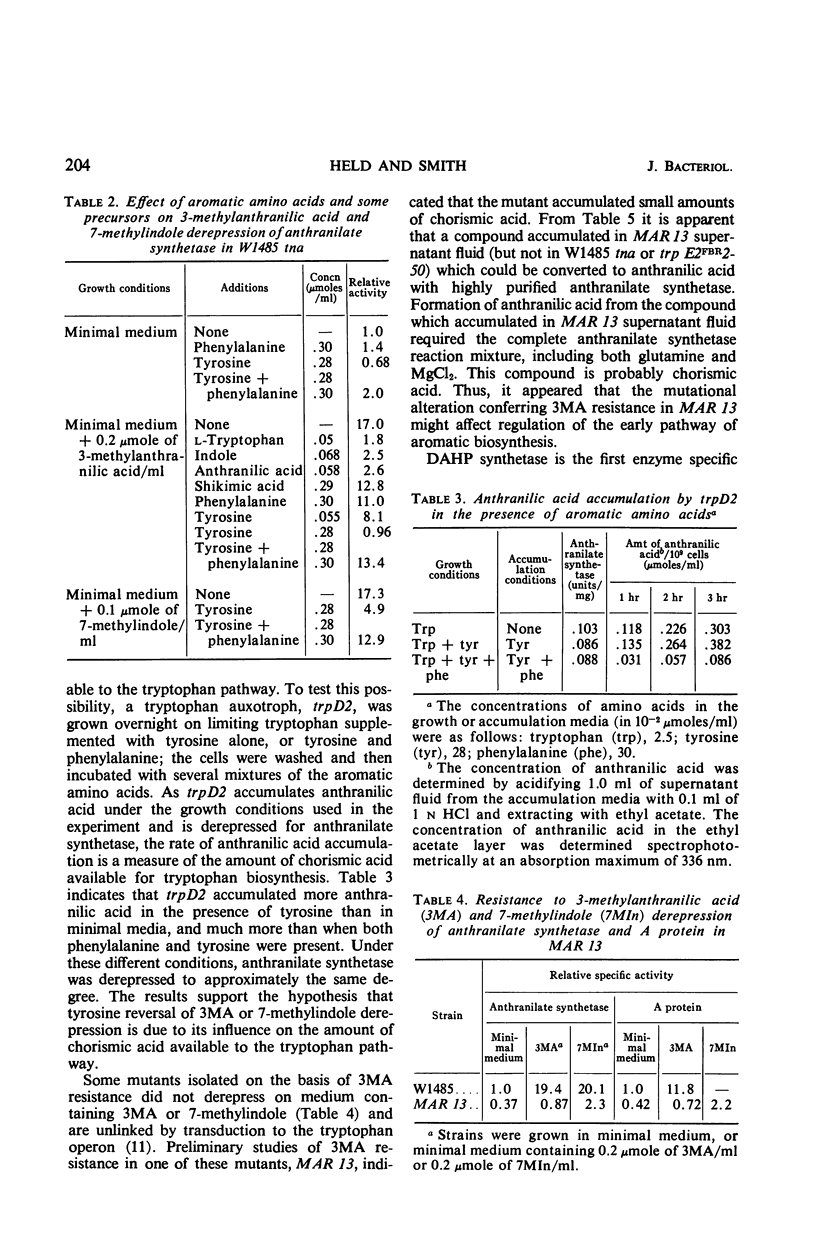
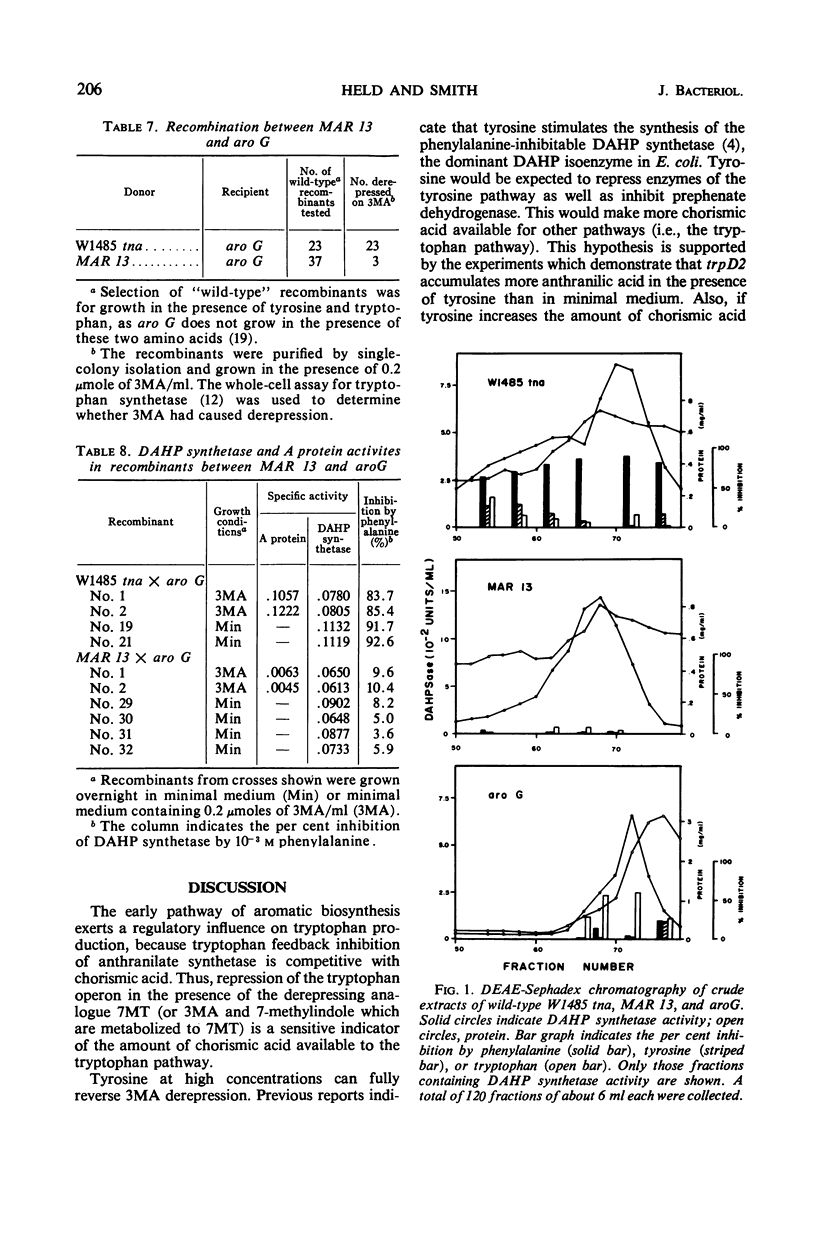
Abstract
7-Methyltryptophan (7MT) or compounds which can be metabolized to 7MT, 3-methylanthranilic acid (3MA) and 7-methylindole, cause derepression of the trp operon through feedback inhibition of anthranilate synthetase. Tyrosine reverses 3MA or 7-methylindole derepression, apparently by increasing the amount of chorismic acid available to the tryptophan pathway. A mutant isolated on the basis of 3MA resistance (MAR 13) was found to excrete small amounts of chorismic acid and to have a feedback-resistant phenylalanine 3-deoxy-d-arabinoheptulosonic acid-7-phosphate (DAHP) synthetase. Genetic evidence indicates that the mutation conferring 3MA resistance and feedback resistance is very closely linked to aroG, the structural gene for the DAHP synthetase (phe). Since feedback inhibition of anthranilate synthetase by l-tryptophan (or 7MT) is competitive with chorismic acid, alterations in growth conditions (added tyrosine) or in a mutant (MAR 13) which increase the amount of chorismic acid available to the tryptophan pathway result in resistance to 7MT derepression. Owing to this competitive nature of tryptophan feedback inhibition of anthranilate synthetase by chorismic acid, the early pathway apparently serves to exert a regulatory influence on tryptophan biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. I., Crawford I. P. Anthranilate synthetase. Partial purification and some kinetic studies on the enzyme from Escherichia coli. J Biol Chem. 1966 Dec 10;241(23):5577–5584. [PubMed] [Google Scholar]
- Brown K. D., Doy C. H. Control of three isoenzymic 7-phospho-2-oxo-3-deoxy-Darabino-heptonate-D-erythrose-4-phosphate lyases of Escherichia coli W and derived mutants by repressive and "inductive" effects of the aromatic amino acids. Biochim Biophys Acta. 1966 Apr 12;118(1):157–172. doi: 10.1016/s0926-6593(66)80153-4. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Doy C. H., Brown K. D. Control of aromatic biosynthesis: the multiplicity of 7-phospho-2-oxo-3-deoxy-D-arabino-heptonate D-erythrose-4-phosphate-lyase (pyruvate-phosphorylating) in Escherichia coli W. Biochim Biophys Acta. 1965 Jul 8;104(2):377–389. doi: 10.1016/0304-4165(65)90343-0. [DOI] [PubMed] [Google Scholar]
- Doy C. H. Tryptophan as an inhibitor of 3-deoxy-arabino-heptulosonate 7-phosphate synthetase. Biochem Biophys Res Commun. 1967 Jan 23;26(2):187–192. doi: 10.1016/0006-291x(67)90232-x. [DOI] [PubMed] [Google Scholar]
- EZEKIEL D. H. FALSE FEEDBACK INHIBITION OF AROMATIC AMINO ACID BIOSYNTHESIS BY BETA-2-THIENYLALANINE. Biochim Biophys Acta. 1965 Jan 11;95:54–62. doi: 10.1016/0005-2787(65)90210-8. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W. A., Smith O. H. Mechanism of 3-methylanthranilic acid derepression of the tryptophan operon in Escherichia coli. J Bacteriol. 1970 Jan;101(1):209–217. doi: 10.1128/jb.101.1.209-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pittard J., Camakaris J., Wallace B. J. Inhibition of 3-deoxy-d-arabinoheptulosonic acid-7-phosphate synthetase (trp) in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1242–1247. doi: 10.1128/jb.97.3.1242-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Chromatography of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) on diethylaminoethyl cellulose: a correction. J Bacteriol. 1967 Oct;94(4):1279–1280. doi: 10.1128/jb.94.4.1279-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


