Abstract
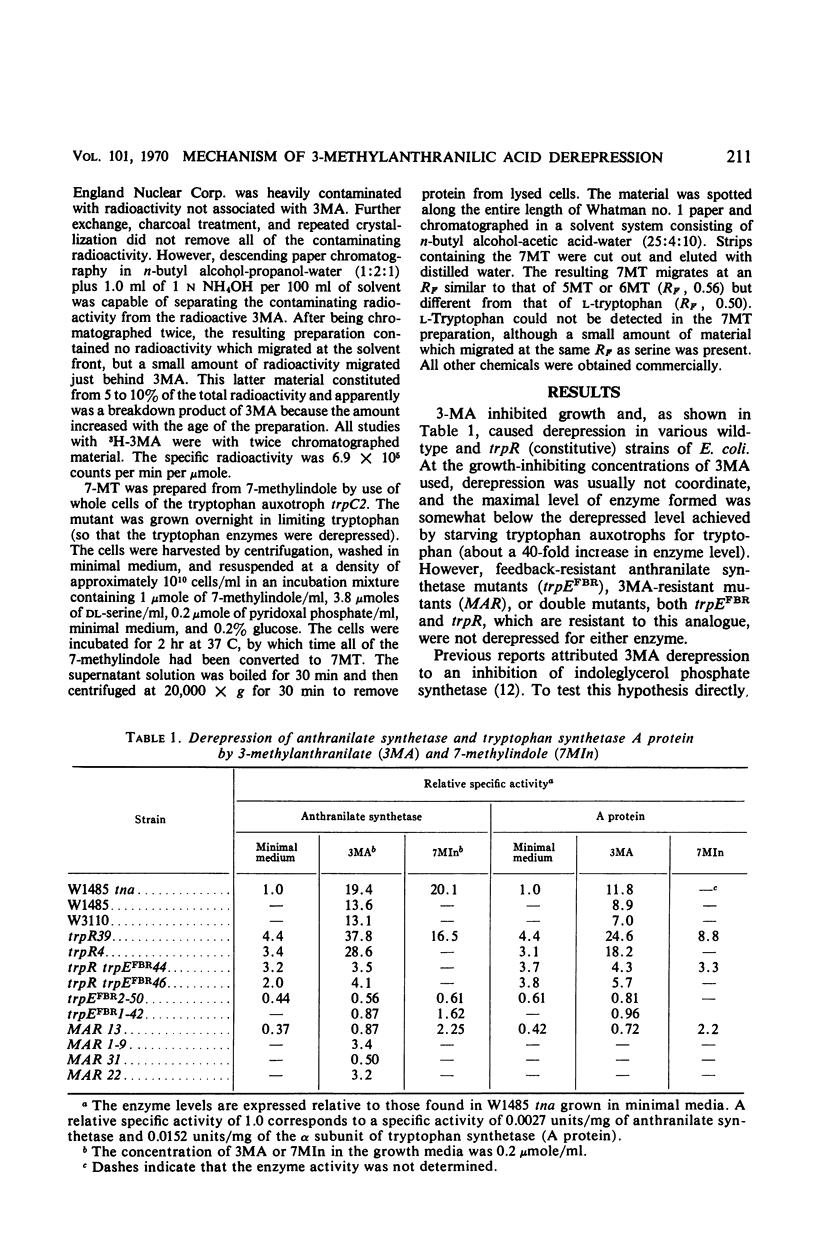
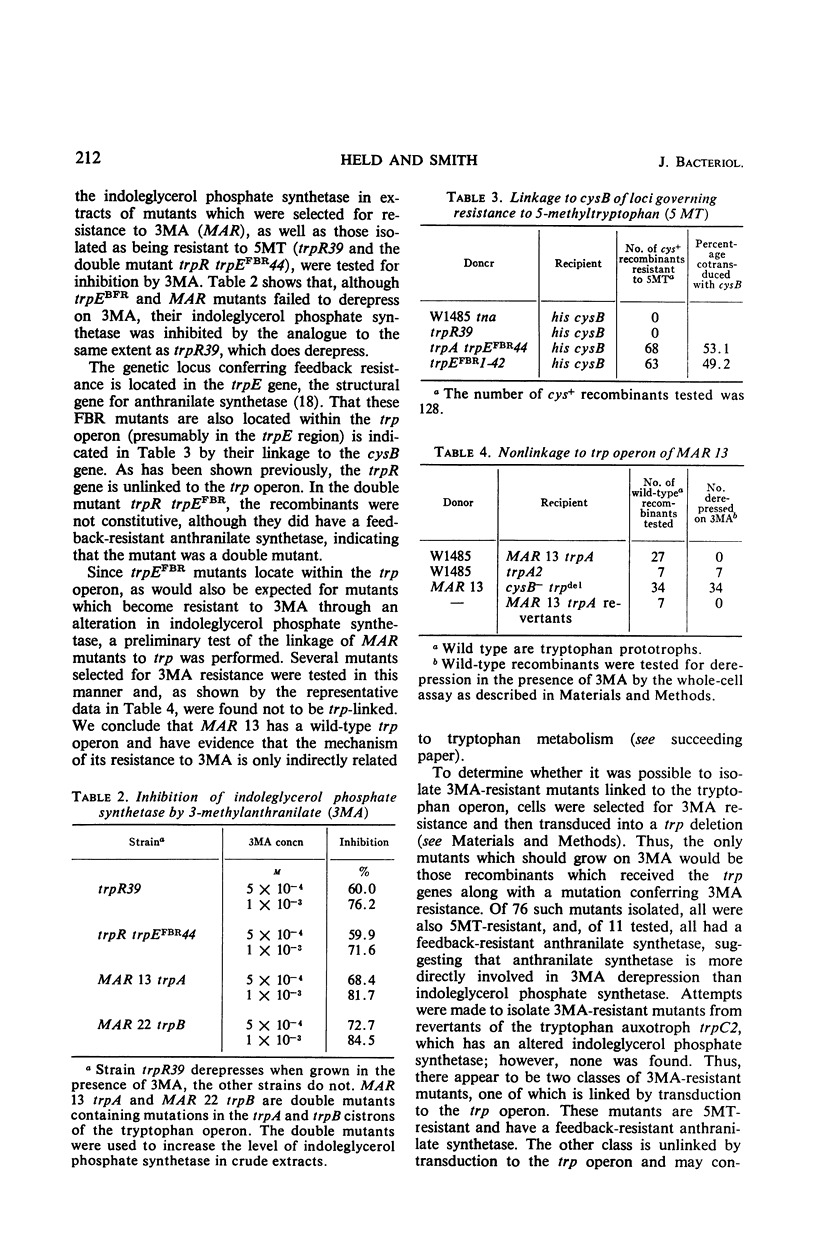
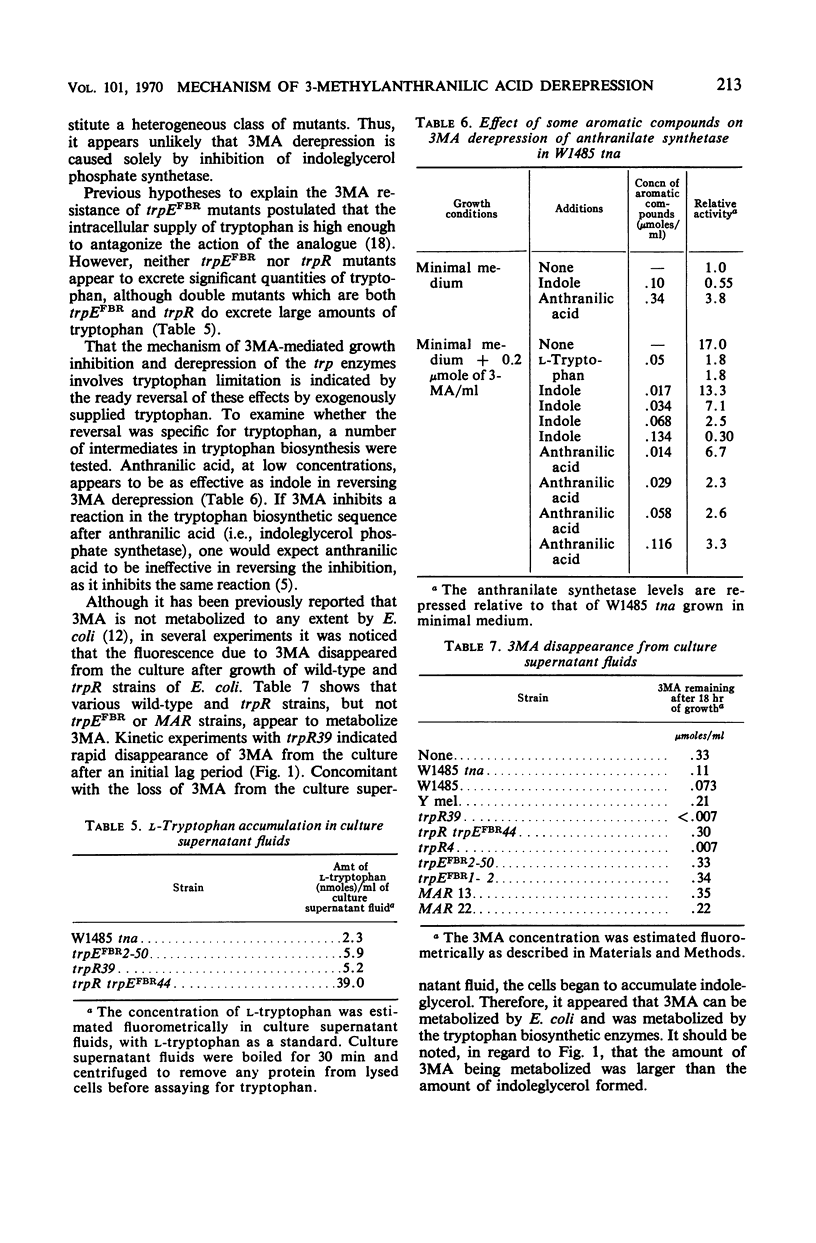
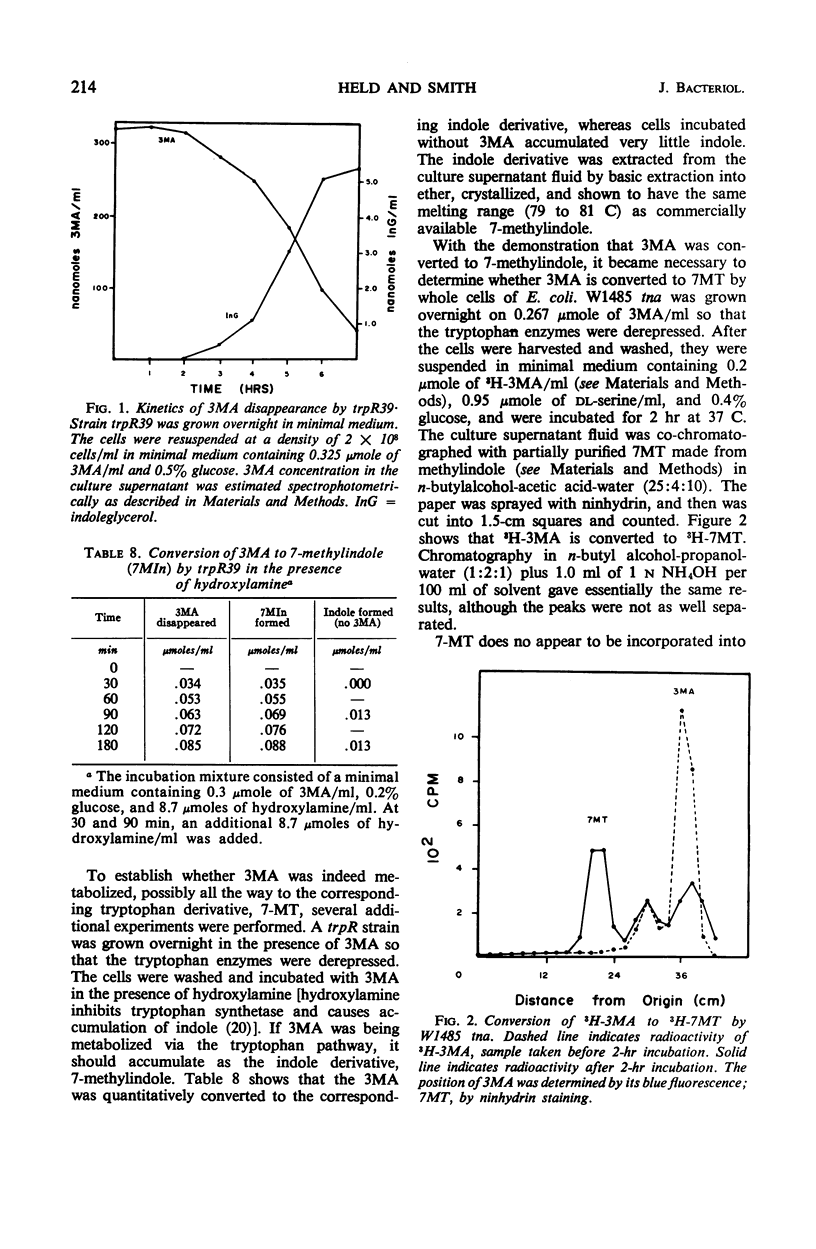
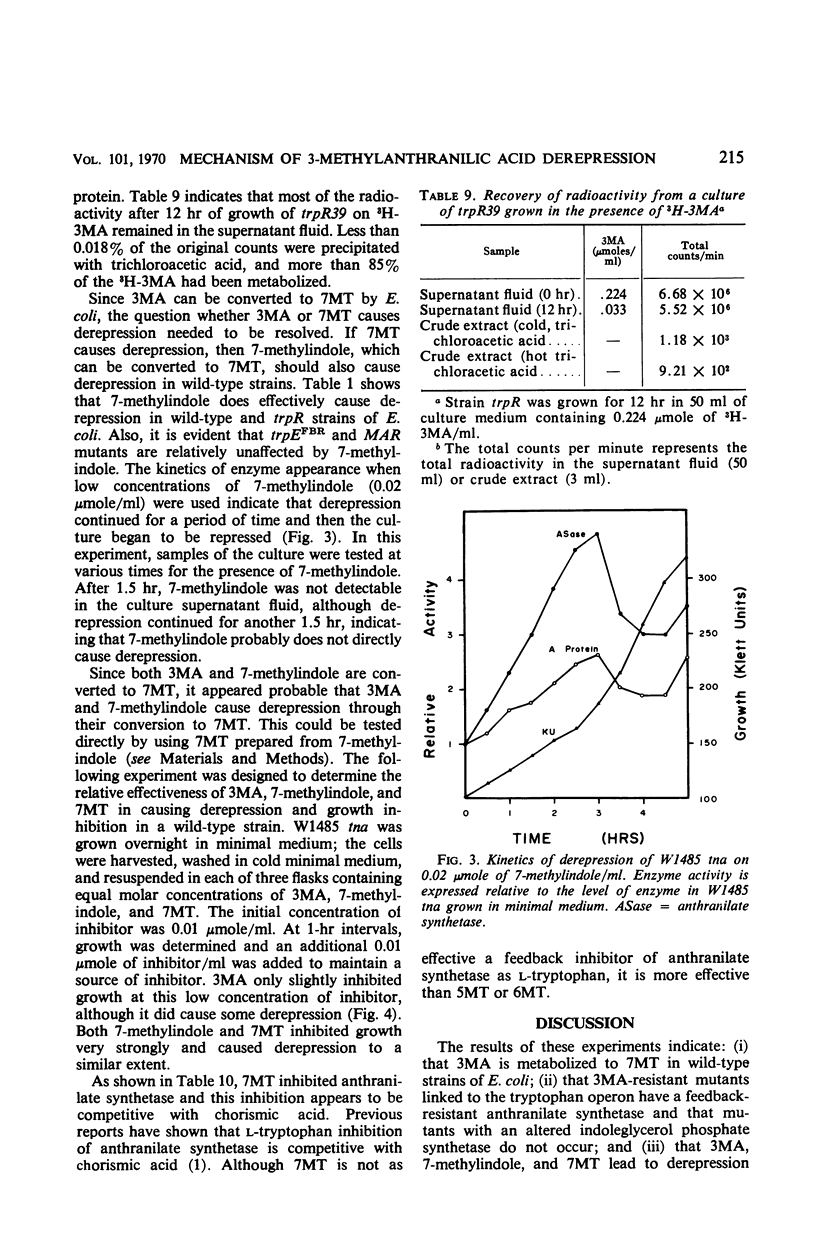
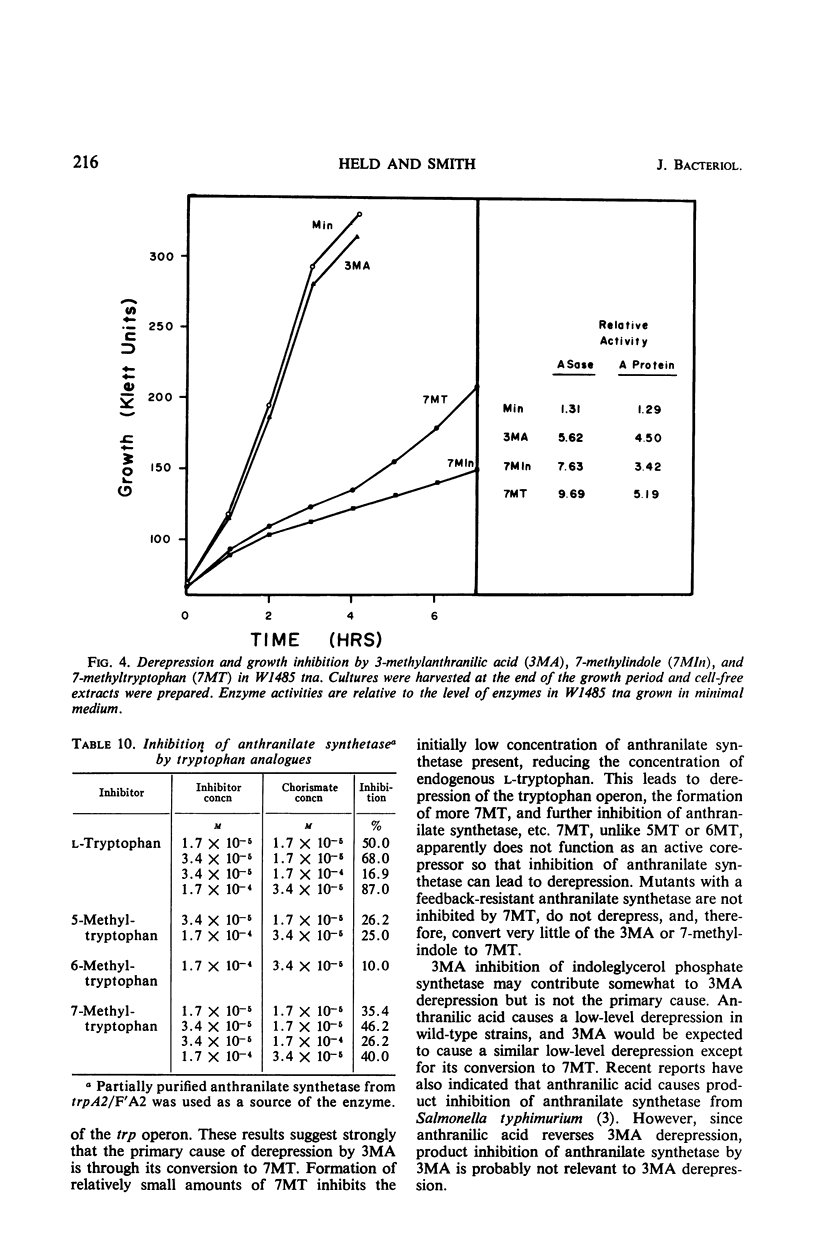
3-Methylanthranilic acid (3MA) inhibits growth and causes derepression of the tryptophan biosynthetic enzymes in wild-type strains of Escherichia coli. Previous reports attributed this effect to an inhibition of the conversion of 1-(o-carboxyphenylamino)-1-deoxyribulose 5-phosphate to indole-3-glycerol phosphate and a consequent reduction in the concentration of endogenous tryptophan. Our studies have shown that 3MA-resistant mutants linked to the tryptophan operon have a feedback-resistant anthranilate synthetase; mutants with an altered indole-3-glycerol phosphate synthetase were not found. 3MA or 7-methylindole can be metabolized to 7-methyltryptophan, and 3MA, 7-methylindole, and 7-methyltryptophan lead to derepression of the tryptophan operon. Furthermore, 3MA-resistant mutants are also resistant to 7-methylindole derepression. These results strongly suggest that the primary cause of derepression by 3MA is through its conversion to 7-methyltryptophan, which can inhibit anthranilate synthetase, thereby decreasing the concentration of endogenous tryptophan. Unlike 5- or 6-methyltryptophan, 7-methyltryptophan does not appear to function as an active corepressor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. I., Crawford I. P. Anthranilate synthetase. Partial purification and some kinetic studies on the enzyme from Escherichia coli. J Biol Chem. 1966 Dec 10;241(23):5577–5584. [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- Cordaro J. C., Levy H. R., Balbinder E. Product inhibition of anthranilate synthetase in Salmonella typhimurium. Biochem Biophys Res Commun. 1968 Oct 24;33(2):183–189. doi: 10.1016/0006-291x(68)90765-1. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON F., YANOFSKY C. The partial purification and properties of indole-3-glycerol phosphate synthetase from Escherichia coli. Biochim Biophys Acta. 1960 Oct 7;43:489–500. doi: 10.1016/0006-3002(60)90471-6. [DOI] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: Comparative studies on the complex and the subunits. J Bacteriol. 1969 Feb;97(2):734–742. doi: 10.1128/jb.97.2.734-742.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. The nature of the anthranilic acid synthetase complex of Escherichia coli. J Biol Chem. 1966 Sep 10;241(17):4112–4114. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lark K. G. Incorporation of 5-methyltryptophan into the protein of Escherichia coli 15T- (555-7). J Bacteriol. 1969 Feb;97(2):980–982. doi: 10.1128/jb.97.2.980-982.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Silbert D. F., Fink G. R., Voll M. J., Antón D., Hartman P. E., Ames B. N. Transfer RNA and the control of the histidine operon. Cold Spring Harb Symp Quant Biol. 1966;31:383–392. doi: 10.1101/sqb.1966.031.01.050. [DOI] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- YANOFSKY C. On the conversion of anthranilic acid to indole. Science. 1955 Jan 28;121(3135):138–139. doi: 10.1126/science.121.3135.138. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



