with 5x108 CFU per 90g rabbit weight of either 905 or 905ΔespFU. Regardless of the infecting strain, all rabbits developed severe diarrhea and exhibited some degree of mucosal damage with acute diffuse suppurative colitis, indistinguishable from that previously described (Ritchie et al., 2003) (data not shown), suggesting that espFU is not essential for the development of disease in this model. Furthermore, two days post-inoculation, there were no differences in the number of 905 or 905ΔespFU CFU recovered from ileal, cecal or mid-colon tissue sections or in the stool of 905-or 905ΔespFU-infected rabbits (Figure 2). However, while the numbers of 905 CFU in the ileum, cecum, and mid-colon were 5- to 30-fold (P<0.01) greater by day 7 than day 2, a significant increase in the number of 905ΔespFU CFU was observed only in the ileum. Thus, at this time point, colonization by 905ΔespFU was significantly impaired compared to 905 in the cecum (4-fold reduction, P≤0.05), mid-colon (6-fold reduction, P≤0.001), and stool (2-fold reduction, P≤0.05). Together these observations suggest that EspFU promotes robust EHEC colonization in the cecum and colon after the first 48 hours of infection.
Figure 2. EspFU is associated with increased efficiency of colonization at day 7 post-infection of infant rabbits.
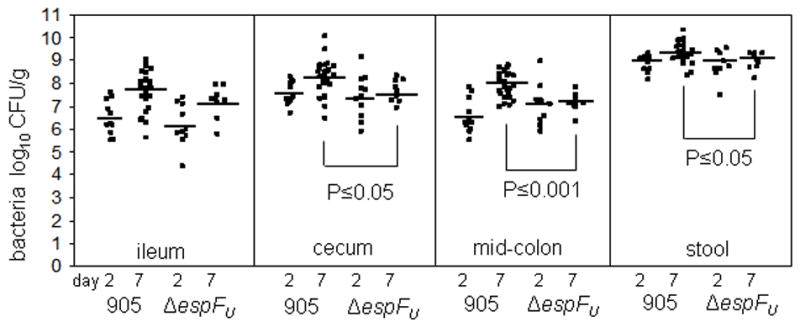
905 or 905ΔespFU were recovered from intestinal segments or stool samples from infected rabbits at 2 and 7 days post-inoculation. Each point represents an individual rabbit and bars represent the geometric mean for each strain. The level of wild type colonization differed significantly between day 2 and day 7 in the ileum (P≤0.01), cecum (P≤0.01), mid colon (P≤0.0001) and stool (P≤0.01), whereas the level of mutant colonization only differed significantly (P≤0.05) in the ileum, when analyzed by the Student’s t-test (two-way).
EspFU may increase the efficiency of, but is not absolutely required for, A/E lesion formation during piglet infection
Gnotobiotic piglets are not a useful model to quantify EHEC intestinal colonization by viable counts because EHEC mutants defective for the expression of intimin, a known colonization factor, or for type III secretion, are still present at levels comparable to the wild type in the piglet intestine ((Tzipori et al., 1995); MB, ST, unpublished observations). However, the absence of competing intestinal microflora in this animal model allows EHEC to be readily visualized within the intestine, making this a particularly attractive system in which to examine the interaction between EHEC and the intestinal epithelium. Therefore, to examine whether EHEC mutants deficient in EspFU interact with the intestinal epithelium in vivo, TUV93-0 or isogenic derivatives lacking espFU or tir (Campellone et al., 2002) were orally inoculated into 1-day old gnotobiotic piglets. One day later, a time point known to yield numerous A/E lesions ((Campellone et al., 2007); data not shown), intestinal tissue samples were removed and assessed for bacterial attachment by staining sections with hematoxylin and eosin (H&E). As previously observed with other EHEC strains (Tzipori et al., 1986), in much of the small intestine and throughout the large intestine, TUV93-0 was closely associated with the host epithelium, appearing to disrupt the regular brush border observed in uninfected tissue (data not shown). In marked contrast, TUV93-0Δtir was not observed in close association with the epithelium (data not shown), as has been reported in previous studies using tir-deficient mutants in different experimental infection models (Sheng et al., 2006; Deng et al., 2003; Ritchie et al., 2003; Marches et al., 2000). Bacterial attachment in tissue samples from piglets infected with the espFU mutant appeared indistinguishable from that seen in samples taken from piglets infected with TUV93-0; thus, H&E staining of cross-sections of piglet intestinal tissue did not reveal a gross defect in the ability of the espFU mutant to associate with epithelial cells in vivo at this time point.
TUV93-0ΔespFU retains the ability to induce actin assembly on cultured Hela cells but with significantly reduced efficiency compared with TUV93-0 (Campellone et al., 2004; Garmendia et al., 2004). To assess the ability of TUV93-0ΔespFU to generate A/E lesions in vivo, samples from infected piglets were analyzed using transmission electron microscopy. In ileal samples, TUV93-0 was frequently associated with raised pedestals under which electron-dense material could be observed (Figure 3A). Ileal samples from piglets infected with TUV93-0ΔespFU also revealed some pedestals, albeit at apparently lower frequency than observed with TUV93-0. Many TUV93-0ΔespFU bacteria in these images appeared to be in close contact with the epithelium in the absence of regions of electron-dense staining characteristic of localized actin assembly (Figure 3B). Infection with TUV93-0ΔespFU harboring a complementing EspFU-encoding plasmid resulted in restoration of high frequency pedestal formation (Figure 3C). Although there are clearly limitations inherent in the analysis of electron micrographs, our observations suggest that TUV93-0ΔespFU has a diminished capacity to generate actin pedestals in vivo; however since some pedestals were observed, our findings also indicate that EspFU is not absolutely essential for pedestal formation in vivo.
Figure 3. EspFU may increase the efficiency of, but is not absolutely required for A/E lesion formation during piglet infection.

Electron micrographs of the ileum of piglets infected with wild type (A), ΔespFU (B) orΔespFU / pespFU (C). Arrows show areas of electron-dense material. Bars equal 1μm.
EspFU promotes EHEC association with the intestinal epithelium in vivo
Because sampling variability in electron micrographic analysis of cross-sections of intestinal tissue limits measurement of bacterial attachment to the host epithelium, we adapted a high resolution, in situ fluorescence-based imaging technique originally developed for en face viewing of the vascular endothelium (Herman et al., 1987; Wong et al., 1983; Herman et al., 1982) as an alternative method. We applied fluorescent anti-O157 antiserum to directly visualize the distribution of attached bacteria over relatively large (0.5cm by 0.5cm) mucosal segments. Pilot experiments revealed that the cecum was the easiest intestinal segment to assess attached TUV93-0 (data not shown). In these tissue samples, non-adherent bacteria could readily be distinguished from adherent bacteria by virtue of the fact that they were usually observed as individual cells and located out of the plane of focus, apparently suspended in the mucus layer. Consistent with this classification, TUV93-0Δtir was generally seen as isolated bacteria that were almost never observed in close apposition to the epithelium (data not shown). In contrast, adherent TUV93-0 was routinely observed in aggregates in the same focal plane as the surface of the epithelial cells (Figure 4A).
Figure 4. EspFU is associated with a larger size of clusters of attached bacteria.

O157-labeled bacteria adherent to the cecum of piglets infected with wild type (A), ΔespFU (B), and ΔespFU / pespFU (C) –infected piglets. Bacteria are stained green and F-actin is stained red. Note the relatively small clusters of attached bacteria in (B). Scale bars are 10μm in each panel. Panel (D): Area of tissue colonized by O157-labeled bacteria divided by area visible with phalloidin staining expressed as a percentage. aStrains are derivatives of TUV93-0. bValues are mean ± standard deviation. Data were compared using the Student’s t-test (two-way) and considered significantly different (*) at P≤0.05.
Comparison of cecal sections from piglets inoculated with TUV93-0 or TUV93-0ΔespFU revealed that there were much larger clusters of TUV93-0 present on the cecal epithelium (compare Figure 4A and 4B). In many sections, clusters of TUV93-0 cells covered most of the visible cecal surface, whereas TUV93-0ΔespFU cells were only observed as relatively small discrete foci covering less of the cecal surface. These qualitative observations were corroborated by more quantitative analyses of these micrographs, in which the percentage of the cecal epithelial surface covered with bacteria was scored in a blinded manner (see methods). In piglets from at least 2 independent experiments and in multiple cecal segments, the area of the cecum covered by TUV93-0 was significantly (P≤0.01) larger than that covered by the espFU mutant (Figure 4D). This defect in epithelial colonization by the espFU mutant could be restored to wild-type levels by the introduction of a plasmid-borne copy of espFU into TUV93-0ΔespFU (Figure 4C and 4D). These observations suggest that espFU promotes EHEC association at the intestinal epithelial surface.
DISCUSSION
Intimate attachment of EHEC to the intestinal epithelium is well established as a requirement for EHEC pathogenesis, since mutations of intimin or Tir preclude EHEC colonization in experimental models of disease (Ritchie et al., 2003; Tzipori et al., 1995; Donnenberg et al., 1993a). However, uncovering the significance of actin assembly during pedestal formation has been elusive. By deleting espFU from EHEC, the role of intimate adherence mediated by intimin and Tir can be partially uncoupled from the function of robust actin polymerization, since EHECΔespFU binds to cultured host cells at normal levels but exhibits a ~20-fold lower frequency of actin assembly into pedestals (Campellone et al., 2004). While an EHEC espFU mutant does not exhibit a colonization defect in calves or lambs, both normal animal reservoirs (Vlisidou et al., 2006), we examined the role of EspFU during infection using two complementary animal models for EHEC disease, rabbits and piglets. We found that EspFU had a significant influence on intestinal colonization in both systems suggesting a link between actin assembly and pathogenesis by these extracellular bacteria.
No defect in colonization by an EHEC espFU mutant was detectable when infant rabbits were co-infected with wild type EHEC (Table 1). However, this observation could reflect trans-complementation by EspFU injected into epithelial cells by the wild type strain during co-colonization. Indeed, we demonstrated highly efficient EspFU trans-complementation in cultured cells (Figure 1). Such trans-complementation in the host cell does not appear to be a universal feature of EHEC type III secreted effectors, since Tir from wild type EHEC is incapable of efficient trans-complementation during co-infection of rabbits or cultured epithelial cells (Table 1 and Figure 1), and strains lacking the type III secreted effectors Map or NleD were not trans-complemented during co-infection experiments in calves (van Diemen et al., 2005; Dziva et al., 2004).
Importantly, when rabbits were singly infected with wild type or EHECΔespFU, the mutant showed a moderate but significant colonization defect at day seven. No defect was observed in any segment of the intestine at day two, suggesting that EspFU does not play an important role in the establishment of infection in this model. Consistent with this, during the competition experiments, when rabbits were co-infected with wild type and mutant at a dose close to the apparent ID50, the number of rabbits that became predominately infected with the mutant was similar to the number that became predominately infected with the wild type strain. Between day 2 and day 7, however, the wild type increased significantly in numbers in all segments of the intestine, whereas the mutant increased only in the ileum, resulting in a 4- to 6-fold colonization defect at this time point. Interestingly, a Citrobacter rodentium strain harbouring a point mutation mutation in tir that specifically diminishes actin pedestal formation also showed a small (~5-fold) colonization defect at 10 days post-infection, but the difference did not reach statistical significance (Deng et al., 2003) The late-stage defect associated with deletion of espFU in EHEC contrasts with lack of a colonization defect previously observed using a different EHECΔespFU strain in calves and lambs (Vlisidou et al., 2006). In this earlier study, neither single nor dual infection experiments of 11 to 15 days duration revealed a colonization phenotype for EHECΔespFU at any time point, as reflected in the number of EHEC CFU found in stool. These disparate findings may be due to the differences in animal host species, the EHEC strain backgrounds, or experimental methodologies. For example, the calf and lamb study monitored colonization by stool CFU. In rabbits, both in previous studies (Ritchie and Waldor, 2005), and in the current study, we have found that colonization defects are usually more pronounced in tissue homogenates than in fecal material.
Although the gnotobiotic piglet model is very different from the infant rabbit model and cannot be easily adapted for quantitative kinetic studies of infection, robust A/E lesions form on the intestinal epithelium one day after high dose oral inoculation of gnotobiotic piglets (Tzipori et al., 1995; Tzipori et al., 1986). Thus, this model provided an attractive means to analyze the role of EspFU in epithelial attachment and colonization. Electron microscopic analysis of tissues from infected piglets revealed that EHECΔespFU were intimately attached to the intestinal epithelium, but appeared to generate typical A/E lesions at a diminished frequency relative to wild type. This observation is consistent with studies indicating that EspFU dramatically increases the efficiency of pedestal formation on cultured cells (Brady et al., 2007). Nevertheless, the mutant was still occasionally associated with A/E lesions in piglets, consistent with the previous observation that an EHECΔespFU mutant generated A/E lesions in bovine ligated ileal loops (Vlisidou et al., 2006) and with the relatively mild colonization defect of the espFU mutant in rabbits. EHECΔtir, which is completely incapable of generating A/E lesions, is severely defective for colonization. That A/E lesions are associated with EHECΔespFU during piglet infection reinforces the finding that mutants of A/E pathogens that are severely defective for pedestal formation in vitro may retain a significant ability to generate such lesions in animal models (Schuller et al., 2007; Deng et al., 2003).
Our findings suggest that EspFU facilitates EHEC’s capacity to establish a larger niche in the rabbit intestine after initial steps in colonization have been achieved, since we observed that the EHECΔespFU colonization defect was detectable after seven (but not two) days of infection. We adapted an in situ fluorescence-based imaging technique originally developed for en face analysis of the vascular endothelium to investigate colonization of the lumenal surface of the piglet intestine to gain insight into how EspFU promotes bacterial colonization. Interestingly, we found that the 4-6-fold colonization defect in rabbits was similar to the 5-6-fold reduction in the area of cecal epithelium covered by discreet masses of attached bacteria in piglets (Figure 4). EspFU appears to have several activities, as its homolog EspF harbors a mitochrondrial localization signal (Nagai et al., 2005) and triggers apoptosis of cultured mammalian cells (Nougayrede and Donnenberg, 2004; Crane et al., 2001), and both EspF and EspFU can promote disruption of tight junctions (Viswanathan et al., 2004; McNamara et al., 2001). Nevertheless, an EPEC strain expressing the Tir Y474F mutant that is diminished in its ability to stimulate actin assembly also appeared to generate smaller bacterial aggregates on human explants (Schuller et al., 2007). Thus, it is tempting to speculate that actin assembly promoted by EspFU facilitates an expansion of primary colonization foci, perhaps by ‘anchoring’ bacteria to the epithelial cytoskeleton or promoting EHEC motility and spread across the intestinal epithelium, similar to the spread of some viral pathogens (Goldberg, 2001).. In fact, EPEC (Sanger et al., 1996), and to a lesser degree EHEC (Shaner et al., 2005), move along the surface of cultured epithelial cells in an actin polymerization-dependent fashion. By combining in vitro analyses of EHEC pedestal formation and motility with the animal-based techniques described in this study, we can test these and other models of processes that contribute to intestinal colonization by A/E.
MATERIALS AND METHODS
Bacterial strains, complementing plasmids and cell culture
Strain 905 is a Stx2-producing E. coli O157:H7 clinical isolate (Ritchie et al., 2003). Deletion of espFU in 905 was preformed using a one-step PCR-based gene inactivation protocol (Datsenko and Wanner, 2000). Briefly, PCR-generated substrates containing the kanamycin resistance gene flanked by 36 nucleotides of espFU targeting sequences were electroporated into 905 containing the lambda-red plasmid, pKD46. Replacement of codons 63 -1116 of espFU with the kanamycin resistance gene was confirmed by PCR. Strain 905Δtir has been previously described (Ritchie et al., 2003). Strain 905ΔlacZ contains a deletion of lacZ codons 36 - 3036 and was made using the one-step PCR-based gene inactivation protocol. TUV93-0 is a Shigatoxin deficient form of the prototype sequenced E. coli O157:H7 strain EDL933, and the isogenic tir and espFU mutants have been described (Campellone et al., 2004; Campellone et al., 2002). EPEC KC12, an O127:H6 strain in which EPEC tir-cesT-eae is replaced with EHEC tir-cesT-eae, and the isogenic tir derivative have been described (Campellone et al., 2002). The complementing plasmid pespFU is based on a medium copy vector pKC321 and was previously described (Campellone et al., 2004).
For routine passage, E. coli strains were cultured on or in LB at 37°C. EHEC or EPEC mutants containing antibiotic resistance genes were grown in media containing the appropriate antibiotic [kanamycin 50 μg ml−1, chloramiphenicol 12.5 μg ml−1]. None of the mutants appeared to have any obvious growth defects when cultured in LB at 37°C (data not shown).
Mammalian culture and trans-complementation assays
HeLa cells were routinely cultured in DMEM plus 10% fetal bovine serum. For trans-complementation experiments, HeLa cell monolayers were co-infected with equal numbers (~106 colony forming units (CFU) / strain) of EHEC and KC12 or derivatives for 6 hours, fixed and permeabilized as previously described (Campellone et al., 2002). For microscopic examination, fixed monolayers were treated with rabbit anti-O157 antisera (1:500 in PBS; Difco) for 30 minutes prior to washing and addition of Alexa488 goat anti-rabbit antisera (1:200; Molecular Probes) to detect EHEC. KC12 and F-actin were detected as described previously (Campellone et al., 2002).
Infant rabbit experiments
Infant rabbit experiments were carried out as has been previously described (Ho and Waldor, 2007; Ritchie et al., 2003). Briefly, 3-day infant rabbits were orogastrically inoculated with 905 and/or isogenic derivatives with deletions of espFU, tir, or lacZ at a total dose equivalent to 5x108 CFU per 90g rabbit weight. Diarrhea was scored as follows: none, no diarrhea; mild, diarrhea consisting of a mix of soft unformed and formed pellets, resulting in light staining of the hind legs; or severe, diarrhea consisting of unformed or liquid stool, resulting in significant staining of the perineum and hind legs. At 2 and 7 days post-infection, infected rabbits were euthanized and their intestines removed. Various intestinal sections were weighed and homogenized before the determination of bacterial numbers by serial dilution and plating on sorbitol MacConkey (SMAC) agar plates. Colonic sections were also evaluated for histologic changes including assessment for infiltration of heterophils, mononuclear cells, the extent of edema, congestion and mucosal damage, and numbers of globlet cells by a comparative pathologist blinded to the sample identity. For co-infection experiments, the SMAC plates were replica plated onto antibiotic containing SMAC plates to allow enumeration of antibiotic-resistant EHEC cells (representing either the espFU, tir or lacZ mutants). Data were expressed as competitive indices (C.I.), the ratio of the number of mutant to wild type bacteria post-infection divided by the number of mutant to wild type bacteria in the inoculum. All single infection and co-infection experiments were repeated in at least two different rabbit litters.
Gnotobiotic piglet experiments
Gnotobiotic piglet experiments were carried out as has been previously described (Tzipori et al., 1992). Briefly, piglets derived by caesarean section and maintained in microbiological isolation, were orally infected with 5 x 109 CFU TUV93-0 or one of its derivatives. After euthanasia at 24hr post-infection, sections of the intestine were removed and fixed in 10% neutral buffered formalin. Bacterial attachment was determined in intestinal cross sections stained with hematoxylin and eosin (H&E) and in unstained segments as described below. Processing of intestinal samples for electron microscopic analysis was performed as described previously (Donnenberg et al., 1993a).
In situ localization and quantitative analysis of EHEC association with the piglet intestinal epithelium
To visualize and quantify bacterial attachment in infected gnotobiotic piglets, we adapted a fluorescence light microscopic in situ imaging technique originally developed for the quantitative analysis of vascular endothelial cytoskeletal arrays (Berceli et al., 1991; Herman et al., 1987; Wong et al., 1983; Herman et al., 1982). In brief, approximately 0.5cm long segments of the intestine were slit length-wise to expose the luminal surface and rinsed 3x in PBS-azide (PBS containing 0.02% Na-azide) for 5 minutes each at room temperature. The tissue samples were then incubated for 90 sec at room temperature in 0.1% Triton-X100, 40mM HEPES (pH 7.1), 1mM MgCl2, 0.5mM EGTA, 50mM PIPES (pH 6.9) and 75mM NaCl, before being rinsed twice in PBS-azide for 5 minutes. The samples were then stained with E. coli O157 antiserum [1:500 in PBS; Difco] for 30 minutes at room temperature. After further rinsing in PBS-azide (2 times), the samples were incubated for 30 minutes with Alexa488 conjugated anti-rabbit IgG (1:500 in PBS; Invitrogen) to enable detection of EHEC and Alexa546-phalloidin (1:50 in PBS; Invitrogen) for visualization of the tissue architecture in control and EHEC-infected intestinal segments. The samples were finally rinsed in PBS-azide before being flattened and mounted under cover slips, which were subsequently anchored to the slide surface to permit en face mucosal surface viewing. The samples were examined using a Zeiss Axiovert 200M Digital light microscope image acquisition system (Molecular Devices Corporation, CA, USA). To quantitatively assess the attachment of EHEC to the intestine, coded cecal samples were scanned for attached bacteria and representative images captured. In each experiment for each bacterial strain, five images from 2 - 3 cecal segments were examined for bacterial attachment. Each experiment was performed on two separate occasions. The area of EHEC attachment (visualized using anti-O157 antibody) was calculated as a percentage of the epithelial surface in the field of view (visualized using fluorescent phalloidin) by an observer who was blinded to the coding system.
Statistical analysis
Bacterial counts from single infection experiments were log transformed and analyzed using the Student’s t-test (two-way), comparing each mutant to the wild type. C.I. values obtained from competition experiments between wild type and the espFU or tir mutants were compared using the Student’s t-test (two-way) to C.I. values obtained from competition experiments between wild type and a lacZ mutant. Differences in the percentages of wild type and mutant bacteria attached to intestinal tissue were also analyzed using the Student’s t-test (two-way).
Acknowledgments
We are grateful to Chris Pearson for performing electron microscopy work and Jennifer Durham for help with the fluorescence microscopy work. We thank Donald Tipper, Brian Skehan, Jon Goguen, Chris Sassetti, Peter Pryciak and Rebekah DeVinney for helpful discussion and careful review of the manuscript. This work was supported by NIH-R01-A146454 and NIH-R01-AI49470 (J.M.L.), NIH R21-AI67827 (J.M.R., T.D.H and M.K.W), HHMI (M.K.W), and Tufts-NEMC Center for Gastroenterology Research on Absorptive and Secretory Processes P30DK-34928.
References
- Abe A, Heczko U, Hegele RG, Brett Finlay B. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J Exp Med. 1998;188:1907–1916. doi: 10.1084/jem.188.10.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berceli SA, Borovetz HS, Sheppeck RA, Moosa HH, Warty VS, Armany MA, Herman IM. Mechanisms of vein graft atherosclerosis: LDL metabolism and endothelial actin reorganization. J Vasc Surg. 1991;13:336–347. doi: 10.1067/mva.1991.25645. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Campellone KG, Ghildiyal M, Leong JM. Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–228. doi: 10.1016/j.devcel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Giese A, Tipper DJ, Leong JM. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol Microbiol. 2002;43:1227–1241. doi: 10.1046/j.1365-2958.2002.02817.x. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Brady MJ, Alamares JG, Rowe DC, Skehan BM, Tipper DJ, Leong JM. Enterohaemorrhagic Escherichia coli Tir requires a C-terminal 12-residue peptide to initiate EspF-mediated actin assembly and harbours N-terminal sequences that influence pedestal length. Cell Microbiol. 2006;8:1488–1503. doi: 10.1111/j.1462-5822.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Roe AJ, Lobner-Olesen A, Murphy KC, Magoun L, Brady MJ, et al. Increased adherence and actin pedestal formation by dam-deficient enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 2007;63:1468–1481. doi: 10.1111/j.1365-2958.2007.05602.x. [DOI] [PubMed] [Google Scholar]
- Crane JK, McNamara BP, Donnenberg MS. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell Microbiol. 2001;3:197–211. doi: 10.1046/j.1462-5822.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Grado M, Abe A, Gauthier A, Steele-Mortimer O, DeVinney R, Finlay BB. Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell Microbiol. 1999;1:7–17. doi: 10.1046/j.1462-5822.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- Deng W, Vallance BA, Li Y, Puente JL, Finlay BB. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol Microbiol. 2003;48:95–115. doi: 10.1046/j.1365-2958.2003.03429.x. [DOI] [PubMed] [Google Scholar]
- DeVinney R, Puente JL, Gauthier A, Goosney D, Finlay BB. Enterohaemorrhagic and enteropathogenic Escherichia coli use a different Tir-based mechanism for pedestal formation. Mol Microbiol. 2001;41:1445–1458. doi: 10.1046/j.1365-2958.2001.02617.x. [DOI] [PubMed] [Google Scholar]
- DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay BB. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect Immun. 1999;67:2389–2398. doi: 10.1128/iai.67.5.2389-2398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg MS, Tzipori S, McKee ML, O'Brien AD, Alroy J, Kaper JB. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Invest. 1993a;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg MS, Tacket CO, James SP, Losonsky G, Nataro JP, Wasserman SS, et al. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993b;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziva F, van Diemen PM, Stevens MP, Smith AJ, Wallis TS. Identification of Escherichia coli O157 : H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology. 2004;150:3631–3645. doi: 10.1099/mic.0.27448-0. [DOI] [PubMed] [Google Scholar]
- Elliott SJ, Yu J, Kaper JB. The cloned locus of enterocyte effacement from enterohemorrhagic Escherichia coli O157:H7 is unable to confer the attaching and effacing phenotype upon E. coli K-12. Infect Immun. 1999;67:4260–4263. doi: 10.1128/iai.67.8.4260-4263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia J, Frankel G, Crepin VF. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect Immun. 2005;73:2573–2585. doi: 10.1128/IAI.73.5.2573-2585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia J, Phillips AD, Carlier MF, Chong Y, Schuller S, Marches O, et al. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell Microbiol. 2004;6:1167–1183. doi: 10.1111/j.1462-5822.2004.00459.x. [DOI] [PubMed] [Google Scholar]
- Goldberg MB. Actin-based motility of intracellular microbial pathogens. Microbiol Mol Biol Rev. 2001;65:595–626. doi: 10.1128/MMBR.65.4.595-626.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosney DL, DeVinney R, Finlay BB. Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals. Infect Immun. 2001;69:3315–3322. doi: 10.1128/IAI.69.5.3315-3322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–859. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- Hartland EL, Batchelor M, Delahay RM, Hale C, Matthews S, Dougan G, et al. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol. 1999;32:151–158. doi: 10.1046/j.1365-2958.1999.01338.x. [DOI] [PubMed] [Google Scholar]
- Hayward RD, Leong JM, Koronakis V, Campellone KG. Exploiting pathogenic Escherichia coli to model transmembrane receptor signalling. Nat Rev Microbiol. 2006;4:358–370. doi: 10.1038/nrmicro1391. [DOI] [PubMed] [Google Scholar]
- Herman IM, Pollard TD, Wong AJ. Contractile proteins in endothelial cells. Ann N Y Acad Sci. 1982;401:50–60. doi: 10.1111/j.1749-6632.1982.tb25706.x. [DOI] [PubMed] [Google Scholar]
- Herman IM, Brant AM, Warty VS, Bonaccorso J, Klein EC, Kormos RL, Borovetz HS. Hemodynamics and the vascular endothelial cytoskeleton. J Cell Biol. 1987;105:291–302. doi: 10.1083/jcb.105.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TD, Waldor MK. Enterohemorrhagic Escherichia coli O157:H7 gal mutants are sensitive to bacteriophage P1 and defective in intestinal colonization. Infect Immun. 2007;75:1661–1666. doi: 10.1128/IAI.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KE, Thorpe CM, Sears CL. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin Infect Dis. 2006;43:1587–1595. doi: 10.1086/509573. [DOI] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Karch H, Tarr PI, Bielaszewska M. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol. 2005;295:405–418. doi: 10.1016/j.ijmm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;1:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Logsdon LK, Mecsas J. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect Immun. 2003;71:4595–4607. doi: 10.1128/IAI.71.8.4595-4607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marches O, Nougayrede JP, Boullier S, Mainil J, Charlier G, Raymond I, et al. Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect Immun. 2000;68:2171–2182. doi: 10.1128/iai.68.4.2171-2182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara BP, Koutsouris A, O'Connell CB, Nougayrede JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Abe A, Sasakawa C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J Biol Chem. 2005;280:2998–3011. doi: 10.1074/jbc.M411550200. [DOI] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougayrede JP, Donnenberg MS. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol. 2004;6:1097–1111. doi: 10.1111/j.1462-5822.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Ritchie JM, Waldor MK. The locus of enterocyte effacement-encoded effector proteins all promote enterohemorrhagic Escherichia coli pathogenicity in infant rabbits. Infect Immun. 2005;73:1466–1474. doi: 10.1128/IAI.73.3.1466-1474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, Thorpe CM, Rogers AB, Waldor MK. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect Immun. 2003;71:7129–7139. doi: 10.1128/IAI.71.12.7129-7139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Chang R, Ashton F, Kaper JB, Sanger JW. Novel form of actin-based motility transports bacteria on the surfaces of infected cells. Cell Motil Cytoskeleton. 1996;34:279–287. doi: 10.1002/(SICI)1097-0169(1996)34:4<279::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schuller S, Chong Y, Lewin J, Kenny B, Frankel G, Phillips AD. Tir phosphorylation and Nck/N-WASP recruitment by enteropathogenic and enterohaemorrhagic Escherichia coli during ex vivo colonization of human intestinal mucosa is different to cell culture models. Cell Microbiol. 2007;9:1352–1364. doi: 10.1111/j.1462-5822.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Sanger JW, Sanger JM. Actin and alpha-actinin dynamics in the adhesion and motility of EPEC and EHEC on host cells. Cell Motil Cytoskeleton. 2005;60:104–120. doi: 10.1002/cm.20047. [DOI] [PubMed] [Google Scholar]
- Sheng H, Lim JY, Knecht HJ, Li J, Hovde CJ. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect Immun. 2006;74:4685–4693. doi: 10.1128/IAI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket CO, Sztein MB, Losonsky G, Abe A, Finlay BB, McNamara BP, et al. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect Immun. 2000;68:3689–3695. doi: 10.1128/iai.68.6.3689-3695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Montanaro J, Robins-Browne RM, Vial P, Gibson R, Levine MM. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60:5302–5306. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Gunzer F, Donnenberg MS, de Montigny L, Kaper JB, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Wachsmuth IK, Chapman C, Birden R, Brittingham J, Jackson C, Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986;154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- van Diemen PM, Dziva F, Stevens MP, Wallis TS. Identification of enterohemorrhagic Escherichia coli O26:H- genes required for intestinal colonization in calves. Infect Immun. 2005;73:1735–1743. doi: 10.1128/IAI.73.3.1735-1743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan VK, Koutsouris A, Lukic S, Pilkinton M, Simonovic I, Simonovic M, Hecht G. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect Immun. 2004;72:3218–3227. doi: 10.1128/IAI.72.6.3218-3227.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlisidou I, Dziva F, La Ragione RM, Best A, Garmendia J, Hawes P, et al. Role of intimin-tir interactions and the tir-cytoskeleton coupling protein in the colonization of calves and lambs by Escherichia coli O157:H7. Infect Immun. 2006;74:758–764. doi: 10.1128/IAI.74.1.758-764.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AJ, Pollard TD, Herman IM. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983;219:867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]


