Abstract
Donor lymphocyte infusion (DLI) is used after both myeloablative and non-myeloablative stem-cell transplantation to treat and prevent relapse, to establish full donor chimerism, and to treat and prevent infections. The major treatment-related complication of DLI is graft-versus-host disease (GVHD). The presentation and treatment of GVHD after DLI is similar to its presentation and treatment after stem-cell transplantation, with some notable exceptions. While GVHD and graft-versus-tumor (GVT) effects are highly correlated after DLI, some patients experience remission without GVHD. Studies to define tumor-specific target antigens and GVT effector cells, as well as strategies of donor T-cell manipulation and optimization of DLI dose and schedule, may ultimately lead to the consistent ability to separate GVHD from GVT activity, improvement in the safety and specificity of DLI, and enhancement of the anti-tumor activity of donor T cells.
Keywords: donor lymphocyte infusion, immunotherapy, graft-versus-host disease, graft-versus-tumor effect, CD8+ depletion, T-cell inactivation, suicide gene
It is well established that the success of allogeneic stem-cell transplantation (SCT) relies in part on the graft-versus-tumor (GVT) effect of donor-derived T cells.[1,2] Donor lymphocyte infusions (DLIs) are frequently used to harness this GVT activity in patients who relapse after SCT and have otherwise limited treatment options. DLI is dramatically effective in inducing sustained remissions and cure in patients with chronic myelogenous leukemia (CML) in chronic phase, but is less successful in inducing initial and sustained remissions in patients with advanced-phase CML or acute leukemia.[3–7] Optimizing the effectiveness of DLI for non-CML disorders is the subject of numerous trials.[8,9]
The broadening applications of allogeneic SCT and expansion of non-conventional conditioning regimens have resulted in the increased use of DLI for several other indications (Table 1). In addition to the treatment of relapsed disease after SCT, DLI is being used as prophylaxis after SCT for patients with a high risk of relapse due either to advanced disease stage or in conjunction with T-cell-depleted grafts.[10–12] In addition, DLI has been used successfully to treat graft failure, post-transplant lymphoproliferative disorder (PTLD), viral infections, and as a method to facilitate immune reconstitution after allogeneic SCT.[13–16] The rapid expansion of non-myeloablative conditioning therapies results in an even greater need for both planned and unplanned DLI to maximize donor chimerism, reverse graft rejection, and treat the higher rate of relapse.[14,17,18] In general, DLI is reserved for patients without active graft-versus-host disease (GVHD), and is given without prophylactic immunosuppression. In certain circumstances, DLI is administered after cytoreductive systemic chemotherapy (either at hematologic nadir or recovery). The timing and dosing of DLI administration is, however, dependent on the indication for therapy.
Table 1.
Indications for donor leukocyte infusion (DLI).
| To treat relapsed disease after stem-cell transplantation (SCT) |
| Prophylactic DLI to prevent relapse and viral reactivation: |
| T-cell-depleted grafts |
| Non-myeloablative SCT |
| Advanced disease stage |
| Induce full donor chimerism |
| Enhance immune reconstitution |
| Treat viral infection after SCT |
| Treat post-transplant lymphoproliferative disorder (PTLD) |
In almost every study reported, disease response to conventional DLI and the presence of GVHD are highly correlated. We review the incidence and clinical presentation of GVHD after DLI and discuss approaches to its management. We also review current strategies to manipulate DLI in attempts to minimize toxicity, maximize efficacy, and separate GVT activity from GVHD.
GRAFT-VERSUS-HOST DISEASE AFTER DLI
The most common and significant toxicity attributable to DLI is GVHD. When unmanipulated, DLI is given for relapsed disease after myeloablative related transplants; the overall incidence of acute GVHD is 40–60% with grade-III–IV GVHD, affecting 20–35% of patients (see Table 2). Chronic GVHD occurs in 33–61% of patients, and deaths attributable to GVHD are in the range 6–11%.[4,5,7,19–22]
Table 2.
Selected reports of the incidence of graft-versus-host disease (GVHD) after unmanipulated donor leukocyte infusion (DLI).
| Reference | N | Disease | Donor source | Acute GVHD | Grade-II–IV GVHD | Grade-III–IV GVHD | Chronic GVHD | GVHD-related mortality | Median time to GVHD | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| DLI after myeloablative SCT: | ||||||||||
| [5] | 125 | Varied | 91% related | 60% | 46% | 22% | 61% | 7% | 32 days | Multicenter retrospective |
| L = 19% | analysis | |||||||||
| E = 22% | (North American) | |||||||||
| [4] | 135 | Varied | 92% related | 59% | 41% | NR | NR | 6% | NR | Multicenter retrospective |
| analysis | ||||||||||
| (European) | ||||||||||
| [19] | 89 | Varied | Related | NR | 34% | 19% | 33% | 8% | NR | Multicenter retrospective |
| analysis | ||||||||||
| (Japanese) | ||||||||||
| [21] | 57 | Advanced | Related | 57% | 45% | 28% | 36% | 9% | NR | Multicenter prospective trial. |
| myeloid | L = 21% | Cytarabine-based | ||||||||
| diseases | E = 14% | chemotherapy before DLI | ||||||||
| [20] | 37 | ALL | 77% related | 49% | 46% | 35% | 25% | 11% | 28 days | Retrospective and prospective |
| data analyzed | ||||||||||
| [22] | 54 | MM | Varied | 57% | 46% | 20% | 47% | NR | Multicenter prospective trial | |
| L = 17% | Most T-cell-depleted grafts | |||||||||
| E = 30% | ||||||||||
| Unrelated DLI: | ||||||||||
| [24] | 56 | Varied | Unrelated | 38% | 33% | 25% | 49% | 9% | NR | Multicenter retrospective |
| L = 9% | analysis | |||||||||
| E = 31% | (North American) | |||||||||
| 51% T-cell-depleted SCT | ||||||||||
| Haploidentical DLI: | ||||||||||
| [27] | 20 | Varied | Haploidentical | 55% | NR | 30% | 64% | 15% | NR | Intolerable rates of grade-III– |
| IV GVHD and GVHD-related | ||||||||||
| mortality in subset of patients | ||||||||||
| treated without GVHD | ||||||||||
| prophylaxis | ||||||||||
| DLI after non-myeloablative SCT: | ||||||||||
| [14] | 53 | Varied | 98% related | 19% | 17% | 6% | 34% | 2% | NR | Multicenter retrospective |
| L = 15% | analysis | |||||||||
| E = 19% | Less GVHD but lower cell | |||||||||
| dose 1 × 107 | ||||||||||
| [18] | 63 | MM | 73% related | 38% | 22% | 16% | 43% | 8% | NR | Retrospective multicenter |
| L = 33% | analysis (European) | |||||||||
| E = 10% | 50% SCT with T-cell-depleted | |||||||||
| graft | ||||||||||
| [17] | 81 | Varied | 88% related | 33% | 28% | 15% | 33% | 9% | NR | Multicenter retrospective |
| L = 7% | analysis | |||||||||
| E = 26% | Only 51 treated for disease | |||||||||
| progression | ||||||||||
N, number of evaluable patients; SCT, stem-cell transplantation; L, limited; E, extensive; ALL, acute lymphoblastic leukemia; MM, multiple myeloma; NR, not reported.
Most studies demonstrate a strong correlation between GVHD and GVT effects. For instance, a retrospective multicenter North American study evaluated outcomes in 140 patients who received DLI for relapsed disease after SCT. In this study 60% of evaluable patients developed acute GVHD (46% with grade-II–IV disease) and 61% developed chronic GVHD. Of 45 patients who achieved a complete response (CR), 42 developed acute GVHD, and 36/41 evaluable patients in CR developed chronic GVHD. Conversely, only three of 23 patients who did not develop GVHD achieved a CR.[5] Similar results were reported by the European Bone Marrow Transplant (EBMT) group which retrospectively reviewed outcomes of 135 patients with relapsed chronic and acute leukemias after allogeneic SCT who received DLI. Grade-II–IV GVHD requiring treatment occurred in 41% of evaluable patients, and death attributable to GVHD occurred in 6% of patients. Of the 46 patients with CML who developed GVHD, 42 had a response while only 13/29 CML patients without GVHD had a response, again highlighting the tight association between GVHD and GVL activity.[4] Notably, a similar multicenter Japanese study found lower incidences of GVHD after DLI, with 34% of patients developing grade-II–IV GVHD and 33% developing chronic GVHD.[19] These findings are consistent with their reports of less GVHD after SCT, and may be due to less genetic diversity in the Japanese population compared with the European and North American populations. While illustrating the high correlation between GVHD and GVT effects, the above studies also reveal the need to minimize the severity of GVHD, and highlight that, at least in some patients, GVT and GVHD effects are separable.
GVHD after unrelated DLI
Due to the higher incidence of major and minor histocompatibility differences and underlying genetic diversity anticipated in unrelated transplants, a higher degree of GVHD might be expected after unrelated DLI (UDLI) in comparison to recipients of related DLI. Several of the retrospective reports included small numbers of unrelated donor recipients, but did not find a higher incidence of GVHD after UDLI.[4,5,23] Similarly, when UDLI was evaluated as an independent risk factor after non-myeloablative transplant, no increased risk of GVHD was found.[17]
To further evaluate the outcomes of UDLI, a retrospective analysis of data reported to the National Marrow Donor Program was performed. Fifty-eight patients were identified who underwent unrelated SCT (with 21% receiving mismatched grafts) followed by UDLI for relapsed acute and chronic leukemias. Acute grade-II–IV GVHD occurred in 33% of patients, chronic GVHD in 41% of patients, and deaths attributable to GVHD occurred in 9% of patients.[24] Interestingly, responses were at least similar to those anticipated after matched sibling DLI. Another retrospective study compared outcomes of 30 patients with CML who received related or unrelated DLI for relapsed disease; no significant difference was found in the incidence of acute GVHD.[25] While limited, these data combined with subset analyses from other studies suggest that the incidence of GVHD after UDLI is not significantly different from GVHD after related donor DLI.
GVHD after haploidentical DLI
Haploidentical SCT is being applied with increasing frequency when well-matched donors cannot be identified. However, there are limited reports on outcomes of DLI after haploidentical SCT.[10,26–28] One group evaluated the role of prophylactic DLI (with initial infusions of 104 CD3+ cells/kg) after T-cell-depleted haploidentical transplants for subjects with refractory malignancies to aid in immune reconstitution and prevent relapse. Various doses and schedules of DLI were evaluated. Low-dose DLI (1 × 104 CD3+/kg) monthly starting at Day 28 improved immune reconstitution and was not associated with GVHD. Higher doses of DLI, either prophylactic or for relapse, were invariable associated with GVHD.[10] Another study recently reported outcomes of 20 patients with relapsed disease after haploidentical SCT who were treated with DLI.[27] The first nine subjects were given DLI in the conventional manner without GVHD prophylaxis. Five of these patients (55%) developed grade-III–IV GVHD, resulting in three deaths. The subsequent 11 subjects were therefore treated with GVHD prophylaxis (cyclosporine A or low-dose methotrexate) for 2–4 weeks. Only one of these patients developed grade-III–IV GVHD. In the total cohort, 55% developed acute GVHD and 64% a chronic GVHD. Fifteen of the 20 patients achieved complete remission, and while numbers were too small to formally evaluate, there did not seem to be a difference in response rate between the group that received GVHD prophylaxis and the group that did not.[27]
DLI and GVHD after non-myeloablative SCT
Non-myeloablative SCT (NST) regimens are being used more frequently in an effort to limit SCT toxicity and broaden potential treatment options for older and sicker patient populations. This therapy is more reliant on the GVT effect of SCT than conventional transplant. Reduced-intensity conditioning regimens commonly induce a state of mixed chimerism, although some evidence suggests that conversion to a full chimerism may be necessary to achieve sustained remissions.[14,17,29] The indications for DLI after NST are therefore somewhat different from the myeloablative setting. In addition to treating relapsed or persistent disease, DLI is often given for persistent mixed chimerism. The use of a less cytotoxic conditioning regimen and presence of mixed chimerism after NST may also predict for a different response to DLI both in terms of a GVT effect and incidence of GVHD. Interesting data from murine models have shown that mixed chimeras can convert to complete donor chimerism after DLI without significant GVHD.[30–32] This suggests that a GVH lymphohematopoietic reaction can occur in the absence of a more generalized GVHD reaction. Further studies in the mouse have demonstrated that the GVT activity of DLI is more potent in mixed chimeras than full chimeras, perhaps due to the persistence of host antigen-presenting cells (APCs) in the mixed chimeras. Again a profound separation of GVT from generalized GVHD was noted.[30]
Efforts to translate these mouse models into humans have thus far failed to elicit similar separations of GVT and GVHD responses. However, what is noted is the suggestion of a slight decrease in the overall incidence of acute GVHD after DLI in the non-myeloablative setting compared to the myeloablative setting. In retrospective analyses the overall incidence of acute GVHD in DLI recipients after non-myeloablative transplants is 19–33%, with grade-III–IV GVHD affecting 6–16% of patients (see Table 2). Chronic GVHD occurs in 33–43% of patients.[14,17,18] While far short of an appropriately powered and designed analysis to determine comparative incidences of GVHD, these findings are intriguing, and raise the possibility that the incidence of GVHD in DLI recipients after non-myeloablative conditioning may be lower than the incidences of overall and severe GVHD in recipients of myeloablative transplants.
GVHD after activated DL
Overall, DLI has been disappointing in the treatment of diseases other than CML after SCT. This could be due in part to inadequate activation of T cells in vivo, limiting any potent GVT response. In an effort to enhance the GVT potential of DLI, we studied the use of ex-vivo CD3 and CD28 co-stimulated and expanded T cells as ‘activated’ DLI.[9] Eighteen subjects were treated in a phase-I study, and high initial remission rates were noted without the expected increased rates of GVHD. The overall complete response rate was 44%, and the overall incidence of acute GVHD was 39%. Chronic GVHD developed in 22% of subjects, and no deaths were attributable to GVHD.[9]
Practice points
DLI is effective in inducing remissions in patients with relapsed chronic-phase CML after SCT, but is less successful in inducing sustained remissions in other relapsed, advanced hematologic malignancies
the incidence of acute GVHD after conventional DLI is in the range 40–60%
the incidence of GVHD after unrelated DLI is similar to the incidence after related DLI
prophylactic immunosuppression should be considered before haploidentical DLI
while GVHD and GVT after DLI are highly correlated, some patients enter a remission without experiencing GVHD
CLINICAL PRESENTATION OF GVHD AFTER DLI
While significant, the toxicity from GVHD after DLI seems to be lower than anticipated if similar doses of T cells are given at the time of SCT.[33] This is important, since DLI – in contrast to SCT – is usually administered without prophylactic immunosuppression. This decreased incidence and severity of GVHD after DLI may be due to several factors, including distancing of DLI from cytotoxic regimens which cause tissue injury, and cytokine release predisposing to GVHD.[34] In addition, the presence of mixed chimerism at the time of DLI may imply an active state of immune tolerance capable of limiting GVHD.
The timing of GVHD after DLI may be delayed when compared to conventional SCT. The onset of acute GVHD after conventional DLI occurs a median of 28–32 days after infusion.[5,20] The range reported, however, is quite broad, and GVHD can occur several months to years after DLI. Interestingly, the timing and severity of GVHD is affected by manipulation of DLI cell number and content.[35] The delayed development of GVHD after low-dose DLI is logical. It is likely that some threshold of GVHD effector cells must be reached before clinical manifestations of GVHD are evident. After low-dose DLI it will take longer before the small number of alloreactive precursor cells recognize allo-antigen and proliferate to numbers sufficient to cause clinical signs of GVHD. As discussed in more detail below, CD8+ T cells are important in GVHD, and recipients of CD8+-depleted DLI have a decreased incidence and delayed onset of GVHD. Conversely, certain chemotherapeutic agents administered before DLI have been associated with an earlier onset and increased severity of GVHD.[8]
In general, the clinical signs and symptoms of GVHD after DLI mirror the signs and symptoms of GVHD occurring after SCT, with the same organs affected. In one study of 81 recipients of DLI after NST, 26% of subjects developed acute skin GVHD, 14% developed acute gut GVHD, and 9% developed acute hepatic GVHD. In this same study, 16% developed chronic skin GVHD, 10% chronic gut GVHD, 16% chronic liver GVHD, and 4% chronic eye GVHD.[17] Some case series have suggested an increased incidence of a hepatitis-like variant of liver GVHD compared to patients who develop GVHD after SCT.[36,37] In these cases patients present with hepatic inflammation and transaminitis rather than cholestasis. One group reported their observation that 15% of 73 recipients of DLI developed this hepatitis-like variant of GVHD associated with marked elevations of serum transaminases.[37] Another study described characteristics of GVHD in 19 subjects after DLI, nine of whom developed isolated liver GVHD. Of these nine patients, six (55%) presented with a hepatitis-like picture. The authors compared these findings with those from 106 patients who developed GVHD after SCT. While the DLI recipients had a higher overall incidence of liver GVHD, there were similar percentages in the two groups having hepatitis-like and classical forms of hepatic GVHD.[36] Further analyses will be needed to determine whether the incidence and presentation of hepatic GVHD after DLI differs significantly from its presentation after SCT.
Practice points
the same target organs are affected by GVHD after DLI as after SCT
the median onset of GVHD after conventional DLI is 28–32 days
RISK FACTORS FOR GVHD AFTER DLI
It has been difficult to predict which patients will develop significant GVHD after DLI. The North American retrospective analysis identified non-white race and the presence of chronic GVHD after SCT as independent risk factors for the development of GVHD after DLI.[5] Interestingly, the presence of acute GVHD after SCT in this and other studies is not predictive of developing GVHD after DLI.[4,5,18,24,38] Other factors which were not shown to be significant predictors of GVHD after DLI included donor–recipient sex mismatch, receipt of DLI more or less than 1 year from SCT, mononuclear cell dose more or less than 4.0 × 108/kg, and use of T-cell-depleted grafts for SCT.[5] In contrast to these data, the EBMT identified receipt of T-cell-depleted grafts at the time of SCT to be associated with a higher incidence of GVHD after DLI. Factors not shown to be independent predictors of GVHD included GVHD after SCT, sex of donor, diagnosis, receipt of DLI more or less than 500 days after SCT, mononuclear cell dose more or less than 3.0 × 108/kg and use of chemotherapy before DLI.[4] In contrast to the findings of these large retrospective analyses, other studies have suggested an increased risk of GVHD with closer proximity of DLI to SCT.[17,30,38] As discussed in detail below, manipulation of DLI cell number and content, in addition to its administration via a dose-escalation regimen, may alter the ability of DLI to induce GVT and GVHD effects.
DLI AND CHEMOTHERAPY
For patients with relapsed chronic-phase CML, DLI is dramatically effective without other therapy. However, when administered for advanced-phase CML or acute leukemia, remission rates are low and often of short duration. DLI is therefore often given in conjunction with cytoreductive chemotherapy in an attempt to improve outcomes in these patients. The optimal timing of DLI in this setting remains to be determined, with the advantages of early administration balanced by the desire to distance DLI from chemotherapy-induced end organ damage and subsequent inflammation.
A large prospective trial evaluated outcomes of 65 subjects who were treated with chemotherapy followed by DLI for refractory or relapsed myeloid malignancies after SCT. Most subjects were treated with combination cytarabine (100 mg/m2 × 7 days) and daunarubicin (30 mg/m2 × 3 days), with DLI administered at the hematologic nadir 10–14 days after onset of chemotherapy. The intent was to administer DLI in the setting of 14 minimal disease. Overall initial response rates were 47%, with an overall survival rate at 2 years of only 19%. Acute GVHD occurred in 59% of evaluable cases, with grade-II–IV GVHD occurring in 45% of subjects. Chronic GVHD affected 36% of participants. Treatment-related mortality was high at 28%, with 10% of overall deaths attributable to GVHD.[21] As these findings are consistent with other reported outcomes of GVHD after DLI, this study does not suggest an increased incidence due to pre-DLI chemotherapy.
Other data suggest that administering DLI shortly after chemotherapy may actually increase the risk of GVHD. Miller and colleagues treated patients with relapsed disease after SCT with cyclophosphamide (50 mg/kg on Day 6) and fludarabine (25 mg/m2 on days −6 to −2) followed shortly by DLI. The goal of this regimen was to lymphodeplete the host to promote in-vivo expansion of donor lymphocytes and enhance the potential GVT effects. Marked donor T-cell proliferation and activation were noted 14 days after DLI, but there was an unacceptably high incidence of grade-III–IV GVHD (47% of 15 patients treated) and GVHD-related mortality (five of 15 subjects treated). Interestingly, the median time to onset of GVHD was 17 days, compared to 34 days in historical controls.[8] It is unclear whether the high incidence of GVHD observed in this trial is due to the close proximity of DLI to the cytotoxic chemotherapy regimen or the induction of a pre-DLI lymphodepleted state. It is of interest that some studies have revealed an increased risk of GVHD in recipients of DLI after a T-cell-depleted graft while others have not.[4,5,18]
A recent large retrospective study by the EBMT has suggested that DLI is less effective when administered shortly after chemotherapy. They evaluated outcomes of 399 patients with relapsed acute myelogenous leukemia (AML) after SCT (171 who received DLI and 228 who did not), and showed that optimal responses to DLI occur in patients who are in a complete remission (rather than at chemotherapy nadir or with active disease).[7] This finding, however, may be due to selection of ‘good-risk’ disease rather than increased toxicity of DLI when administered in these other settings. Its important to note that while this study showed an improved overall survival rate in recipients of DLI compared with recipients of other salvage treatments, the overall complete response rate remained low at 34%, with a 2-year overall survival rate of 20% in the group that received DLI. Other large retrospective analyses have failed to identify pre-DLI chemotherapy as an independent risk factor for the development of GVHD.[4,24]
Rather than increasing the risk, some systemic chemotherapies may be used in combination with DLI to decrease the risk of GVHD without adversely affecting GVT activity. Thalidomide (100 mg daily) was used concurrently with DLI for 18 patients with persistent or relapsed multiple myeloma after prior SCT and DLI. The overall response rate was 67%, with 22% achieving CR. This was higher than historical response rates to single-agent therapy, implying a synergistic effect of dual-modality therapy. Interestingly, the incidence of GVHD in these subjects was very low, with only two of 18 subjects experiencing grade-I GVHD.[39] An interesting hypothesis requiring further testing is that thalidomide might inhibit GVHD without limiting GVT activity.
Practice points
for treatment of relapsed advanced-phase CML and acute leukemia, DLI is often given in combination with chemotherapy
the optimal timing of DLI in relationship to chemotherapy remains to be determined
certain chemotherapeutic regimens and DLI dose schedules may increase the risk of GVHD after DLI
certain concurrent therapies may inhibit GVHD activity without inhibiting GVT activity
SEPARATION OF GVT FROM GVHD
The promise of effective cellular immunotherapy has been realized with the remarkable activity of DLI in early-phase CML, but has yet to be realized with any consistency for the majority of patients who relapse with diseases other than CML. In addition, although GVT and GVHD responses after DLI are highly correlated, there are patients who experience potent GVT effects without generalized GVHD. This has inspired several novel approaches designed to both maximize the anti-tumor potential of DLI and to GVHD from GVT activity (Table 3).
Table 3.
Selected methods to separate graft-versus-tumor (GVT) and graft-versus-host (GVHD) effects of donor leukocyte infusion (DLI).
| Method | Comments |
|---|---|
| Dose-escalation schemes | Significant separation of GVT from GVHD effects in patients with CML and other indolent diseases |
| CD8 T-cell depletion | Less GVHD compared to recipients of conventional DLI; numbers too small to assess for differences in remission rates |
| Intra-BM injection | Encouraging preclinical results |
| Ex-vivo T-cell inactivation | Encouraging preclinical and preliminary clinical results |
| In-vivo T-cell inactivation: suicide genes | Administration of ganciclovir to recipients of HSV-TK-transduced donor T cells effectively abrogates GVHD; numbers too small to assess for differences in remission rates. Other suicide genes under exploration |
| Tumor-specific DLI | Efforts are ongoing to make candidate leukemic antigens the targets of cell-based immunotherapy |
CML, chronic myelogenous leukemia; BM, bone marrow; HSV-TK, herpes simplex virus thymidine kinase.
Dose escalation of DLI
Many have hypothesized that there may be a minimal effective dose of DLI which can induce remission without triggering GVHD. This hypothesis assumes that both GVT and GVHD effects are dependent on T-cell dose, and that the differences between these T-cell numbers are large enough that they can be taken advantage of clinically. In SCT the number of T cells in the donor graft correlates with the incidence of GVHD.[40] Interestingly, while a minimal T-cell dose of DLI is required to exert GVT and GVHD responses, there is no clear association between DLI T-cell dose and incidence or severity of GVHD.
The strongest evidence supporting the hypothesis that dose manipulation can separate GVT effects from GVHD comes from studies using a DLI dose-escalation scheme to treat patients with relapsed CML after SCT.[23,41–43] In one study, 22 subjects with relapsed CML after T-cell-depleted SCT were each treated with escalating doses of DLI until they achieved a clinical response or dose-limiting GVHD. In total, eight dose levels – from 1 × 105/kg T cells to 5 × 108/kg T cells – were used. Nineteen of the 22 subjects achieved a remission from doses ranging from 1 × 107/kg to 5 × 108/kg T cells. Interestingly, using this dose-escalation method, 52% of subjects achieved a complete remission without developing GVHD. This is in contrast to the >90% correlation between GVHD and remission described with conventional DLI. The incidence of GVHD in this study was also dependent on T-cell dose. Only one of eight patients who went into remission with a T-cell dose of 1 × 107/kg T cells developed GVHD, while eight of eleven patients who went into remission with a T cell dose of ≥5 × 107/kg developed GVHD.[42] A large multicenter retrospective study evaluated the effect of DLI initial cell dose on outcome in 298 patients with relapsed CML after SCT. Three different initial dose levels were evaluated: mononuclear cell dose (MNC) ≤0.2 × 108/kg, MNC 0.2–2.0 × 108/kg, and MNC ≥2.0 × 108/kg. This study found that a lower initial cell dose was associated with significantly lower rates of GVHD and improved overall survival. While no difference in response rates were noted, patients treated with lower initial cell doses required an increased number of infusions to achieve a response.[23]
Another study compared outcomes of 48 patients with relapsed CML who were treated with a conventional single-dose regimen of DLI or with a T-cell dose-escalation scheme. Between 1990 and 1995, subjects treated were treated with a single dose of DLI (median T-cell dose 1.5 × 108/kg), and between 1996 and 1998 subjects were treated with a dose-escalation regimen (doses ranging from 1× 106 cells/kg to 1× 108 cells/kg). While no statistically significant differences in response rates were noted between the two groups, a significant reduction in GVHD was noted in the group treated with dose escalation (10% versus 40%, P = 0.011) (Figure 1). Subsets of patients from each group who received the same total T-cell dose were then compared. The group being treated with the dose-escalation scheme had less GVHD, implying that the decrease in GVHD in the dose-escalation group was not a direct effect of a low T-cell dose but rather a result of sequential T-cell administration, with early low-dose infusions conferring a degree of anergy.[41] Recently this same group reported follow-up on 82 patients with relapsed CML after SCT treated with an escalated-dose regimen of DLI. A multivariate analysis was performed to identify risk factors for developing GVHD. The overall incidence of GVHD remained low when compared to conventional DLI, with grade-II–IV GHVD affecting 15% of subjects and chronic GVHD affecting 29% of subjects. No correlation was found, however, between cell dose and incidence of GVHD.[38] Also of interest, several larger studies employing conventional DLI failed to find a correlation between T-cell dose and the development of GVHD (Figure 2).[4,17,18,24] In part, some of these discrepancies between studies may be attributable to different T-cell dose thresholds examined. Another possibility remains that the decreased incidence of GVHD identified in some of these studies may be due to the immunological effects of the sequential dosing schedule rather than cell dose. It should also be noted that a strategy of low-dose DLI followed by dose escalation is most appropriate for patients with CML or indolent diseases. Patients with more aggressive tumors are unlikely to tolerate the delayed GVT effect inherent in these strategies
Figure 1.
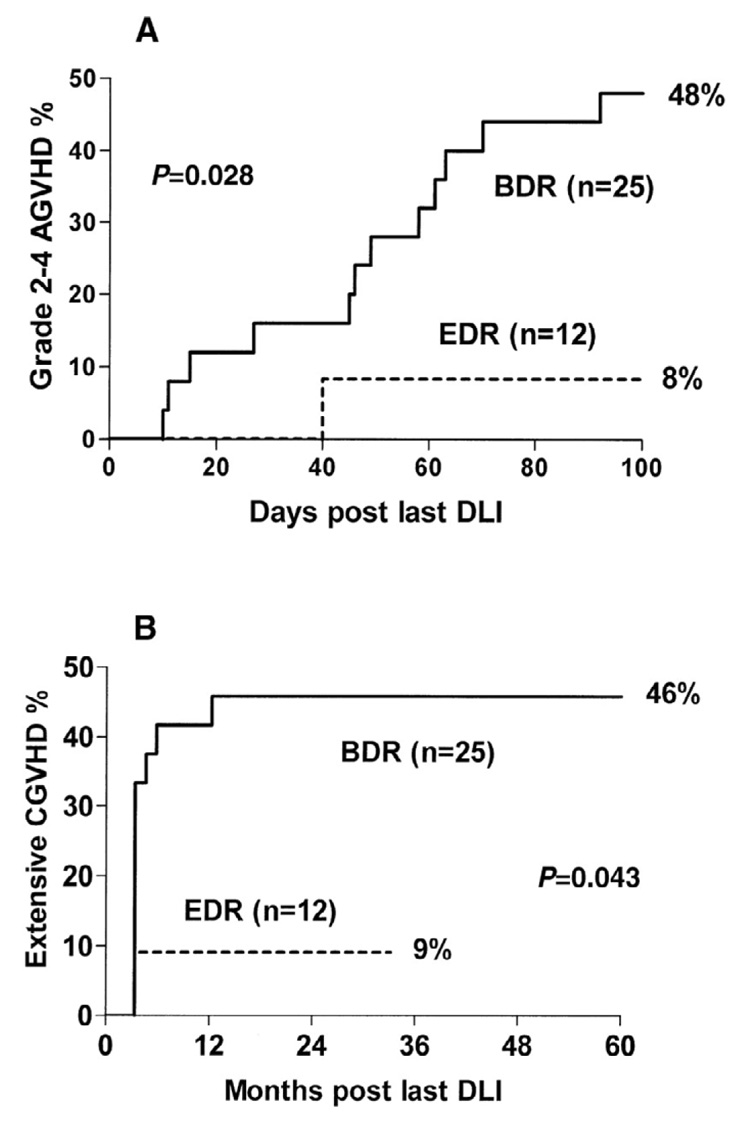
Probability of acute and chronic graft-versus-host disease (AGVHD and CGVHD) after bulk-dosing regimen (BDR) versus escalating-dose regimen (EDR) donor leukocyte infusion (DLI). Reprinted from Dazzi et al (2000, Blood 95: 67–71) with permission.
Figure 2.
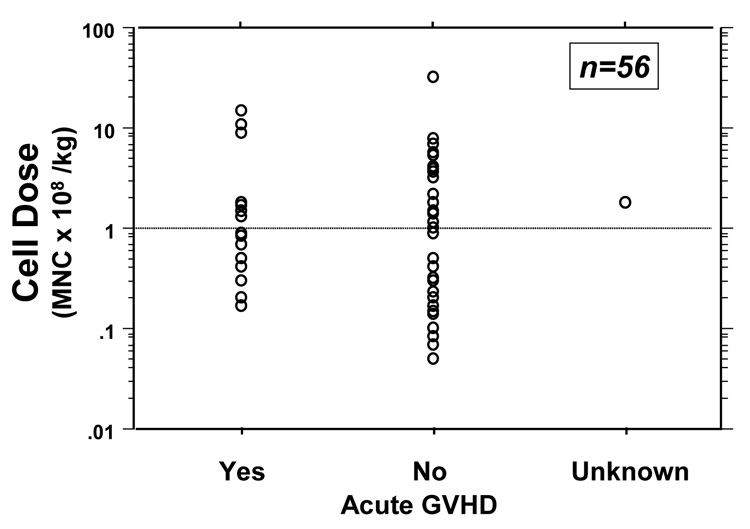
Correlation of donor leukocyte infusion (DLI) cell dose with acute graft-versus-host disease (GVHD) after unrelated stem-cell transplantation (USCT). After unrelated DLI, no correlation between cell dose and the incidence of acute GVHD was identified. Reprinted from Collins et al (2000, Bone Marrow Transplantation 26: 511–516) with permission.
CD8 depletion
Preclinical models predict that different T-cell subsets may differentially effect GVT and GVHD responses.[44–46] A mouse model linking CD8+ T cells to GVHD, and the clinical observation that circulating CD8+ T cells in human subjects predicts clinical GVHD, inspired several investigators to evaluate the role of CD8+-depleted stem-cell grafts.[46,47] In some cases, the use of CD8+-depleted bone-marrow grafts results in less GVHD without an obvious loss of GVT activity, at least in chronic-phase CML.[48–50] These findings have led to several investigations evaluating the role of CD8+-depleted DLI for disease relapse after SCT.[35,51–53] One study analyzed outcomes of 40 patients with relapsed hematologic malignancies after SCT who were treated with CD8+-depleted DLI at three CD4+ dose levels. The overall incidence of acute GVHD was 24% and the incidence of chronic GVHD was 16%, with only one death attributable to GVHD or infection. While all subjects who developed GVHD experienced GVT, 48% of subjects who had a disease response did not develop GVHD, suggesting some degree of separation of GVT and GVHD effects.[35] In the subset of patients with chronic-phase CML, the probability of a complete cytogenetic response was 87% at 1 year, suggesting a similar GVT effect to conventional unfractionated DLI. Also noted in this study was a delay in time to GVHD (median of 11 weeks) and disease response when compared to conventional DLI.[35] Another small randomized trial compared outcomes of prophylactic unmanipulated DLI versus CD8+-depleted DLI administered 6 months after T-cell-depleted SCT. Of the nine subjects receiving conventional DLI, 67% developed GVHD compared with 0% of subjects in the CD8+-depleted group. Numbers were to too small to evaluate for meaningful differences in relapse rates.[53]
Intra-bone-marrow injection
In mouse models the injection of intra-bone-marrow stem-cells results in establishment of donor-derived hematopoiesis, eradication of disease, and a low incidence of GVHD.[54] Similar findings have been reported in the mouse using intra-bone-marrow injection of DLI.[55] Whether this procedure can be translated into clinical transplantation remains to be determined.
Inactivation of GVHD effector cells
The inactivation of donor T cells through radiation or photochemical modification has been used as a mechanism to separate the GVT and GVHD effects of DLI. These approaches are based on the hypothesis that the inactivated T cells may induce initial GVT activity with minimal GVHD due to their inability to proliferate in response to allo-antigens. Studies using these methods in mismatched murine models have shown improvement in engraftment and GVL outcomes without significant GVHD.[56,57]
The insertion of a suicide gene into donor lymphocytes provides a very elegant mechanism for inactivating allo-reactive T cells in vivo once signs of GVHD develop. Presumably these cells can induce a GVT effect before therapy is given to turn off GVHD. For instance, insertion of the herpes simplex virus thymidine kinase (HSV-TK) results in the death of transduced donor T cells with administration of ganciclovir.[58–60] Of 12 evaluable patients treated with HSV-TK-transduced donor T cells for disease relapse after SCT, two developed grade-II or higher acute GVHD, and one developed chronic GVHD. GVHD in these subjects was successfully treated with ganciclovir alone.[59] Other suicide genes (including Fas and CD20-based genes) are also being explored in this clinical context.[61,62]
Tumor-specific DLI
Efforts to improve the overall effectiveness of DLI with tumor-specific donor lymphocytes may also prove to be one of the most effective methods to separate GVT and GVHD effects. The clinical observation that some patients experience GVT without GVHD, the isolation of leukemia-specific lymphocytes, and the successful treatment of relapsed CML with leukemia-reactive donor lymphocytes suggests that such an approach to treatment is possible.[63–65] A major limitation to this mode of therapy has been the inability to isolate tumor-specific target antigens. In many cases, GVT induction is likely the result of differences in host and donor minor histocompatibility antigens, and hence it is logical that GVT and GVHD are linked.[66] However, for leukemia, candidate tumor-specific antigens such as Wilm’s tumor protein, the PR-1 epitope of proteinase 3, PRAME and RHAMM can induce tumor-specific immune responses, making them potential targets for cell-based immunotherapy.[67–69] While laborious, efforts to bring tumor-specific DLI into clinical practice are ongoing.
Practice points
there is no clear association between T-cell dose and incidence of GVHD after DLI
an escalating T-cell dosing schedule for DLI is a successful method to decrease GVHD and preserve GVT in patients with CML
selective CD8+ depletion of DLI results in a decreased incidence of GVHD
transduction of donor T cells with an HSV-TK suicide gene offers a successful method of abrogating GVHD after DLI
leukemic antigens have been identified as potential targets of cell-based immunotherapy
MANAGEMENT OF GVHD AFTER DLI
Although development of GVHD correlates with leukemia response in many cases, no data suggest that severity of GVHD correlates with a better response to DLI. Accordingly, there are currently no data to suggest that GVHD after DLI should be managed differently from GVHD after SCT. At least in CML patients, treatment of GVHD at the time of onset does not appear to impair GVT activity.[6] This may be because intensive immunosuppression is more active against generalized GVH-reactive cells and less suppressive for leukemia-reactive cells. Alternatively, the maximal GVT reaction may have already occurred by the time GVHD is clinically apparent. In addition, since GVHD remains the major treatment-related cause of death after DLI, and there is no practical way to titrate GVHD or GVT activity by either partially treating GVHD or allowing symptoms and manifestations to progress, GVHD after DLI should be managed similarly to GVHD after SCT.
CONCLUSIONS
DLI is increasingly being used after both myeloablative and non-myeloablative SCT to treat and prevent relapse, to establish full donor chimerism, and to treat and prevent infections. While GVHD remains the major non-relapse complication of conventional DLI, the observation that some patients enter remission without experiencing GVHD supports the ultimate promise of separating GVH and GVT activity. Numerous techniques already are actively being studied to both enhance GVT responses while limiting toxicity from DLI. In addition, future studies will determine the most advantageous cell dose and schedule for specific indications of DLI. Despite current limitations, DLI remains one of the most powerful methods of adoptive immunotherapy of cancer, and no doubt a clearer definition of the target antigens and effector cells for GVT activity will lead to new, more effective, and safer methods of DLI.
ACKNOWLEDGEMENTS
This work was supported in part by grants from The Leukemia & Lymphoma Society (7000-02) and NIH (K24 CA11787901) (DLP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990 Feb 1;75(3):555–562. [PubMed] [Google Scholar]
- 2.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. The New England journal of medicine. 1981 Jun 18;304(25):1529–1523. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 3.Drobyski WR, Keever CA, Roth MS, Koethe S, Hanson G, McFadden P, et al. Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood. 1993 Oct 15;82(8):2310–2318. [PubMed] [Google Scholar]
- 4.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995 Sep 1;86(5):2041–2050. [PubMed] [Google Scholar]
- 5.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997 Feb;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 6.Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. The New England journal of medicine. 1994 Jan 13;330(2):100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- 7.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007 Nov 1;25(31):4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 8.Miller JS, Weisdorf DJ, Burns LJ, Slungaard A, Wagner JE, Verneris MR, et al. Lymphodepletion followed by donor lymphocyte infusion (DLI) causes significantly more acute graft-versus-host disease than DLI alone. Blood. 2007 Oct 1;110(7):2761–2763. doi: 10.1182/blood-2007-05-090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Bunin N, Stadtmauer EA, Luger SM, Goldstein S, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006 Feb 15;107(4):1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 10.Lewalle P, Triffet A, Delforge A, Crombez P, Selleslag D, De Muynck H, et al. Donor lymphocyte infusions in adult haploidentical transplant: a dose finding study. Bone marrow transplantation. 2003 Jan;31(1):39–44. doi: 10.1038/sj.bmt.1703779. [DOI] [PubMed] [Google Scholar]
- 11.Lee CK, deMagalhaes-Silverman M, Hohl RJ, Hayashi M, Buatti J, Wen BC, et al. Donor T-lymphocyte infusion for unrelated allogeneic bone marrow transplantation with CD3+ T-cell-depleted graft. Bone marrow transplantation. 2003 Jan;31(2):121–128. doi: 10.1038/sj.bmt.1703803. [DOI] [PubMed] [Google Scholar]
- 12.Dey BR, McAfee S, Colby C, Sackstein R, Saidman S, Tarbell N, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003 May;9(5):320–329. doi: 10.1016/s1083-8791(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. The New England journal of medicine. 1994 Apr 28;330(17):1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 14.Bethge WA, Hegenbart U, Stuart MJ, Storer BE, Maris MB, Flowers ME, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004 Feb 1;103(3):790–795. doi: 10.1182/blood-2003-07-2344. [DOI] [PubMed] [Google Scholar]
- 15.Yoshihara S, Kato R, Inoue T, Miyagawa H, Sashihara J, Kawakami M, et al. Successful treatment of life-threatening human herpesvirus-6 encephalitis with donor lymphocyte infusion in a patient who had undergone human leukocyte antigen-haploidentical nonmyeloablative stem cell transplantation. Transplantation. 2004 Mar 27;77(6):835–838. doi: 10.1097/01.tp.0000119603.59880.47. [DOI] [PubMed] [Google Scholar]
- 16.Kishi Y, Kami M, Oki Y, Kazuyama Y, Kawabata M, Miyakoshi S, et al. Donor lymphocyte infusion for treatment of life-threatening respiratory syncytial virus infection following bone marrow transplantation. Bone marrow transplantation. 2000 Sep;26(5):573–576. doi: 10.1038/sj.bmt.1702559. [DOI] [PubMed] [Google Scholar]
- 17.Marks DI, Lush R, Cavenagh J, Milligan DW, Schey S, Parker A, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002 Nov 1;100(9):3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 18.van de Donk NW, Kroger N, Hegenbart U, Corradini P, San Miguel JF, Goldschmidt H, et al. Prognostic factors for donor lymphocyte infusions following non-myeloablative allogeneic stem cell transplantation in multiple myeloma. Bone marrow transplantation. 2006 Jun;37(12):1135–1141. doi: 10.1038/sj.bmt.1705393. [DOI] [PubMed] [Google Scholar]
- 19.Shiobara S, Nakao S, Ueda M, Yamazaki H, Takahashi S, Asano S, et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: indications and dose escalation. Ther Apher. 2001 Feb;5(1):40–45. doi: 10.1046/j.1526-0968.2001.005001040.x. [DOI] [PubMed] [Google Scholar]
- 20.Collins RH, Jr, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone marrow transplantation. 2000 Sep;26(5):511–516. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 21.Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002 Jan 15;20(2):405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 22.Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004 Jun 1;103(11):4362–4364. doi: 10.1182/blood-2003-11-3862. [DOI] [PubMed] [Google Scholar]
- 23.Guglielmi C, Arcese W, Dazzi F, Brand R, Bunjes D, Verdonck LF, et al. Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: prognostic relevance of the initial cell dose. Blood. 2002 Jul 15;100(2):397–405. doi: 10.1182/blood.v100.2.397. [DOI] [PubMed] [Google Scholar]
- 24.Porter DL, Collins RH, Jr, Hardy C, Kernan NA, Drobyski WR, Giralt S, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000 Feb 15;95(4):1214–1221. [PubMed] [Google Scholar]
- 25.van Rhee F, Savage D, Blackwell J, Orchard K, Dazzi F, Lin F, et al. Adoptive immunotherapy for relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: equal efficacy of lymphocytes from sibling and matched unrelated donors. Bone marrow transplantation. 1998 May;21(10):1055–1061. doi: 10.1038/sj.bmt.1701224. [DOI] [PubMed] [Google Scholar]
- 26.Ferster A, Bujan W, Mouraux T, Devalck C, Heimann P, Sariban E. Complete remission following donor leukocyte infusion in ALL relapsing after haploidentical bone marrow transplantation. Bone marrow transplantation. 1994 Aug;14(2):331–332. [PubMed] [Google Scholar]
- 27.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica. 2007 Mar;92(3):414–417. doi: 10.3324/haematol.10570. [DOI] [PubMed] [Google Scholar]
- 28.Pati AR, Godder K, Lamb L, Gee A, Henslee-Downey PJ. Immunotherapy with donor leukocyte infusions for patients with relapsed acute myeloid leukemia following partially mismatched related donor bone marrow transplantation. Bone marrow transplantation. 1995 Jun;15(6):979–981. [PubMed] [Google Scholar]
- 29.Spitzer TR, McAfee S, Sackstein R, Colby C, Toh HC, Multani P, et al. Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant. 2000;6(3A):309–320. doi: 10.1016/s1083-8791(00)70056-5. [DOI] [PubMed] [Google Scholar]
- 30.Mapara MY, Kim YM, Wang SP, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002 Sep 1;100(5):1903–1909. doi: 10.1182/blood-2002-01-0023. [DOI] [PubMed] [Google Scholar]
- 31.Pelot MR, Pearson DA, Swenson K, Zhao G, Sachs J, Yang YG, et al. Lymphohematopoietic graft-vs.-host reactions can be induced without graft-vs.-host disease in murine mixed chimeras established with a cyclophosphamide-based nonmyeloablative conditioning regimen. Biol Blood Marrow Transplant. 1999;5(3):133–143. doi: 10.1053/bbmt.1999.v5.pm10392959. [DOI] [PubMed] [Google Scholar]
- 32.Sykes M, Sheard MA, Sachs DH. Graft-versus-host-related immunosuppression is induced in mixed chimeras by alloresponses against either host or donor lymphohematopoietic cells. The Journal of experimental medicine. 1988 Dec 1;168(6):2391–2396. doi: 10.1084/jem.168.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan KM, Storb R, Buckner CD, Fefer A, Fisher L, Weiden PL, et al. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. The New England journal of medicine. 1989 Mar 30;320(13):828–834. doi: 10.1056/NEJM198903303201303. [DOI] [PubMed] [Google Scholar]
- 34.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992 Dec 15;80(12):2964–2968. [PubMed] [Google Scholar]
- 35.Alyea EP, Soiffer RJ, Canning C, Neuberg D, Schlossman R, Pickett C, et al. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood. 1998 May 15;91(10):3671–3680. [PubMed] [Google Scholar]
- 36.Ma SY, Au WY, Lie AK, Ng IO, Leung AY, Tse EW, et al. Liver graft-versus-host disease after donor lymphocyte infusion for relapses of hematologic malignancies post allogeneic hematopoietic stem cell transplantation. Bone marrow transplantation. 2004 Jul;34(1):57–61. doi: 10.1038/sj.bmt.1704522. [DOI] [PubMed] [Google Scholar]
- 37.Akpek G, Boitnott JK, Lee LA, Hallick JP, Torbenson M, Jacobsohn DA, et al. Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood. 2002 Dec 1;100(12):3903–3907. doi: 10.1182/blood-2002-03-0857. [DOI] [PubMed] [Google Scholar]
- 38.Fozza C, Szydlo RM, Abdel-Rehim MM, Nadal E, Goldman JM, Apperley JF, et al. Factors for graft-versus-host disease after donor lymphocyte infusions with an escalating dose regimen: lack of association with cell dose. British journal of haematology. 2007 Mar;136(6):833–836. doi: 10.1111/j.1365-2141.2007.06501.x. [DOI] [PubMed] [Google Scholar]
- 39.Kroger N, Shimoni A, Zagrivnaja M, Ayuk F, Lioznov M, Schieder H, et al. Low-dose thalidomide and donor lymphocyte infusion as adoptive immunotherapy after allogeneic stem cell transplantation in patients with multiple myeloma. Blood. 2004 Nov 15;104(10):3361–3363. doi: 10.1182/blood-2004-05-2031. [DOI] [PubMed] [Google Scholar]
- 40.Kernan NA, Collins NH, Juliano L, Cartagena T, Dupont B, O'Reilly RJ. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986 Sep;68(3):770–773. [PubMed] [Google Scholar]
- 41.Dazzi F, Szydlo RM, Craddock C, Cross NC, Kaeda J, Chase A, et al. Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood. 2000 Jan 1;95(1):67–71. [PubMed] [Google Scholar]
- 42.Mackinnon S, Papadopoulos EB, Carabasi MH, Reich L, Collins NH, Boulad F, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995 Aug 15;86(4):1261–1268. [PubMed] [Google Scholar]
- 43.Bacigalupo A, Soracco M, Vassallo F, Abate M, Van Lint MT, Gualandi F, et al. Donor lymphocyte infusions (DLI) in patients with chronic myeloid leukemia following allogeneic bone marrow transplantation. Bone marrow transplantation. 1997 May;19(9):927–932. doi: 10.1038/sj.bmt.1700762. [DOI] [PubMed] [Google Scholar]
- 44.Fowler DH, Breglio J, Nagel G, Eckhaus MA, Gress RE. Allospecific CD8+ Tc1 and Tc2 populations in graft-versus-leukemia effect and graft-versus-host disease. J Immunol. 1996 Dec 1;157(11):4811–4821. [PubMed] [Google Scholar]
- 45.Truitt RL, Atasoylu AA. Contribution of CD4+ and CD8+ T cells to graft-versus-host disease and graft-versus-leukemia reactivity after transplantation of MHC-compatible bone marrow. Bone marrow transplantation. 1991 Jul;8(1):51–58. [PubMed] [Google Scholar]
- 46.Korngold R, Sprent J. T cell subsets and graft-versus-host disease. Transplantation. 1987 Sep;44(3):335–339. doi: 10.1097/00007890-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Soiffer RJ, Gonin R, Murray C, Robertson MJ, Cochran K, Chartier S, et al. Prediction of graft-versus-host disease by phenotypic analysis of early immune reconstitution after CD6-depleted allogeneic bone marrow transplantation. Blood. 1993 Oct 1;82(7):2216–2223. [PubMed] [Google Scholar]
- 48.Nimer SD, Giorgi J, Gajewski JL, Ku N, Schiller GJ, Lee K, et al. Selective depletion of CD8+ cells for prevention of graft-versus-host disease after bone marrow transplantation. A randomized controlled trial. Transplantation. 1994 Jan;57(1):82–87. doi: 10.1097/00007890-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Champlin R, Giralt S, Przepiorka D, Ho W, Lee K, Gajewski J, et al. Selective depletion of CD8-positive T-lymphocytes for allogeneic bone marrow transplantation: engraftment, graft-versus-host disease and graft-versus leukemia. Progress in clinical and biological research. 1992;377:385–394. discussion 95–8. [PubMed] [Google Scholar]
- 50.Champlin R, Ho W, Gajewski J, Feig S, Burnison M, Holley G, et al. Selective depletion of CD8+ T lymphocytes for prevention of graft-versus-host disease after allogeneic bone marrow transplantation. Blood. 1990 Jul 15;76(2):418–423. [PubMed] [Google Scholar]
- 51.Giralt S, Hester J, Huh Y, Hirsch-Ginsberg C, Rondon G, Seong D, et al. CD8-depleted donor lymphocyte infusion as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation. Blood. 1995 Dec 1;86(11):4337–4343. [PubMed] [Google Scholar]
- 52.Meyer RG, Britten CM, Wehler D, Bender K, Hess G, Konur A, et al. Prophylactic transfer of CD8-depleted donor lymphocytes after T-cell-depleted reduced-intensity transplantation. Blood. 2007 Jan 1;109(1):374–382. doi: 10.1182/blood-2006-03-005769. [DOI] [PubMed] [Google Scholar]
- 53.Soiffer RJ, Alyea EP, Hochberg E, Wu C, Canning C, Parikh B, et al. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol Blood Marrow Transplant. 2002;8(11):625–632. doi: 10.1053/bbmt.2002.v8.abbmt080625. [DOI] [PubMed] [Google Scholar]
- 54.Kushida T, Inaba M, Hisha H, Ichioka N, Esumi T, Ogawa R, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001 May 15;97(10):3292–3299. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 55.Fukui J, Inaba M, Ueda Y, Miyake T, Hosaka N, Kwon AH, et al. Prevention of graft-versus-host disease by intra-bone marrow injection of donor T cells. Stem cells (Dayton, Ohio) 2007 Jun;25(6):1595–1601. doi: 10.1634/stemcells.2006-0234. [DOI] [PubMed] [Google Scholar]
- 56.Truitt RL, Johnson BD, Hanke C, Talib S, Hearst JE. Photochemical treatment with S-59 psoralen and ultraviolet A light to control the fate of naive or primed T lymphocytes in vivo after allogeneic bone marrow transplantation. J Immunol. 1999 Nov 1;163(9):5145–5156. [PubMed] [Google Scholar]
- 57.Waller EK, Ship AM, Mittelstaedt S, Murray TW, Carter R, Kakhniashvili I, et al. Irradiated donor leukocytes promote engraftment of allogeneic bone marrow in major histocompatibility complex mismatched recipients without causing graft-versus-host disease. Blood. 1999 Nov 1;94(9):3222–3233. [PubMed] [Google Scholar]
- 58.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science (New York, NY. 1997 Jun 13;276(5319):1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 59.Ciceri F, Bonini C, Marktel S, Zappone E, Servida P, Bernardi M, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007 Jun 1;109(11):4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 60.Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007 Jun 1;109(11):4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 61.Introna M, Barbui AM, Bambacioni F, Casati C, Gaipa G, Borleri G, et al. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Human gene therapy. 2000 Mar 1;11(4):611–620. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- 62.Thomis DC, Marktel S, Bonini C, Traversari C, Gilman M, Bordignon C, et al. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood. 2001 Mar 1;97(5):1249–1257. doi: 10.1182/blood.v97.5.1249. [DOI] [PubMed] [Google Scholar]
- 63.Clark RE, Dodi IA, Hill SC, Lill JR, Aubert G, Macintyre AR, et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001 Nov 15;98(10):2887–2893. doi: 10.1182/blood.v98.10.2887. [DOI] [PubMed] [Google Scholar]
- 64.Bocchia M, Korontsvit T, Xu Q, Mackinnon S, Yang SY, Sette A, et al. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood. 1996 May 1;87(9):3587–3592. [PubMed] [Google Scholar]
- 65.Falkenburg JH, Wafelman AR, Joosten P, Smit WM, van Bergen CA, Bongaerts R, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999 Aug 15;94(4):1201–1208. [PubMed] [Google Scholar]
- 66.Voogt PJ, Goulmy E, Veenhof WF, Hamilton M, Fibbe WE, Van Rood JJ, et al. Cellularly defined minor histocompatibility antigens are differentially expressed on human hematopoietic progenitor cells. The Journal of experimental medicine. 1988 Dec 1;168(6):2337–2347. doi: 10.1084/jem.168.6.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996 Oct 1;88(7):2450–2457. [PubMed] [Google Scholar]
- 68.Rosenfeld C, Cheever MA, Gaiger A. WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia. 2003 Jul;17(7):1301–1312. doi: 10.1038/sj.leu.2402988. [DOI] [PubMed] [Google Scholar]
- 69.Greiner J, Schmitt M, Li L, Giannopoulos K, Bosch K, Schmitt A, et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood. 2006 Dec 15;108(13):4109–4117. doi: 10.1182/blood-2006-01-023127. [DOI] [PubMed] [Google Scholar]


