Abstract
Objectives
Donor site morbidity, including pneumothorax, can be a considerable problem when harvesting cartilage grafts for laryngotracheal reconstruction (LTR). Tissue-engineered cartilage may offer a solution to this problem. This study investigated the feasibility of using Hyalograft C combined with autologous chondrocytes to tissue engineer cartilage grafts for LTR in rabbits.
Study Design
Animal study.
Methods
Eighteen New Zealand white rabbits underwent LTR: 12 rabbits received autologous tissue-engineered cartilage grafts and 6 animals, serving as a positive control group, native auricular cartilage. To determine any differences in response to the site of implantation and any potential immune response to the scaffold, a second piece of engineered neocartilage and a non-cell-loaded scaffold were inserted paralaryngeally into a subset of the rabbits. The rabbits were sacrificed 3, 6, 8, 10, and 12 weeks after the LTR and their larynx examined.
Results
None of the 18 rabbits showed signs of respiratory distress. A smooth, noninflammatory scar was visible intraluminally. Histologically, the native auricular cartilage implants showed excellent integration without any signs of inflammation or cartilage degradation. In contrast, all tissue-engineered grafts and empty scaffolds revealed marked signs of an unspecific foreign body reaction, leading to a complete degradation of the neocartilage, whether implanted para- or intralaryngeally.
Conclusion
In contrast to the success with which Hyalograft C has been applied in articular defect repair, our results indicate that, in rabbits, Hyalograft C initiates a foreign body reaction if implanted intra- or paralaryngeally, leading to cartilage degradation and possible graft failure. These findings suggest limitations on the environment in which Hyalograft C can be applied.
Keywords: Laryngotracheal reconstruction, cartilage implant, tissue engineering, subglottic stenosis, Hyalograft C, hyaluronan-based scaffold
INTRODUCTION
Treatment of laryngotracheal stenoses (LTS) in pediatric patients can be challenging, and, as a result, these infants often require tracheotomy. Despite advances in modern pediatric tracheostomy care, complications (including death) still occur.1 In this light, tracheotomy should be avoided whenever possible. Various techniques to enlarge the stenotic, subglottic space have been introduced. The traditional procedure for LTS has been laryngotracheal reconstruction (LTR) with anterior or posterior cartilage grafting, which has proven to be successful for mild and moderate graded stenoses in pediatric and adult patients.2,3 Costal cartilage is the preferred grafting material for LTR; however, costal cartilage harvest can produce comorbidity, such as pneumothorax, donor site pain, and scarring.4 Great progress has been made in the area of tissue engineering during the last decade and may provide significantly improved therapies to patients. The need to harvest costal cartilage can potentially be eliminated by combining chondrocytes with a biocompatible, biodegradable matrix to fabricate new living cartilage in vitro, which can then be used as a graft for LTR.
Hyaluronic acid (HA)-based scaffolds, such as Hyalograft C, have been widely used in the past in the treatment of articular cartilage defects. HA-containing scaffolds support the growth of chondrocytes in vitro and support the expression of their chondrogenic phenotype together with a high degree of biocompatibility by inducing formation of hyaline cartilage in the absence of inflammation in vivo.5,6 Hyalograft C has been approved by the Food and Drug Administration and has been used successfully for the treatment of articular cartilage defects in over 3,600 patients.7,8 However, until now, there are no reports of the use of Hyalograft C to engineer a cartilage graft for LTR. Previous experiments in this laboratory suggest that Hyalograft C is a suitable carrier matrix for in vitro fabrication of cartilage grafts from auricular chondrocytes and that the fabricated tissue is mechanically suitable for implantation in anterior grafting in LTR in rabbits.9 The purpose of this study was to determine the feasibility of using Hyalograft C to tissue-engineer cartilage grafts for LTR in rabbits.
MATERIALS AND METHODS
Chondrocyte Isolation and Expansion
Twelve New Zealand white adult male rabbits, weighing 3.0 to 3.5 kg and at 12 to 14 months of age, were used to harvest a 5 × 5 mm piece of auricular cartilage under sterile conditions. The perichondrium was carefully removed to minimize potential contamination with fibroblastic cells. The cartilage was then cut into approximately 1 mm3 pieces, enzymatically digested, and the chondrocytes were expanded, as previously described.9 The cells were frozen in expansion medium containing 10% dimethyl sulfoxide (Sigma, St. Louis, MO) and stored in liquid nitrogen for following experiments.
Preparation of Scaffold and Cell Seeding
Prior to the bioreactor culture, the chondrocytes were thawed and expanded in 175 cm2 culture flasks. Chondrocytes from the first passage were distributed onto a 10 × 10 × 2 mm Hyalograft C scaffold (Fidia Advanced Biopolymers, Terme, Italy) at a loading density of 4 million cells (in 150 μL of Bioreactor Medium) and allowed to attach for 2 hours at 37°C and 5% CO2, with the scaffolds flipped after 45 and 90 minutes.
Bioreactor Culture
The scaffold was placed into the bioreactor and cultured for 3 weeks, as previously described.9 The bioreactor was perfused with growth media at a rate of 0.25 mL an hour while mounted on a rocker (30° arc at 0.5 Hz) to improve mixing, with the entire system located inside an incubator at 37°C and 7.5% CO2. After 3 weeks, samples were removed from the bioreactor and used for LTR.
LTR With Anterior Cartilage Grafting
Eighteen New Zealand white rabbits underwent LTR with anterior cartilage grafting. The animal care committee of Case Western Reserve University, Cleveland, approved the protocol. In 12 animals, autologous tissue-engineered grafts were used (group I), and in the remaining 6 animals, which served as a positive control group, native auricular cartilage was implanted (group II). Costal cartilage of rabbits has proven to be an insufficient grafting material for LTR because of size limitations. An oval template, measuring 10 mm in length and 4 mm wide at the center and tapering toward the ends, was used to trim all native and engineered cartilage grafts to a standard size.
The rabbits were anesthetized by an intramuscular injection of ketamine hydrochloride (70 mg/kg) and xylazine (7 mg/kg), and the necks were shaved and disinfected with 10% povidoneiodine and 70% ethanol. A 3-cm midline incision was made, the strap muscles were separated, and the laryngotracheal segment exposed. An anterior, midline laryngofissure was made through the cricoid cartilage and the upper three tracheal rings followed by implantation of the graft into the defect. Perichondrium was harvested from the ear and was laid over the tissue-engineered cartilage implant for reinforcement, which allowed us to secure the graft tightly with 5–0 Vicryl sutures (Fig. 1). In 9 of the 12 rabbits of group I, a second piece of engineered cartilage with similar dimensions, but no perichondrium, was inserted next to the larynx to determine any possible differences in response to the site of implantation. An unexpected foreign body reaction observed at 3 weeks and 6 weeks prompted modification of the schedule for animal sacrifice to determine whether the tissue response was related to Hyalograft C scaffold material. The original study design was modified such that the remaining rabbits, which had not been operated on yet, were implanted with a 10 × 4 mm non–cell-loaded piece of the scaffold into a paralaryngeal pouch. For the following 3 days, the rabbits were given 2 mg dexamethasone and 10 mg/kg enrofloxacin as a prophylaxis. Post-operatively, the animals were observed for approximately 2 hours before returning to the cages, where water and standard feed were available. The animals were monitored carefully for any signs of respiratory distress, infection, pneumonia, or stridor. The rabbits were weighed weekly. The initial study design planned evaluation of the tissue-engineered grafts at 3, 6, and 12 weeks, with sacrifice of four rabbits of group I and two rabbits of group II at each time point. Because of an unexpected inflammatory response of the tissue-engineered grafts after 3 weeks, the time points for group I were modified to allow a more detailed chronological assessment of the immune reaction. Two rabbits were sacrificed after 3, 6, 8, 10, and 12 weeks. The rabbits were sedated and euthanized by an intracardiac injection of 150 mg/kg phenytoin sodium.
Fig. 1.
Laryngotracheal reconstruction with anterior interposition of engineered cartilage graft. An anterior incision was made through the cricoid cartilage and the upper tracheal ring. Left side of the graft has been sutured to the cricoid and the tracheal ring and is lifted up. Perichondrium, harvested from the ear, was attached to the graft for reinforcement. *Cricoid ring. TC = thyroid cartilage.
Endoscopy
The laryngotracheal segments of the 18 operated animals of group I and II were examined endoscopically and histologically and compared with nonoperated rabbits, which were available from a different research study. The rabbits were euthanized, examined endoscopically (0° optic, 2.6 mm diameter, Storz, Tuttlingen, Germany), and photographs of their laryngotracheal segments taken (Nikon D50, Melville, NY).
Histology
For histology, the laryngotracheal segment and the paralaryngeal implants (tissue-engineered cartilage and empty Hyalograft C scaffolds), if present, were removed and fixed in formalin. Because of an early degradation of the non–cell-loaded Hyalograft C, only three of the six implanted scaffolds could be harvested after 3 weeks. All specimens were then embedded in paraffin, cut into 5 μm thick sections, and stained with hematoxylin-eosin, toluidine blue, Masson's trichrome, and safranin-O. The specimens were examined on a bright field microscope (Leika DM6000B, Hamburg, Germany) and images recorded.
RESULTS
Clinical Findings
In total, 18 rabbits underwent LTR with anterior cartilage implantation. Six animals received a native auricular cartilage graft, serving as a positive control group, and in 12 experimental rabbits, a tissue-engineered cartilage graft was implanted. All animals survived without complications until they were sacrificed. None of the rabbits demonstrated any signs of stridor, respiratory distress, wound infection, or inflammatory reaction.
Endoscopy
None of the operated rabbits showed granulation tissue, chondromalacia, or subglottic collapse. Mucosa was intact, and there were no signs of inflammation in either of the groups. The site of graft implantation could be identified by a smooth scar in the subglottic space (Fig. 2). The reconstructed subglottic lumen was comparable with the nonoperated animals.
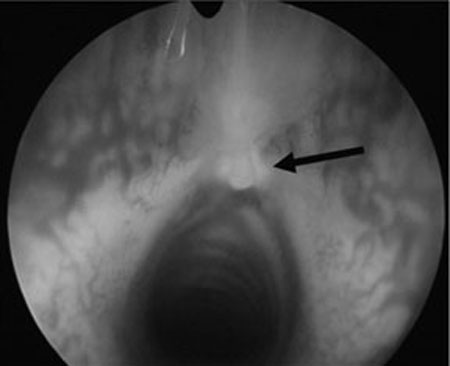
Fig. 2.
Endoscopic picture of the subglottis 12 weeks after implantation of tissue engineered graft. Implant is covered with respiratory mucosa. Shape and size of the subglottis are comparable with that of a nonoperated rabbit (arrow points toward implant).
Histology
All empty Hyalograft C implants provoked an inflammatory immune response. After 3 weeks, the empty scaffolds were completely invaded by macrophages, multinucleated foreign body cells, and fibroblasts, resulting in partial degradation of the scaffold (Fig. 3). This process led to complete absorption of Hyalograft C by 6 weeks. At 6 weeks, the site of implantation in the neck showed no macroscopic signs of inflammation, no formation of fibrous tissue, or other abnormal reaction that might be related to the implanted scaffold.
Fig. 3.
Non–cell-loaded Hyalograft C scaffold 3 weeks postimplantation at paralaryngeal site. Scaffold provoked a foreign body reaction with infiltration of inflammatory cells. Hyalograft C scaffold fibers (arrows) can be recognized, surrounded by macrophages.
In group II, the 3, 6, and 12 week animals all revealed a well-integrated native auricular cartilage graft with distinct neocartilage growth and no signs of inflammation. The grafts were covered with regular respiratory mucosa. The engineered grafts of group I suffered from erosion and degradation, leading to shrinkage and deformation of the graft (Fig. 4). All engineered cartilage grafts, whether implanted intra- or paralaryngeally, had provoked a non-specific foreign body reaction. Mononuclear leukocytes, macrophages, foreign body giant cells, and fibroblasts were observed. After 3 weeks, mononuclear monocytes, macrophages, and some multinucleated foreign body cells surrounded the implant and invaded the scaffold superficially. After 6 weeks, inflammatory cells penetrated the scaffold throughout, and a fibrous capsule had developed. Most of the tissue-engineered cartilage had degraded and was replaced by fibrous tissue, leading to a softening and deformation of the graft (Fig. 4A). After 8, 10, and 12 weeks, the chronic inflammation had gradually led to a complete chondrolysis of the tissue-engineered cartilage with some scaffold remaining, which was surrounded by dense fibrous tissue (Fig. 4B). In all cases, the implants were covered with respiratory mucosa.
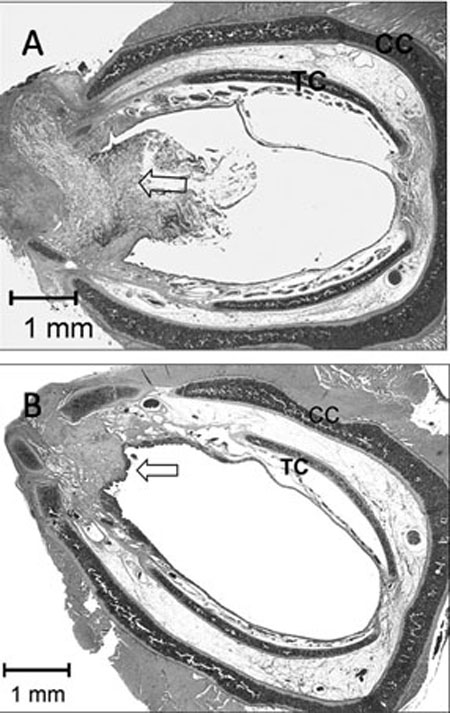
Fig. 4.
Histologic sections of operated cricoid ring after interposition of tissue engineered graft at 3 (A) and 12 (B) weeks. (A) After 3 weeks, large amounts of extracellular cartilage matrix have degraded, resulting in decreased biomechanical properties of the implant. Graft has deformed, resulting in S-curved shape. Most of the scaffold is still visible. (B) After 12 weeks, all neocartilage and most of the scaffold has been absorbed. As a result of foreign body reaction, fibrotic tissue has developed. Implant is covered with normal respiratory mucosa. Arrow = graft, TC = tracheal cartilage, CC = cricoid cartilage.
DISCUSSION
This rabbit model was designed to determine the suitability of autologous tissue-engineered cartilage grafts for LTR. Our findings indicate that Hyalograft C initiated an immune response, resulting in degradation and significant deformation of the cartilage graft. Hyalograft C has been widely studied, mainly for the purpose of articular cartilage repair. However, to the authors' knowledge, there are no previous reports on the use of Hyalograft C for tissue-engineering cartilage implants for LTR. Various scaffolds have been used for cartilage tissue engineering, such as alginate, chitosan, polyglycolic acid, or polylactic acid, with some of them causing a foreign body reaction if used in vivo.10–12 This inflammatory process may lead to a loss of the original three-dimensional shape of the engineered cartilage and a reduction of its initial mass. However, it has been shown that hyaluronan-based scaffolds, such as Hyalograft C, if used in joints, support the formation of new cartilage and that its degradation occurs without inflammatory response, which is essential for the success of grafting materials.5 Clinical studies have reported on the safety and efficacy of Hyalograft C scaffolds in managing articular cartilage defects in the knees.7,8
In the present study, the engineered grafts were used in a nonarticular environment, which may explain the less-promising results. Auricular chondrocytes were seeded on a Hyalograft C fleece and cultured in a perfusion bioreactor for 3 weeks. The engineered cartilage was used as an autologous implant for LTR in a rabbit model. The subglottic space and cartilaginous framework of the larynx in adult rabbits are comparable with human in fants and therefore may provide a suitable model for pediatric airway studies.
Rabbit auricular chondrocytes have shown to be an efficient cell source for cartilage tissue engineering. In a previous study from this laboratory, different chondrocyte subtypes (nasal, auricular, articular) were tested for their suitability to produce cartilage grafts in vitro for LTR in rabbits.9 Biomechanical testing and histologic, immunohistochemical, and biochemical assays indicated that only rabbit auricular chondrocytes produced tissue-engineered cartilage suitable for in vivo testing in LTR. However, surgical handling of the engineered grafts during implantation for LTR was different from native cartilage. The grafts appeared to be fragile and less resistant to tear forces, which was particularly evident during suturing. To overcome these issues, auricular perichondrium was used for reinforcement of the graft.
In this study, tissue-engineered cartilage grafts (group I) were compared with native auricular cartilage grafts (group II) for their efficacy for LTR in rabbits. In group II, the cartilage showed excellent incorporation without any inflammatory response. New cartilage had formed around the graft, which was covered with healthy respiratory mucosa, and the cricoid cartilage remained widened. None of the rabbits showed displacement of the graft, foreign body reaction, or cartilage degradation. In contrast, none of the tissue-engineered cartilage grafts succeeded in vivo despite their good biomechanical properties in vitro. Regardless of whether they were implanted para- or intralaryngeally, a nonspecific foreign body response was observed, which then lead to chondrolysis and replacement with fibrous tissue. Because similar histologic findings were also seen in the cell-free scaffolds, it appears that the foreign body response was related to the Hyalograft C and not to the cartilage matrix or the chondrocytes. These data demonstrate how critical degradable biomaterials are to the success of tissue-engineered grafts for LTR. After 3 weeks, a fibrous capsule had formed around the grafts and the plain scaffold. After 6 weeks in vivo, the engineered cartilage was largely replaced by fibrous tissue. There was a loss of the original three-dimensional shape and significant reduction of cartilage mass along with noticeable softening of the graft. In this study, graft failure was determined on histologic criteria rather than clinically because rabbits with a normal subglottic lumen were used. The cricoid split converged significantly, returning the cartilage framework to its original dimensions. If the same engineered graft had been implanted in a stenotic laryngotracheal segment, formation of granulation tissue, subglottic scarring, and ultimate restenosis would likely have resulted.
In this study, we tried to evaluate the response of engineered cartilage grafts related to two different environments. To address this issue in the same rabbit, engineered cartilage samples were implanted into two different locations. One cartilage graft was implanted into the laryngotracheal defect, where it was exposed to compression force, whereas the paralaryngeally inserted implant was placed in a nonloaded environment. Independent of the location, all implants elicited an immune response leading to a degradation of the cartilage. As a result, the effect of different implantation sites on the matrix synthesis of tissue-engineered cartilage could not be determined.
The question remains as to why Hyalograft C produces no observed inflammatory response when used in articular cartilage defects. Cartilage tissue is bradytrophic and avascular, suggesting that the immunologic environment in the joint is different from in the neck, where a rich blood supply is present and the scaffold is exposed not only to different cells but also at different differentiation stages and concentrations. Further study is indicated to understand the underlying causes of the inflammatory response observed in the present study as well as related differences in the response of different tissue environments to tissue-engineered cartilage grafts. However, it should be considered that the immune response in rabbits may differ from humans, and, therefore, no direct conclusion about an extraarticular application of Hyalograft C in humans can yet be made.
CONCLUSION
Living cartilage grafts can be fabricated in vitro using Hyalograft C as a scaffold. However, Hyalograft C initiates a foreign body reaction in rabbit larynges, leading to degradation of engineered cartilage and graft failure. Our results stand in contrast to other animal and clinical studies that have reported a favorable microenvironment of these scaffolds for the reparative process of articular cartilage defects.
Acknowledgments
The authors thank Nell Ginley and Lisa Walsh for their excellent technical assistance, Jean Welter and Kitsie Penick for their help with the bioreactors, and Fidia for providing the Hyalograft C.
This study was supported by the National Health Grant RO1 DE 015322-01.
Footnotes
James H. Henderson is a recipient of an Arthritis Foundation Post-doctoral Fellowship.
BIBLIOGRAPHY
- 1.Carr MM, Poje CP, Kingston L, Kielma D, Heard C. Complications in pediatric tracheostomies. Laryngoscope. 2001;111:1925–1928. doi: 10.1097/00005537-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Cotton RT, Gray SD, Miller RP. Update of the Cincinnati experience in pediatric laryngotracheal reconstruction. Laryngoscope. 1989;99:1111–1116. doi: 10.1288/00005537-198911000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Schick B, Weidenbecher M, Miller R, Iro H. Experience with laryngotracheal reconstruction in subglottic stenosis in a 30 years time period. Laryngorhinootologie. 2007;86:358–364. doi: 10.1055/s-2006-945002. [DOI] [PubMed] [Google Scholar]
- 4.Lusk RP, Kang DR, Muntz HR. Auricular cartilage grafts in laryngotracheal reconstruction. Ann Otol Rhinol Laryngol. 1993;102:247–254. doi: 10.1177/000348949310200402. [DOI] [PubMed] [Google Scholar]
- 5.Grigolo B, Roseti L, Fiorini M et al. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001;22:2417–2424. doi: 10.1016/s0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 6.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17:205–213. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 7.Marcacci M, Berruto M, Brocchetta D et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96–105. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 8.Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3–8. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Henderson JH, Welter JF, Mansour JM, Niyibizi C, Caplan AI, Dennis JE. Cartilage tissue engineering for laryngotracheal reconstruction: comparison of chondrocytes from three anatomic locations in the rabbit. Tissue Eng. 2007;13:843–853. doi: 10.1089/ten.2006.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britt JC, Park SS. Autogenous tissue-engineered cartilage: evaluation as an implant material. Arch Otolaryngol Head Neck Surg. 1998;124:671–677. doi: 10.1001/archotol.124.6.671. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297–304. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Zhang M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J Biomed Mater Res A. 2005;75:485–493. doi: 10.1002/jbm.a.30449. [DOI] [PubMed] [Google Scholar]