Abstract
Serotonin (5-HT) has long been implicated in social behavior and impulsivity, but the mechanisms through which it modulates self-control remain unclear. We observed the effects of manipulating 5-HT function on behavior in the Ultimatum Game, where players must decide whether to accept or reject fair or unfair monetary offers from another player. Participants with depleted 5-HT levels rejected a greater proportion of unfair, but not fair offers, without showing changes in mood, fairness judgments, basic reward processing or response inhibition. Our results suggest that 5-HT plays a critical role in regulating emotion during social decision-making.
One of the first social rules we learn as children is the golden one: treat others as you wish to be treated. Unfortunately, our peers do not always deserve gold stars for their behavior, which tempts us to retaliate. Resisting aggressive impulses may be difficult, but successfully navigating social life sometimes requires self-regulation in the face of perceived injustice.
Serotonin (5-HT) has long been implicated in social behavior, including impulsive aggression, but its precise involvement in impulse control is controversial (1). Since social interactions can evoke strong emotions, it is plausible that 5-HT modulates impulsivity via emotion regulation mechanisms. Emotion regulation during social interactions has been studied with the Ultimatum Game (UG), in which one player (the proposer) proposes a way to split a sum of money with another player (the responder). If the responder accepts the offer, both players are paid accordingly. If the responder rejects the offer, neither player is paid. Responders tend to reject offers less than 20-30% of the total stake, despite the fact that such retaliation is costly (2), and rejection decisions are predicted by the intensity of the aversive response to the unfair offer (3,4).
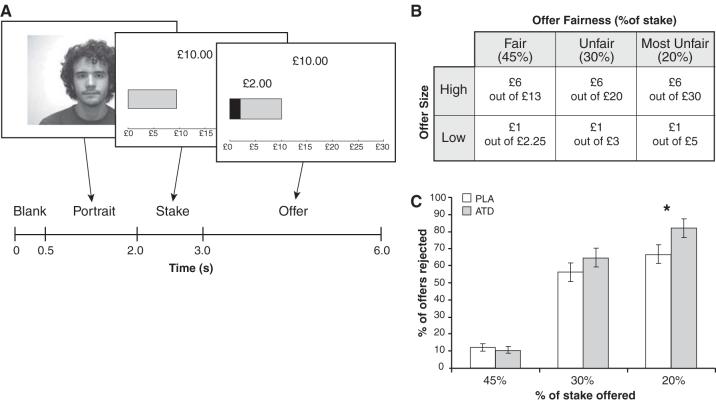
We investigated the effects of manipulating 5-HT function on rejection behavior in the UG. We used a double-blind, placebo-controlled acute tryptophan depletion (ATD) procedure to temporarily lower 5-HT levels in 20 healthy volunteers (5). Once following ATD and once following placebo, participants played the role of responder during several one-shot UGs (Fig. 1A, 5). Offers fell into one of three “fairness” categories: 45% of stake (fair), 30% of stake (unfair), or 20% of stake (most unfair). We independently manipulated social reward (fairness) and basic monetary reward (offer size) by varying both the offer amount and the stake size across trials (Fig. 1B, 5).
Fig. 1.
(A) Diagram (adapted from (4)) illustrating the structure of each one-shot UG. While each offer was on the screen, participants pressed one button to accept, or another button to reject. (B) Types of offers. (C) Rejection rates for fair, unfair, and most unfair offers following ATD and placebo (PLA) treatments. Error bars represent standard errors of the difference between means. *P=0.01 difference between treatments.
Rejection rates (% offers rejected) were calculated for each subject at each level of fairness, during ATD and placebo treatments. Repeated-measures analysis of variance revealed a highly significant interaction between treatment and fairness (F=6.891, P=0.003). Compared to placebo, ATD significantly increased rejection rates, and this effect was restricted to unfair offers (Fig. 1C, supporting online text). In contrast, ATD did not interact significantly with offer size (F=1.164, P=0.294). Controlling for fairness, participants tended to reject low offers more frequently than high offers, regardless of treatment (supporting online text).
The increased rejection of unfair offers following ATD cannot easily be attributed to changes in mood, fairness judgments, or basic response inhibition. As found previously (1), there was no effect of ATD on self-reported mood (5, supporting online text). On each session, we asked participants to indicate the size of a fair offer for each stake, and ATD did not affect these judgments (F=0.648, P=0.431). Finally, consistent with past research (1), we found no effect of ATD on Go/No-go performance, a standard test of response inhibition (5, supporting online text).
These results show that manipulating 5-HT function can selectively alter reactions to unfairness in a laboratory model of self-regulation. Temporarily lowering 5-HT levels increased retaliation to perceived unfairness without affecting mood, fairness judgments, basic reward processing or response inhibition. Our results illuminate the neural mechanisms underlying emotion regulation in the UG. Neuroimaging studies of the UG have implicated both dorsolateral prefrontal cortex (DLPFC) and ventral PFC (VPFC) in regulating reactions to unfair offers (3, 4). While disrupting DLPFC function with transcranial magnetic stimulation leads to decreased rejection of unfair offers (6), patients with VPFC damage reject a higher proportion of unfair offers than controls (7). The present effects of ATD mirror those of VPFC lesions, and are consistent with other data (8) indicating a critical neuromodulatory role for 5-HT in this region.
Supplementary Material
Acknowledgments
This work was completed within the University of Cambridge Behavioural and Clinical Neuroscience Institute, funded by a joint award from the Medical Research Council and the Wellcome Trust.
References and Notes
- 1.Evers EAT, et al. Psychopharmacology. 2006;187:200. [Google Scholar]
- 2.Guth W, Schmittberger R, Schwarze B. J Econ Behav Org. 1982;3:367. [Google Scholar]
- 3.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. Science. 2003 Jun 13;300:1755. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 4.Tabibnia G, Satpute AB, Lieberman MD. Psychological Science. 2008;19:339. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 5.Materials and methods are available on Science online.
- 6.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Science. 2006;314:829. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 7.Koenigs M, Tranel D. J Neurosci. 2007 Jan 24;27:951. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke H, et al. J Neurosci. 2005 Jan 12;25:532. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.