Abstract
In the rat kidney, aquaporin (AQP) 6 is localized in the intracellular vesicle membranes of type-A intercalated cells of the collecting duct; mouse AQP6 (mAQP6) has not been characterized. Although mAQP6 was originally cloned from cDNA in a mouse cerebellum library (GenBank NM 175087), we have independently cloned a cDNA encoding mAQP6 from an adult kidney cDNA library (C57BL/6J strain). We identified two different spliced variants of mAQP6: mAQP6a and mAQP6b. The mAQP6a isoform is almost identical to rat AQP6, whereas mAQP6b is identical to that reported in the mouse cerebellum library mentioned above. We found that the mRNA expression of these two spliced variants is regulated in a tissue-specific and age-dependent manner. Functional analyses of water and ion permeation revealed that mAQP6a functions like rat AQP6 and that mAQP6b does not function as either a water channel or an ion channel under our experimental conditions.
Keywords: Aquaporin, splice variant
1. Introduction
The aquaporins (AQPs) are a family of membrane proteins that largely function as water channels [1]. In mammals, 13 members of the AQP family (AQP0-12) have been reported [2]. AQP6 has been isolated from rat and human kidneys [3, 4], and in rat studies we have shown that AQP6 is functionally distinct from other known AQPs in the following ways. First, AQP6 is localized in intracellular membranes in type-A intercalated cells in the kidney [5]. Second, AQP6 functions not as a water channel but as an anion channel [5–7]: ion permeability, including that of hydronium ions, is not a general feature of AQPs[7, 8]. Third, AQP6 is activated by Hg2+, a well-known water channel inhibitor [1]. AQP6 has also been cloned from mouse cDNA (GenBank NM 175087) derived from the cerebellum. Interestingly, we found that the C-terminal domain of this clone exhibits no significant homology to known sequences of rat or human AQP6; also, no rat or human AQP6 has been reported in the cerebellum. Furthermore, no studies to date have examined the function of mouse AQP6 (mAQP6). To address these issues and provide a foundation for further studies of AQP6, we independently cloned mAQP6 from an adult kidney cDNA library (C57BL/6J strain). We identified two spliced variants of mAQP6. The two isoforms display distinct tissue-specific distribution, developmental regulation, and functional characteristics.
2. Materials and methods
2.1 Isolation of mAQP6 Isoforms
Primers for the full length of the mAQP6 coding region were designed from the cDNA retrieved from GenBank (accession no. NM 175087) with the EcoRI site as the upstream primer and the NheI site as the downstream primer. The sequence of the upstream primer was 5′-ATAGAATTCCATGGAGCCAGGGCTGTGTAGC-3′ and that of the downstream primer was 5′-AATAGCTAGCTCAGCAAAGGCCAAGCGTGAATG-3′. All animal experiments were conducted under protocols approved by the Johns Hopkins Animal Care and Use Committee. Total RNA was extracted from the kidneys of adult (3- to 4-month-old) C57BL/6J mice using the RNeasy kit with optional DNase treatment (Qiagen, Valencia, CA). An aliquot of 5 μg of total RNA was then used for cDNA synthesis with the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA), and 2.5 μL of the resulting cDNAs was subsequently amplified using the Expand High Fidelity PCR system (Roche Applied Science, Penzberg, Germany). All PCR products were purified with a Qiaquick Gel Extraction Kit (Qiagen) and subcloned into the multiple cloning regions of pXβG-myc and pcXβG3 vectors at the EcoRI and NheI sites. The pcXβG3 vector had been constructed by cloning the Xenopus β-globin 5′- and 3′-untranslated regions into the pcDNA3 vector (and was kindly supplied by Michael Caterina, Johns Hopkins University, Baltimore, MD, USA). The pXβG-myc vector was constructed by adding a c-myc tag at the BglI site of the pXβG vector. Sequencing was used to verify the DNA sequence of the constructs.
2.2 Analysis of mAQP6 mRNA expression using RT-PCR
RNA extraction and RT-PCR was performed as described above using cerebella and kidneys from 1-day-old (P1) and adult mice. This time 2 μL of the resulting cDNAs was amplified with primers synthesized according to the sequence of mAQP6 cDNA. The upstream primer used for mAQP6 cDNA was 5′-ACTGGCTGTTCCATGAACCC-3′, and the downstream primer was 5′-AGGAAGTGGCCAGGAGGTACT-3′. The PCR was cycled 40 times, using a 1-minute, 94°C denaturing step; a 1-minute, 61°C annealing step; and a 1-minute, 72°C extension step. The PCR products were visualized by ethidium bromide staining and electrophoresis in 2% agarose.
2.3 Expression in Oocytes and Measurement of Pf
Capped cRNAs were synthesized in vitro from XbaI-linearized pcXβG3 plasmids with T7 RNA polymerase and purified with the RNeasy kit (Qiagen). Five nanograms (50 nL) of cRNA or 50 nL of diethyl pyrocarbonate-treated water was injected into defolliculated mature Xenopus laevis oocytes (controls). The oocytes were incubated for 2 to 3 days at 18°C in 200 mOsm modified Barth’s solution (MBS). An oocyte-swelling assay was used to measure Osmotic water permeability (Pf) was measured from the time course of oocytes swelling in response to a 3-fold dilution of MBS with distilled water as in previous studies [9]. For Hg2+ studies, oocytes were preincubated for 5 minutes in 200 mOsm MBS containing 0.5 mM HgCl2 before the swelling assay, whereas the controls were preincubated without HgCl2.
2.4 Oocyte Immunofluorescence and Confocal Microscopy
Three days after injection oocytes were incubated in fixing solution (80 mM Pipes [pH 6.8], 5 mM EGTA, 1 mM MgCl2, 3.7% formaldehyde, and 0.2% Triton X-100) at room temperature for 4 hours, transferred to methanol at −20°C for 24 hours, equilibrated in PBS at room temperature for 2 hours, incubated in PBS with 100 mM NaBH4 at room temperature for 24 hours, and bisected with blades. The oocytes were blocked by 2% BSA in PBS for 1 hour at room temperature, incubated at 4°C sequentially with rabbit anti-AQP6 antibody [10] and Alexa Fluor 488 goat anti-rabbit IgG (invitrogen) in blocking buffer (each for 24 hours), and mounted in Fluoromount-G (SouthernBiotech, Birmingham, AL, USA). Micrographs were obtained with a confocal laser-scanning microscope (UltraView LCI, PerkinElmer, Wellesley, MA, USA).
2.5 Oocyte Membrane Extraction and Immunoblotting
Ten oocytes were homogenized by pipetting them up and down in hypotonic lysis buffer (7.5 mM sodium phosphate, 1 mM EDTA, pH 7.5) including a protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO, USA). The oocyte yolk was removed by discarding the pellet after centrifugation at 735g and 4°C for 10 minutes. The supernatant was centrifuged again at 200,000g and 4°C for 1 hour; the membrane was harvested by collecting the pellet. The oocyte membrane was solubilized with 2% SDS, normalized by total protein amount following the BCA method (Pierce, Rockford, IL, USA), and subjected to 12% SDS PAGE. The proteins were transferred to a polyvinylidene-difluoride) membrane, probed with a rabbit anti-rat AQP6 antibody and a horseradish peroxidase-conjugated donkey anti-rabbit IgG (Amersham Biosciences, Pittsburgh, PA, USA). An enhanced chemiluminescence detection system (ECL-plus, Amersham Biosciences) was used to visualize the specific immunoreactive proteins by exposure to autoradiographic films.
2.6. Electrophysiological measurements of oocytes were performed with iso-osmotic NaCl solution (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM Hepes [pH 7.5]) or iso-osmotic NaNO3 solution (100 mM NaNO3, 2 mM KCl, 1 mM MgCl2, and 5 mM Hepes [pH 7.5]). The membrane potential of oocytes was controlled by the two-microelectrode voltage clamp technique. The command voltage was applied with a two-microelectrode voltage clamp amplifier (Axoclamp-2A, Axon Instruments, Foster City, CA) controlled by an IBM-compatible computer running pCLAMP software (Axon Instruments). Current signals were sampled at 100 μsec. In most experiments the membrane potential was held at Vhold = −50 mV. To determine the current-voltage relationship, the membrane potential was rapidly stepped up from the holding potential to a series of values generating between +50 and −130 mV, each differing by 20 mV. The pulse duration was 100 msec, and currents from 10 runs were averaged to reduce noise. All measurements were performed at room temperature.
3. Results
By RT-PCR experiments using mouse kidney cDNA as a template, we obtained two different full-length cDNA fragments. One of them (named mAQP6a) (GenBank DQ 826418) was almost identical to rAQP6, whereas the other clone (named mAQP6b) was identical to the previously reported mAQP6 (GenBank NM 175087). The mAQP6a cDNA results from a retention of intron 4, creating a novel 3′ end (Fig. 1A). Nucleotide sequencing analysis revealed that the mAQP6 gene encodes proteins with 276 and 293 amino acid residues and that the difference is observed only at the C-terminus (Fig. 1B). The mAQP6b C-terminal domain exhibits no significant homology to known sequences from other AQPs.
Fig. 1.
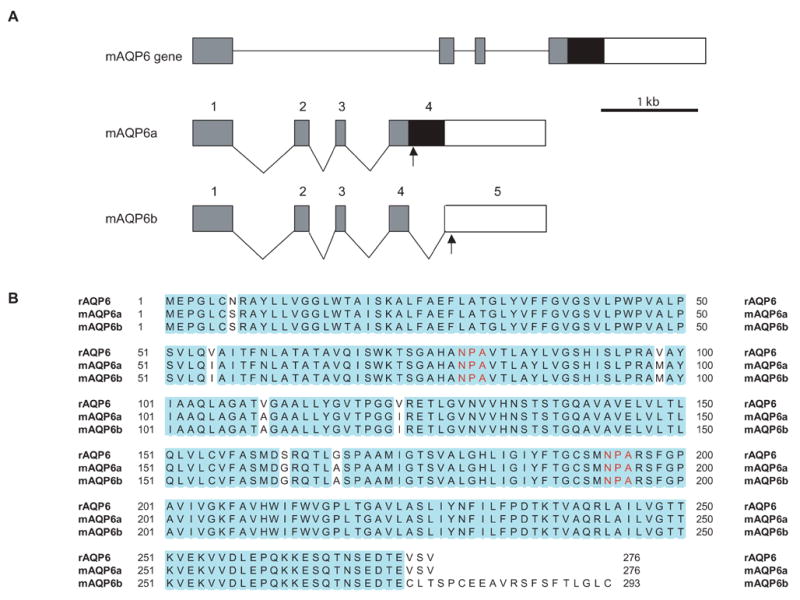
Isolation of mAQP6 splicing variants. A: Exon-intron organization of the mouse AQP6 gene. A segment of the genomic AQP6 clone is shown at the top of the figure. The gray rectangles represent common translated regions of AQP6. Exon 4 of mAQP6a contains a black rectangle with a stop codon in it (arrow). mAQP6b has another intron (intron 4) and exon 5; the stop codon is in exon 5. B: Sequence alignments of rat AQP6, mAQP6a, and mAQP6b. The blue residues are identical. Red ‘NPA’s are a motif of AQPs.
Expression of mAQP6 in the cerebellum and kidney
To investigate the age-related and tissue-specific expression of mAQP6a and mAQP6b, we performed RT-PCR with primers that are designed to amplify 727-bp and 364-bp fragments for mAQP6a and mAQP6b, respectively (Fig. 2). In the cerebellum, mAQP6b was detected only in P1 mice, from which mAQP6b was originally cloned, whereas mAQP6a was expressed not in the P1 but in the adult cerebellum. In the kidney, mAQP6a and mAQP6b were expressed in both P1 and adult mice. Expression of mAQP6a was higher in adult mice, in both the cerebellum and kidney.
Fig. 2.
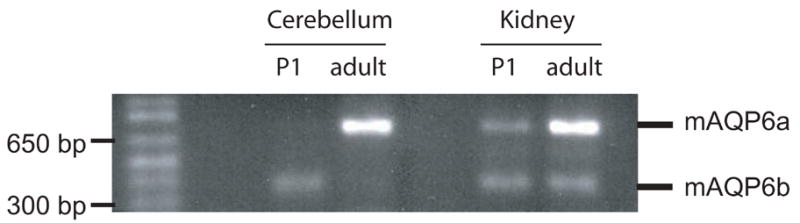
Developmental regulation of mAQP6 transcripts. RT-PCR analysis of mAQP6 expression in the mouse kidney and cerebellum. Total RNA of different developmental points; neonatal (P1) and adult mice subjected to RT-PCR using specific primers for mAQP6.
Functional studies
The functional properties of mAQP6 were evaluated by expression in Xenopus oocytes. Swelling was monitored after the oocytes had been transferred from 200 mOsm to 70 mOsm modified Barth’s solution, and the coefficients of osmotic water permeability (Pf) were calculated. Like rAQP6, mAQP6a has low water permeability, which is activated by Hg2+ (Fig. 3A). On the other hand, Hg2+ does not activate mAQP6b, which also has low basal water permeability. We evaluated the ionic permeation of oocytes expressing mAQP6 using a two-electrode voltage clamp. Oocytes expressing mAQP6a exhibit current under Hg2+ treatment, which is also the case with of oocytes expressing rAQP6 (Fig. 3B). On the other hand, of oocytes expressing mAQP6b did not exhibit any current either with or without Hg2+ treatment. Confocal microscopy demonstrated that both mAQP6a and mAQP6b are located at the oocyte plasma membrane (Fig. 4A). It should be noted that the anti-AQP6 antibody recognized a 30-kDa and a 28-kDa band from oocytes into which mAQP6a had been injected and also recognized a 31-kDa and a 29-kDa band from oocytes into which mAQP6b had been injected (Fig. 4B).
Fig. 3.
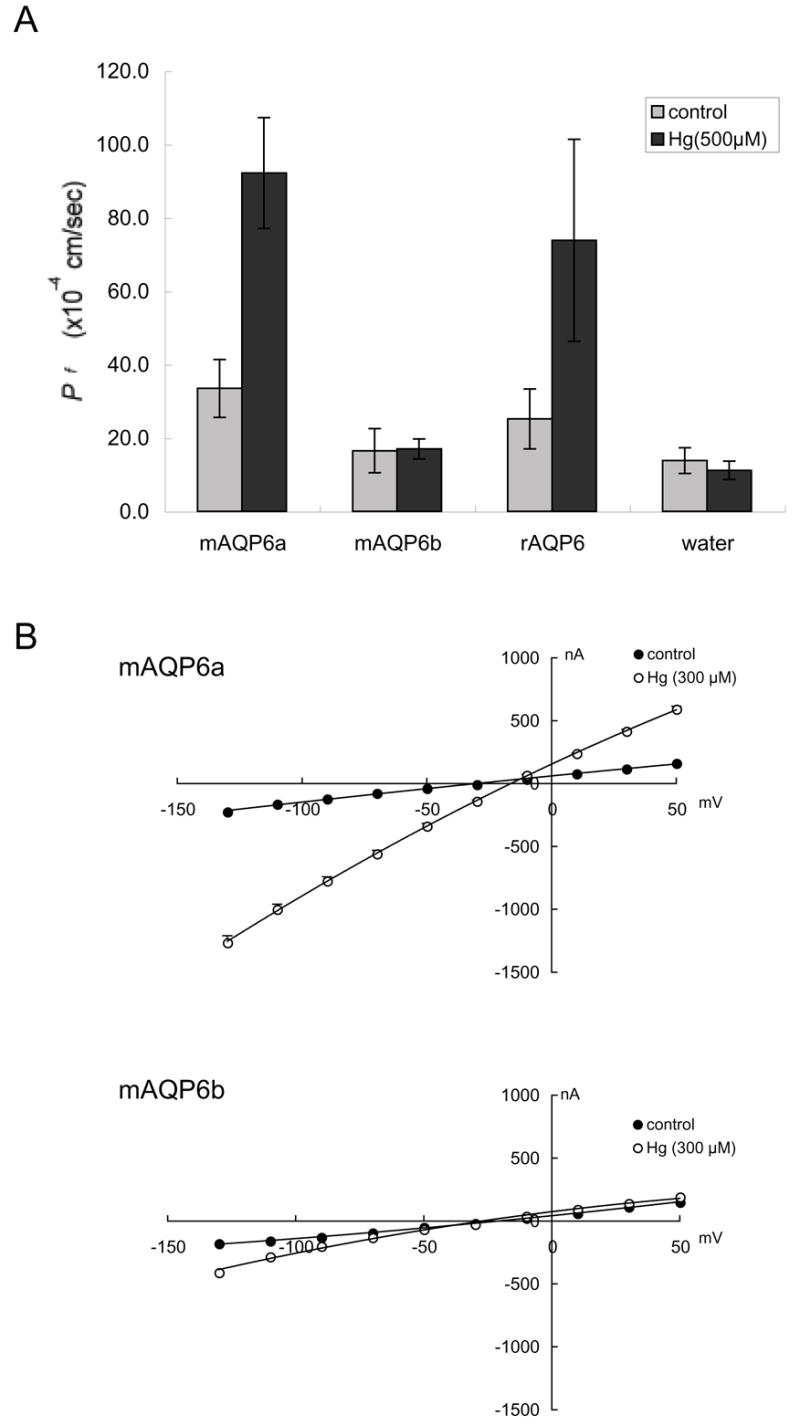
A: The coefficient of osmotic water permeability (Pf) of mAQP6a, mAQP6b, and rAQP6. Mercury treatment activated water permeability in mAQP6a and rAQP6 but not in mAQP6b. B: Electrophysiological analyses of mAQP6a and mAQP6b. One-volt curves between +50 mV and −130 mV were plotted for data obtained from mAQP6a (upper panel) and mAQP6b (lower panel) oocytes. Electrophysiological experiments were performed with a two-electrode voltage clamp system. The membrane potential was held at −50 mV and rapidly stepped up to test potentials from +50 mV to −130 mV at 20-mV intervals. Currents at each voltage were measured with pClamp software.
Fig. 4.
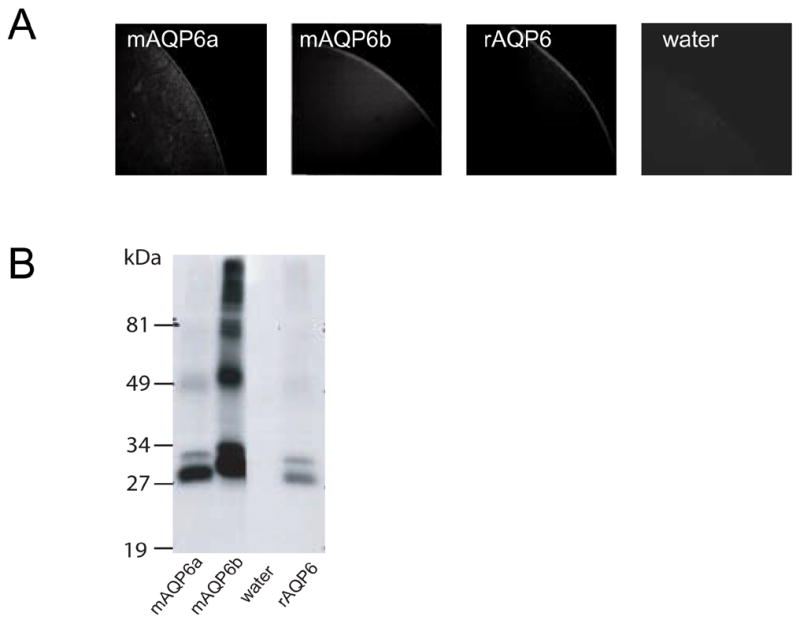
mAQP6/rAQP6 expressed in oocytes. A: Confocal microscope images of oocytes into which water, mAQP6a, mAQP6b, or rAQP6 had been injected. The oocytes were stained with an anti-rat AQP6 antibody. B: Immunoblotting of membrane fractions from oocytes into which mAQP6a, mAQP6b, water, or rAQP6 had been injected.
4. Discussion
We have previously characterized rat AQP6 [5–7, 10] and found that it is localized in the acid-secreting cells of the renal collecting ducts [10] and functions as an anion channel [5–7]. Mouse AQP6 was cloned from a cerebellum library during a large-scale full-length cDNA expression analysis in mice carried out by RIKEN as part of the FANTOM project (GenBank NM 175087) [11, 12]. The FANTOM project represents one of the most comprehensive surveys of a mammalian transcriptome using a full-length cDNA set. However, as was pointed out at the time, it is necessary to perform experimental analyses to verify the functions of genes predicted by computational analyses. The primary sequence revealed that this clone is very different from that of rat or human AQP6. In this study we independently cloned and characterized mAQP6. We found that a new spliced variant of mAQP6 resulted from intron retention and an alternative in-frame stop codon. The new isoform (termed mAQP6a) is almost identical to rat AQP6, whereas the other clone (here called mAQP6b), which has a different C-terminal amino acid, is identical to the one reported in the RIKEN study. Interestingly, we found that both function and distribution differ between mAQP6a and mAQP6b. Sequence homology showed that when expressed in oocytes mAQP6a, like rat AQP6, is activated by Hg2+ and permeated by ions, whereas mAQP6b does not function either as a water channel or an ion channel. The abundance of mAQP6b in neonatal mouse cerebella is consistent with the isolation of the previously reported mouse AQP6 clone from the cerebella of neonatal mice.
We also found that the expression of the mRNA of these two splicing variants is regulated in a tissue-specific and age-related manner, suggesting that mAQP6 may be important during development. The expression of mAQP6a and mAQP6b in the cerebellum is provocative. Immunocytochemical studies with mAQP6-specific antibodies will be needed to determine the physiological relevance of AQP6 to cerebellar function.
Functional analysis revealed that mAQP6a functions as an anion channel, as does rat AQP6. Although mAQP6b was expressed in the plasma membranes of oocytes, we could not demonstrate any water or ion transport. Thus, the biological function of mAQP6b remains unclear. The C-terminus of mAQP6b has a putative casein kinase II phosphorylation site at Ser 277, which may regulate channel gating. One could speculate that mAQP6b forms a heterologous oligomer with mAQP6a, which may have a dominant negative effect on both proteins. However, when mAQP6a and mAQP6b were expressed together in oocytes, no dominant negative effects were detected (data not shown).
Our immunofluoresence studies revealed that both mAQP6a and mAQP6b are expressed in the plasma membranes of oocytes. Although the C-terminus of rat AQP6 is presumably less important for trafficking than is the N-terminus [6], these two isoforms might traffic differently when expressed in mammalian cells. To clarify the precise distribution of mAQP6b, we plan to develop an antibody that recognizes mAQP6b but not mAQP6a. mAQP6b may have distinct cellular distributions and functions during development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels--from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh T, Rai T, Kuwahara M, Ko SB, Uchida S, Sasaki S, Ishibashi K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res Commun. 2005;330:832–838. doi: 10.1016/j.bbrc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Ma T, Frigeri A, Skach W, Verkman AS. Cloning of a novel rat kidney cDNA homologous to CHIP28 and WCH-CD water channels. Biochem Biophys Res Commun. 1993;197:654–659. doi: 10.1006/bbrc.1993.2529. [DOI] [PubMed] [Google Scholar]
- 4.Ma T, Yang B, Kuo WL, Verkman AS. cDNA cloning and gene structure of a novel water channel expressed exclusively in human kidney: evidence for a gene cluster of aquaporins at chromosome locus 12q13. Genomics. 1996;35:543–550. doi: 10.1006/geno.1996.0396. [DOI] [PubMed] [Google Scholar]
- 5.Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402:184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem. 2002;277:39873–39879. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- 7.Hazama A, Kozono D, Guggino WB, Agre P, Yasui M. Ion permeation of AQP6 water channel protein. Single channel recordings after Hg2+ activation. J Biol Chem. 2002;277:29224–29230. doi: 10.1074/jbc.M204258200. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti N, Roux B, Pomes R. Structural determinants of proton blockage in aquaporins. J Mol Biol. 2004;343:493–510. doi: 10.1016/j.jmb.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 10.Yasui M, Kwon TH, Knepper MA, Nielsen S, Agre P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc Natl Acad Sci U S A. 1999;96:5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai J, et al. Functional annotation of a full-length mouse cDNA collection. Nature. 2001;409:685–690. doi: 10.1038/35055500. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki Y, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]


