Abstract
Respiratory infections with both seasonal as well as potential pandemic Influenza viruses represent a significant burden on human health. Furthermore, viruses such as Influenza are increasingly recognized as important etiologic agents in community acquired pneumonia. Within the United States alone ~12.9 million people are heavy drinkers and chronic abuse of alcohol is known to increase the risk and severity of community acquired pneumonia. Given the lack of knowledge regarding Influenza disease in this population, we determined the effects of chronic alcohol consumption on Influenza virus infection. Herein, we report that mice exposed to chronic ethanol have sharp increases in morbidity, mortality, and pulmonary virus titers relative to controls. These increases in influenza severity correspond with inhibited pulmonary influenza-specific CD8 T cell responses. Further, chronic ethanol consumption results in an enhanced pulmonary lesion severity, similar to that recently described for pandemic influenzas. Together, our results suggest that chronic alcohol consumption may increase the risk for severe influenza virus infections by altering the pulmonary inflammatory environment and CD8 T cell response.
Keywords: Viral, Lung, T cells-cytotoxic, Neutrophils
Introduction
Influenza A virus infections represent a serious challenge to human health and are increasingly recognized as an important agent in community acquired pneumonia (CAP)3(1, 2). In addition to primary disease, influenza infection is known to predispose individuals, particularly persons with underlying medical conditions, to an increased incidence of secondary pneumonias, which in turn leads to more severe disease outcomes (3–6). A recent study of CAP hospitalizations during influenza season demonstrated that those patients with a history of prior influenza vaccination had a significantly reduced mortality compared to unvaccinated patients, which portrays the increasingly recognized role of influenza in CAP (7). Individuals chronically abusing alcohol have a predisposition for CAP and are more prone to severe disease outcomes (8, 9). While bacterial pneumonias are among the best-studied examples of CAP in individuals with chronic alcohol abuse, resulting in a 2- to 7-fold greater incidence in mortality and morbidity compared to non-alcoholic pneumonia patients, there is a lack of studies investigating influenza disease and chronic alcohol abuse (10–14). Within the United States alone, ~65 % of the population has consumed alcohol within the last year. Among these individuals, ~16 % are classified as heavy drinkers with another ~21% classified as moderate drinkers (15). Given this prevalence of alcohol consumption and the current threat of both seasonal, epidemic, and potential pandemic influenza (i.e. H5N1 Avian influenza)(3–5), a better understanding of the lesions within pulmonary adaptive immune responses among heavy alcohol-consuming populations could aid in designing strategies to boost immunity in these individuals to this important human pathogen.
While the role of chronic alcohol consumption in increasing the severity of respiratory bacterial infections is well recognized, its effects on viral infections such as influenza virus is lacking. It is well accepted that chronic ethanol (EtOH) ingestion is immunosuppressive and exerts general inhibitory effects on the adaptive immune response -particularly within the CD8 T cell compartment (9, 16–23). However these studies have largely focused upon examining the immunosuppression of cell-mediated immunity in the spleen, skin, and liver. Importantly, for many pathogens immune responses may be tissue specific and those in the respiratory and interstitial mucosa are distinct from that generated following intravenous, intraperitoneal or subcutaneous exposures. Therefore, what progression of lesions is found in the respiratory adaptive immune response within alcoholics and the consequences of these lesions, particularly during virus infections, remains in question.
Materials and Methods
Mice
Six to seven week old female C57Bl/6 and BALB/c mice were obtained from the National Cancer Institute (Frederick, MD). All mice were housed and maintained in the animal care facility at the University of Iowa. All experiments were performed in accordance with regulatory standards and guidelines and were approved by the institutional (The University of Iowa) Animal Care and Use Committee.
Ethanol administration
After 1 week of acclimation, mice from the same purchase lot were separated into two groups of equal size, with one group to be phased on to ethanol (pharmaceutical grade). Ethanol was provided in the drinking water at 10% (weight/volume) for 2 days, 15% for 5 days, and 20% for C57BL/6 or 18% for BALB/c mice for 4 to 8 weeks. All time points in the results are listed as the length of time at the final ethanol concentration. Mice were studied at four and eight week time points. The mice were provided laboratory chow ad libitum in all cases, and control animals were given the same double-distilled water as that used for mixing the ethanol solution. Mice were maintained on the same water or ethanol protocol during influenza infection.
Influenza Virus Infection
Mouse-adapted influenza A viruses A/PuertoRico/8/34 (H1N1) and A/JAPAN/305/57 (H2N2) were grown in the allantoic fluid of 10 day old embryonated chicken eggs for 2 days at 37°C, as previously described (24). Allantoic fluid was harvested and stored at −80°C. Mice were anesthetized with Isoflurane or a mineral oil mixture containing 33% Halothane (Halocarbon, River Edge, NJ). C57BL/6 and BALB/c mice were infected i.n. with 3.155×104 pfu dose of A/PR/8/34 and a 1.985×105 pfu dose of A/JAPAN/305/57, respectively. Morbidity, as measured by weight loss, and mortality were monitored daily.
T cell analysis of intracellular IFNγ production
Single-cell suspensions of lungs were cultured at 5 ×105 cells/well with either media, influenza peptides individually (PA224 and NP366 for C57BL/6 mice or HA204, HA529, and NP147 for BALB/c mice) or an influenza peptide mix for 6 hours at 37°C. Cells were fixed with FACS Lysis Solution (BD, San Diego, CA), permeabilized with Saponin (Acros Organics, New Jersey) and stained with rat anti-mouse CD8α PE (53-6.7), rat anti-mouse CD3ε (145-2C11) FITC, and rat anti-mouse IFNγ APC (XMG1.2). All antibodies were purchased from BD Pharmingen, San Diego, CA.
T cell analysis of MHCI-tetramer binding
Tetramers HA204 (H-2K(d)/LYQNVGTYV); HA529 (H-2K(d)/IYATVAGSL); and NP147 (H-2K(d)/TYQRTRALV) for BALB/c or PA224 (H-dD(b)/SSLENFRAYV)and NP366 (H-2D(b)/ASNENMETM) for C57BL/6 strains were purchased from BioSynthesis Incorporated (Lewisville, TX) and formed into APC or PE tetramers by The NIAID Tetramer Facility (Germantown, MD). CD8α+ influenza-specific T cells were enumerated through tetramer+ staining. Single cell suspensions of lungs were divided into 1×106 cells/100ul/well with anti-CD8α (53-6.7) PE, anti-CD3ε (145-2C11) FITC, and the appropriate tetramer and incubated for 30 minutes at 4°C. Finally, cells were fixed with FACS Lysis Solution (BD, San Diego, CA).
Histopathology Examination
Lungs were inflated with PBS containing heparin, tied off and removed from infected animals on days 5, 6, and 8 post infection and placed in 10% formalin. After 10 days in formalin the lungs were initially assessed grossly for percentage of lung affected and then embedded into paraffin, sectioned, and examined by H&E. All gross and microscopic lesions were examined by a veterinary pathologist blinded from the study. 8 sections including 4 depths per lung were scored as follows: Atelectasis - 0, absent; 1, detectable; 2, mild foci; 3, multifocal areas; 4, widespread to severe; Alveolar edema - 0, absent; 1, detectable; 2, mild foci; 3, moderate; 4, severe
Lung Virus Titer
Lungs from infected mice were rapidly homogenized, snap frozen, and stored at −80° C. Subsequently serial dilutions of homogenized lungs were injected into groups of 10 day old fertile eggs and then the eggs were incubated for an additional 48 hrs at 35° C. A hemagglutination assay was then performed by mixing 25μl of allantoic fluid with 25μl of 1% chicken blood and incubating the mixture for 20 min at room temperature. Virus titer was quantified using the Reed-Muench accumulative method.
Influenza NP ELISA
The concentration of NP in homogenized influenza infected lungs was determined by sandwich ELISAs on days 6, and 8 post infection. The anti-NP mAb (H19-S24-4.3, capture) and biotinylated anti-NP (H16-L10-4R5, secondary) antibodies were provided by Walter Gerhard (The Wistar Institute) and purified NP standard was affinity purified following lysis of A/PR/8/34 virions.
Results
Chronic ethanol consumption increases influenza-associated morbidity, mortality, and pulmonary viral loads
Based on the work of Meadows and colleagues, we have established an EtOH-in-drinking-water mouse model of chronic alcohol intake (hereafter referred to as the Meadows-Cook model) (16, 17, 20, 21). Importantly, this model results in a range of immune and tissue lesions that are normally observed in chronic alcoholics without inducing an overt stress response (16). Likewise experimental influenza infection of mice with mouse-adapted influenza strains offers a well-characterized model that allows careful study of the pathogenesis and immune mechanisms that allow control and elimination of the virus (24–33). Therefore experimental influenza infection of mice on the Meadows-Cook protocol serves as a good model in which to determine how ethanol affects the immune response and severity of influenza infections. Hence, BALB/c mice were placed on chronic EtOH for 4 weeks (wk) and then infected intranasally (i.n.) with a 0.01LD50 dose of Influenza A (mouse-adapted A/JAPAN/305/57, H2N2). As shown in Figure 1, chronic EtOH consumption results in substantial increases in both morbidity (Fig 1A) and mortality (Fig 1B) compared to water controls. It is important to note that in healthy BALB/c mice this inoculum dose of influenza normally results in only low to moderate weight loss (morbidity) and the death of 1 out of ~100 inoculated mice. In contrast, our results demonstrate that chronic EtOH mice have substantially greater weight loss (Fig 1A) and death (50% of the EtOH mice succumb to the infection, Fig 1B). Interestingly, those chronic EtOH mice that survived past day 12, unlike the water controls, again lost weight for approximately another 3 days following their initial recovery (see day 14). These mice subsequently regained weight and survived out to 45 days post-infection (p.i.) when the experiment was terminated (Fig 1A, and data not shown). These results suggest that, like bacterial pneumonia infections in alcoholics and EtOH animal models, chronic EtOH exposure greatly increases influenza-associated severity and undesired outcomes.
Figure 1.
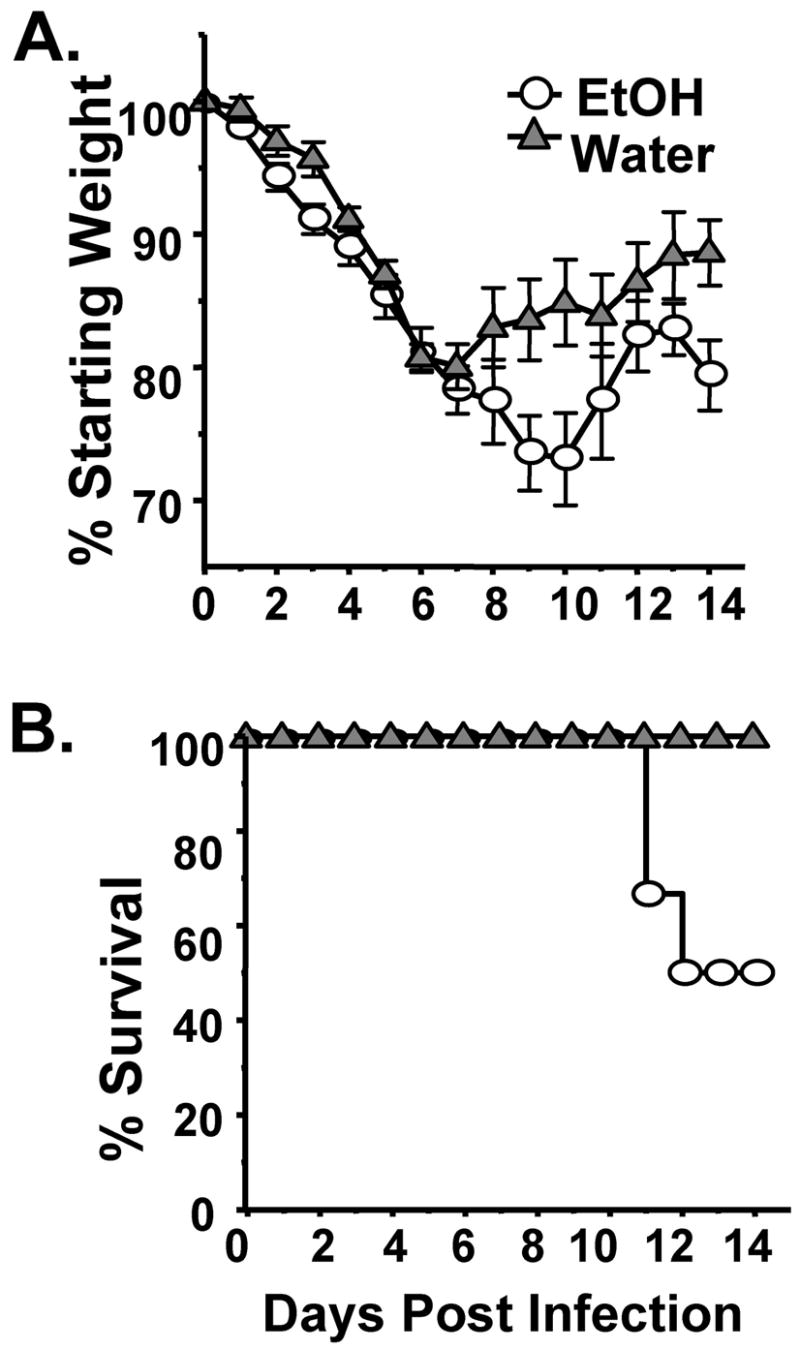
Chronic EtOH consumption increases the severity of influenza virus infections. EtOH (circle) and water (triangles) conditioned groups of BALB/c mice were infected with a 0.01LD50 dose of influenza virus and then assessed for morbidity as measured by weight loss (A), mortality (B). One representative experiment of 2 (8–10 mice/group) is shown in (A, B).
Increased influenza viral load is often associated with more severe disease and outcomes. Therefore, in order to determine if the increase in disease severity in chronic EtOH mice would likewise be reflected in altered influenza pulmonary viral titers and antigen load, we again infected 4 week EtOH or water control BALB/c mice with a 0.01LD50 dose of influenza virus. Our results show that the increase in severity of infection in EtOH mice (Fig 1) correlates with significantly increased pulmonary influenza titers (Fig 2A) and sustained increases in antigen load (Fig 2B).
Figure 2.
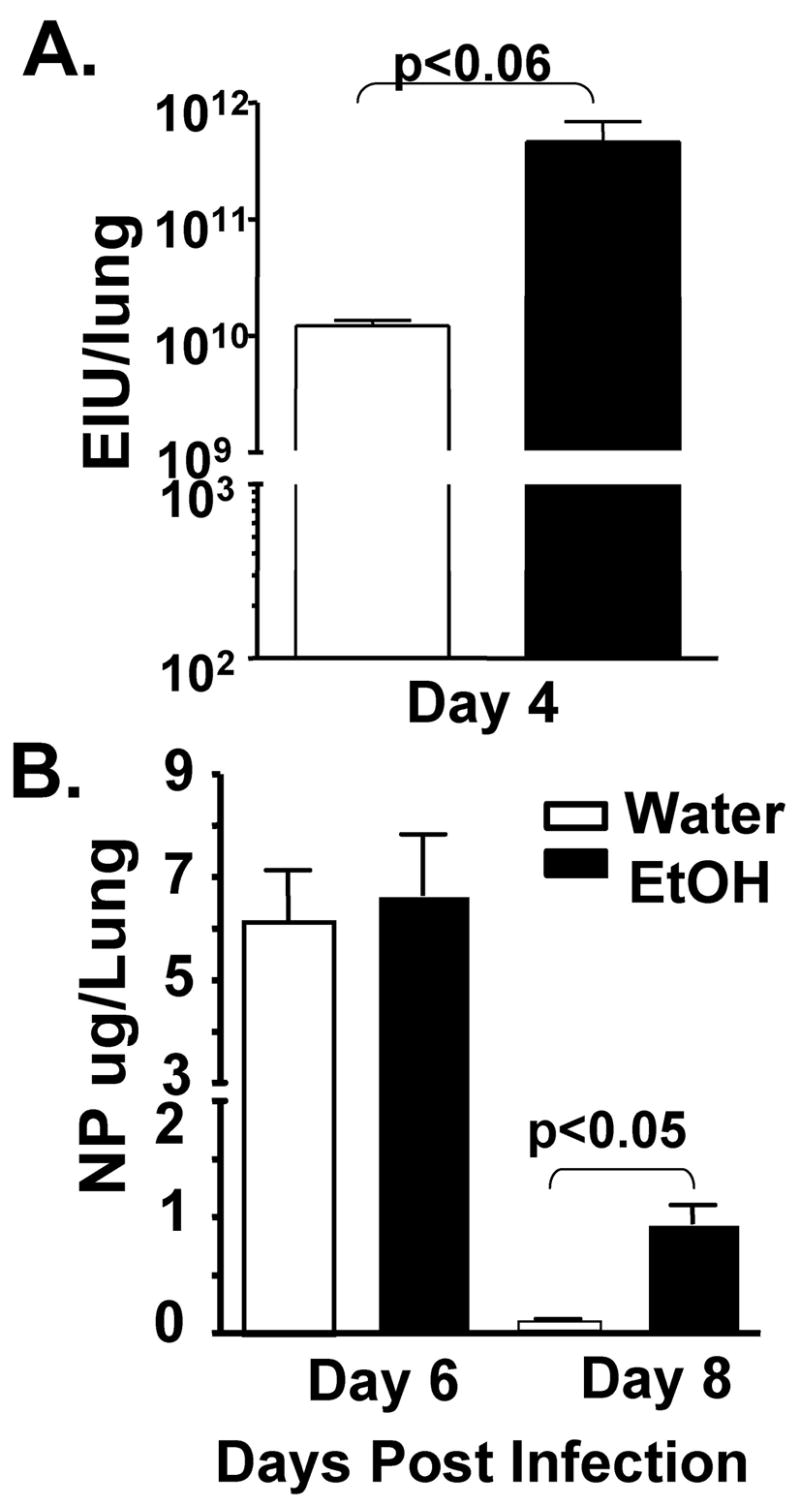
Chronic EtOH consumption increases the magnitude and duration of influenza virus infections. EtOH (black bars) and water (open bars) conditioned groups of BALB/c mice were infected with a 0.01LD50 dose of influenza virus and then assessed for pulmonary virus titer (A), and influenza nucleocapsid protein (NP) loads (B). Data are the mean ± sem of 5–9 mice/group.
Chronic ethanol consumption decreases influenza-specific CD8 T cell responses
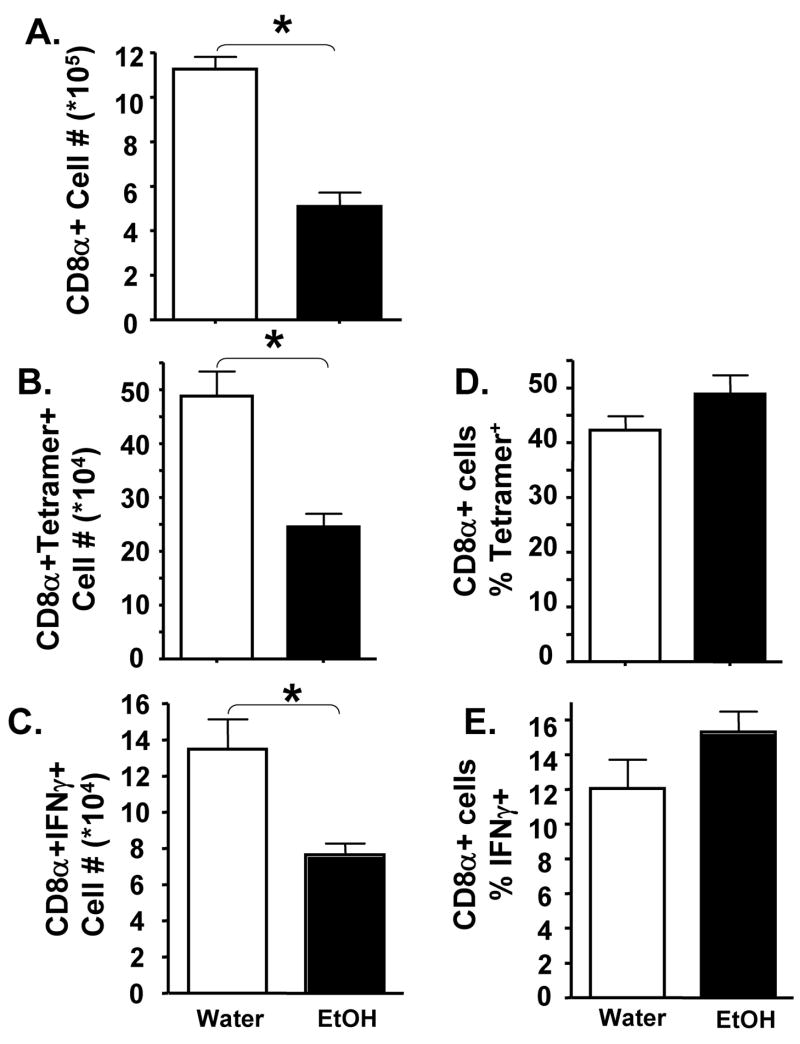
Many studies have shown that protective immunity to primary influenza virus infection involves the clearance of infected epithelial cells by CD8+ T cells through Fas and perforin dependent direct killing mechanisms (27, 34). These T cells first appear in the lungs around day 4 p.i. where their continued expansion and accumulation corresponds with virus clearance (24). The importance of CD8 T cells in protection from influenza infections is further highlighted by the fact that CD8 T cells mediate resistance to and protection from lethal influenza virus infections even in the absence of B cells, CD4 T cells, and antibody (26, 30). Therefore, given the increased pulmonary virus titers observed above, we determined if chronic EtOH consumption altered the pulmonary influenza-specific CD8 T cell response after only 4 weeks of alcohol consumption. Indeed, chronic EtOH exposure decreases the total number of CD8 T cells within the lungs by ~50% after infection (Fig 3A). Further, this decrease in total CD8 T cells, as measured by influenza-peptide MHC tetramer analysis (Fig 3B) or intracellular IFNγ staining (Fig 3C), is reflected in a corresponding reduction in the total number of influenza-specific CD8 T cells. However when the influenza-specific response is examined as a frequency of the total CD8 T cells within the lungs (Fig 3D, E) there is no difference between the water and EtOH groups. This result implies that both the lung environment and secondary lymphoid tissues were in a general immunocompromised state due to chronic EtOH consumption, as previously described (16, 17, 20, 35). Importantly, the compromised immune status of the chronic EtOH host resulted in a poor response and reduced total numbers of influenza-specific effector T cells. Given the role influenza-specific effector CD8 T cells play in elimination of influenza infected cells (26, 36), the loss of these T cells suggests that pulmonary influenza-specific cytotoxicity would also be reduced - an outcome that could be responsible for the increases in influenza virus titers and antigen loads observed in these mice (Fig 2).
Figure 3.
Chronic EtOH inhibits pulmonary Influenza-specific CD8+ T cell immune responses. Four week water (open bars) or EtOH (closed bars) conditioned BALB/c mice were infected with influenza A virus. On day 8 p.i., the number of total pulmonary CD8 T cells (A) as well as the number (B, C) and percentage (D, E) of influenza-specific CD8+ T cells were determined by flow cytometry. Cells were identified by binding of influenza-specific MHC-tetramers (B, D) or by influenza-peptide (HA204, HA529, NP147 or control RSV M280) induced intracellular IFNγ staining (C, E). One representative experiment of 2 (minimum of 4 mice/group, mice analyzed individually) is shown. Data are the mean ± sem. *=p<0.05
As chronic alcoholics are from genetically diverse backgrounds and multiple strains of influenza A virus are responsible for disease, we next determined if the observed inhibition in influenza-specific T cell immunity was unique to BALB/c mice or the mouse-adapted H2N2 strain of influenza A virus used. To this end, we infected 4 wk water and EtOH treated C57Bl/6 mice with mouse-adapted A/PR/8/34 (H1N1) influenza A virus. As shown in Figure 4, the total number of pulmonary CD8α+ (Fig 4A) and influenza-specific CD8 T cells (Fig 4D) were reduced in EtOH mice similar to the results observed in BALB/c mice infected with the H2N2 Influenza A virus (Fig 3A–C). These results indicate that the effects of chronic EtOH on influenza immunity and disease are not unique to either the mouse or influenza virus strain used.
Figure 4.
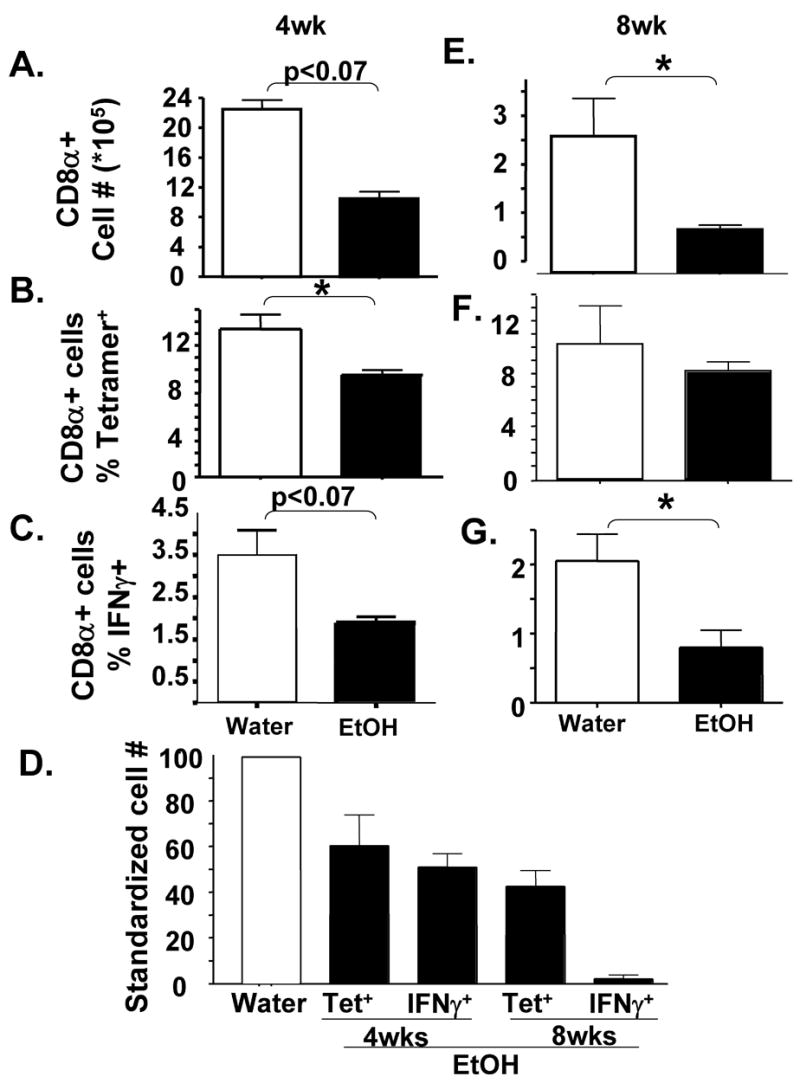
Inhibition of pulmonary Influenza-specific CD8+ T cell immune responses increases with the chronicity of EtOH consumption. Water (open bars) or EtOH (closed bars) conditioned C57Bl/6 mice were infected with influenza A virus. On day 8 p.i., the number of total pulmonary CD8 T cells (A, F) as well as the percentage (B, C, F, G) of influenza-specific CD8+ T cells were determined by flow cytometry (B, F: tetramers; C, G: influenza HA204, HA529, NP147 vs. control RSV M280 peptide induced intracellular IFNγ staining). (D). Comparison of the pulmonary T cell response. The number of influenza-specific CD8 T cells from EtOH mice were each normalized to their corresponding water control, with the water value equal to 100. One representative experiment of 2–3 (minimum of 4 mice/group, mice analyzed individually) is shown. Data are the mean ± sem. *=p<0.05
Influenza-specific CD8 T cell responses become dysregulated during extended chronicity of ethanol consumption
Previous studies have suggested that many of the defects EtOH has on the immune system worsen with the chronicity of EtOH exposure (16). Therefore in order to determine if the inhibition in T cell immunity observed at 4 wk of EtOH was maintained, lost, or strengthened by further EtOH exposure we infected C57Bl/6 mice that had been conditioned with EtOH for 8 weeks. As shown in Figure 4A, chronic EtOH for 4 wk reduces the total pulmonary CD8α+ T cell response to influenza by ~ 50%. This inhibition is further increased to ~70% by 8 wk of EtOH exposure (Fig 4E). These results indicate that the defects in pulmonary CD8 T cell immunity increase with the length of exposure to EtOH.
Next we determined if the chronicity of EtOH altered the number and effector capacity of influenza-specific CD8 T cells. Similar to what occurs in BALB/c mice (Fig 3), the C57Bl/6 EtOH mice have substantially fewer influenza-specific CD8 T cells (Fig 4D) than water controls. This appears to be due primarily to the loss in total pulmonary CD8α+ cells in 4 wk EtOH mice (Fig 4A) as the % of tetramer+ (Fig 4B) or IFNγ+ (Fig 4C) cells among pulmonary CD8+ cells is not significantly different between the water and EtOH mice. Interestingly, however, the influenza-specific CD8 T cell response in 8 wk EtOH mice shows a defect in addition to the above-described reduction in the total number of cells. Whereas the percentage of tetramer+ influenza-specific T cells among total CD8 T cells is equal between the water and EtOH groups (Fig 4F), the EtOH, but not water, 8 wk T cells are dysregulated and do not make IFNγ in response to stimulation with influenza virus peptides (Fig 4G). This dysregulation results in a significant loss of IFNγ+ influenza-specific T cells from the lungs (Fig 4D). Interestingly, however when the 8-week EtOH T cells were stimulated in vitro with PMA/ionomycin, instead of influenza virus peptides, equivalent percentages of CD8 T cells produced IFNγ+ (Fig 5). Since PMA and ionomycin bypass the TCR/CD3 complex to deliver stimulatory signals to T cells by activating protein kinase C (PKC) and raising cytoplasmic calcium concentrations, these results suggest that a defect in signaling upstream of PKC and Ca++ flux leads to the dysregulated ability 8 week EtOH influenza-specific effector CD8 T cells to produce IFNγ.
Figure 5.
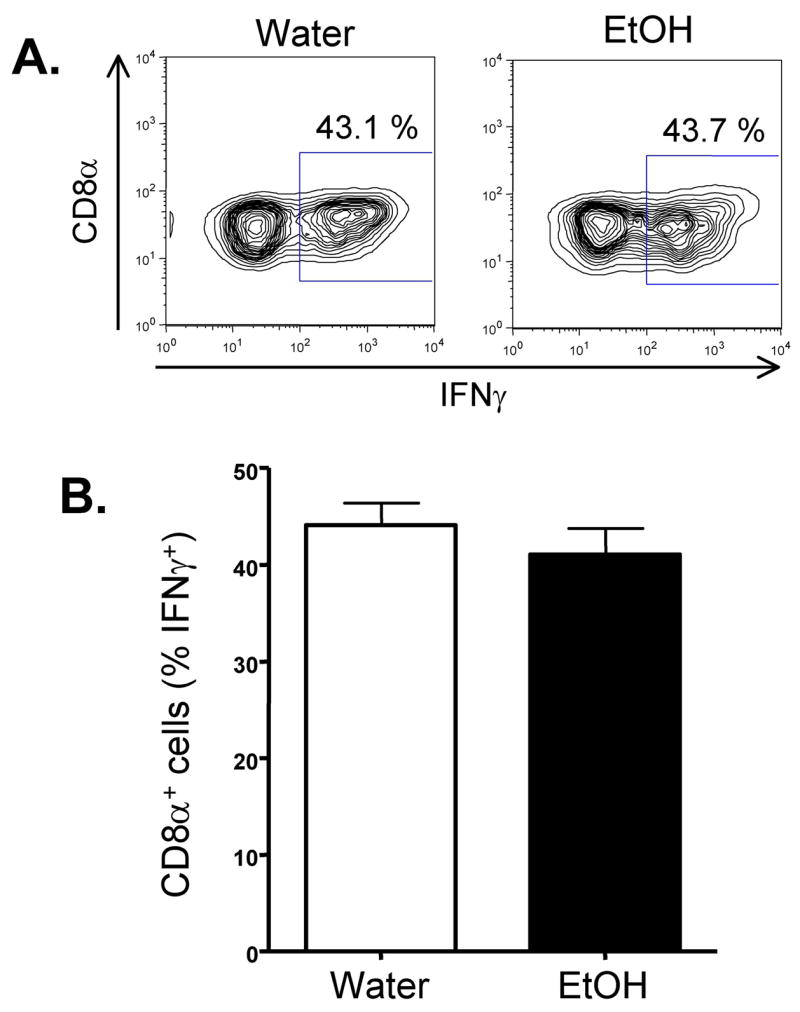
Chronic EtOH consumption does not alter pulmonary CD8 T cell IFNγ production in response to PMA/ionomycin. 8 wk EtOH and water conditioned C57Bl/6 mice (5/group) were infected with influenza A virus. On day 8-post infection the lungs were removed and the ability of CD8+ T cells to produce IFNγ in response to PMA/ionomycin stimulation was determined by intracellular cytokine staining. (A) Representative plots showing the % of IFNγ+ cells of CD8+ gated cells. (B) Data are the mean % of IFNγ+ cells ± sem of CD8+ gated cells from the indicated groups of mice. One representative experiment of 2 (minimum of 4 mice/group, mice analyzed individually) is shown.
Together our results indicate that the influenza-specific T cell response develops progressively greater defects both in the number of cells available as well as in their effector phenotype as the length of exposure to EtOH increases. Given the integral role CD8 T cells play in the elimination of virally infected cells during primary influenza infections (24, 27, 30), these results when combined with those in Figures 1 and 2, suggest that the increased severity of influenza virus infections following chronic EtOH consumption might in part be related to the decrease in influenza-specific T cell immunity Similar results showing increased morbidity and mortality as well as inhibited CD8 T cell and influenza-specific CD8 T cells and function relative to age matched water controls were observed when BALB/c were examined following 8 wks of chronic EtOH ingestion (data not shown).
Chronic ethanol consumption is associated with neutrophilic and severely edematous lungs during influenza infections
Recent studies on pandemic (i.e. 1918 H1N1 Spanish influenza) or potential pandemic (H5N1, Avian influenza) strains have shown that in the absence of adaptive CD8 T cell responses or overwhelming innate immune responses the pulmonary pathology associated with influenza infections is significantly increased (25, 28, 37). Therefore, in order to determine if the reduced numbers of influenza-specific CD8 T cells observed in chronic EtOH mice would lead to a similar increase in pulmonary disease, we infected 4 wk and 8 wk EtOH or water conditioned C57Bl/6 with A/PR/8/34 and performed histopathological analysis. At both the gross (Fig 6A, B) and microscopic level (Fig 6C–H), lungs from 8 week EtOH mice had more inflammation, edema, and consolidation at days 5, 6, and 8 post-infection than that observed in water controls (Fig 6 and data not shown; alveolar edema, p< 0.01; atelectasis, p<0.01). By day 8 post-infection, while recovery was exhibited in the lungs of water mice (28.8% percent of the lung affected in day 8 groups vs. 41.5% at day 6 groups by gross examination, Fig 6A and data not shown) disease in EtOH mice continued to progress (53.5% of the lung affected in day 8 groups vs. 46.5% in day 6 groups Fig 6B and data not shown). At the microscopic level this difference in the severity of the lesion on day 8 p.i. was prominently localized to the alveoli with larger differences observed in alveolar atelectasis (water 1.8±0.3, EtOH 3.2±0.7; p<0.05) and edema (water 0.5±0.3, EtOH 2.4±0.7; p<0.05). Similar increases in histopathology scores were observed in the lungs of 4 wk EtOH infected mice (data not shown). Recently the increases in severe influenza disease have been observed to associate with an increase in neutrophilic infiltrate (25). Therefore we enumerated the number of neutrophils present in the influenza lesions of 8-wk water and EtOH mice. Morphologically as shown in Figure 7A, B and quantified in Figure 7C, chronic EtOH consumption leads to increased neutrophil recruitment into the lungs during influenza virus infections.
Figure 6.
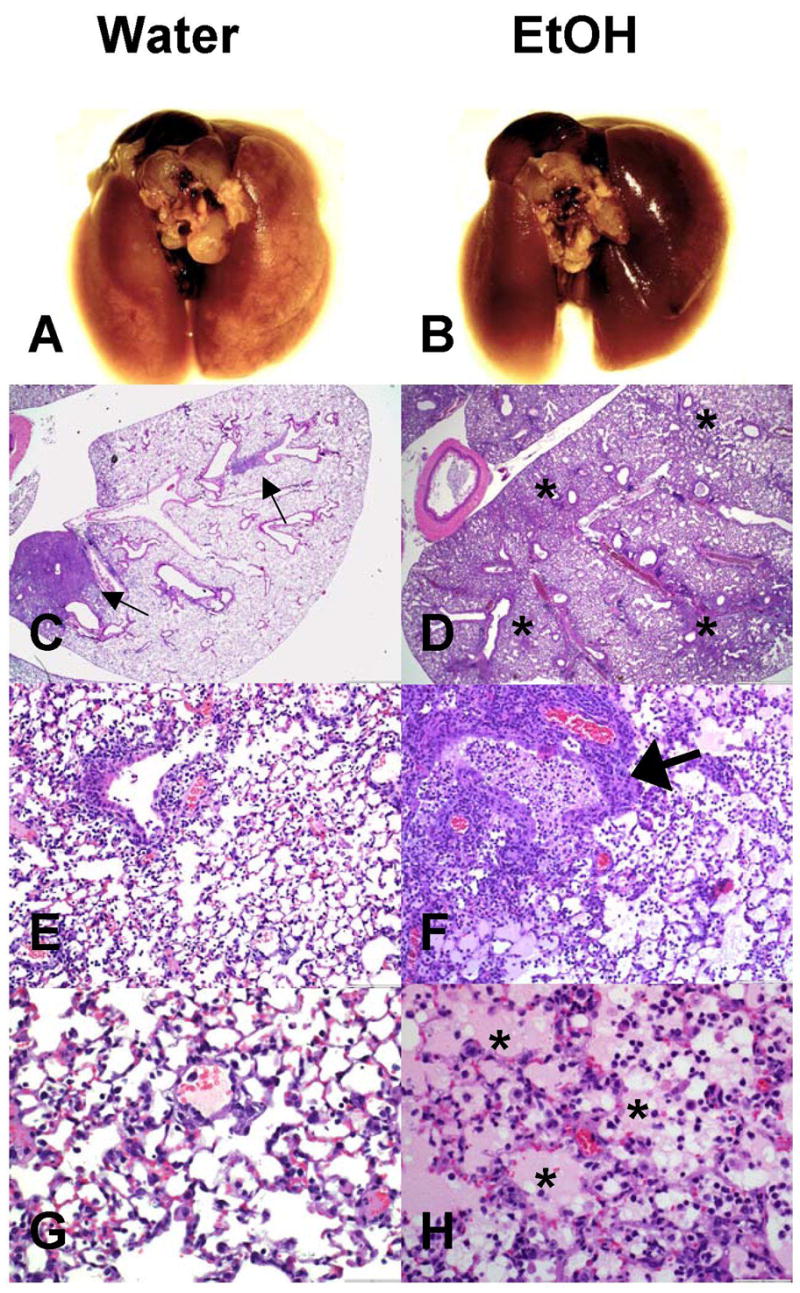
Chronic EtOH consumption increases the severity of the pulmonary lesions associated with influenza infections. On day 8 post influenza infection, 8 wk water-control C57Bl/6 mice (A,C) have localized multifocal consolidation (C, arrows) whereas ethanol mice (B,D) have more widespread and coalescing consolidation (D, *). Water mice had characteristic influenza lesions including necrotizing bronchiolitis (E) and moderate interstitial pneumonia (G). Ethanol mice had more pronounced inflammation including increased necrosis and numbers of inflammatory cells (F, arrow) and pulmonary edema (H, *). Sections were negative for bacterial involvement as modified gram stains were negative for bacteria (data not shown). 8 sections including 4 depths per lung were scored independently from 3 mice per group.
Figure 7.
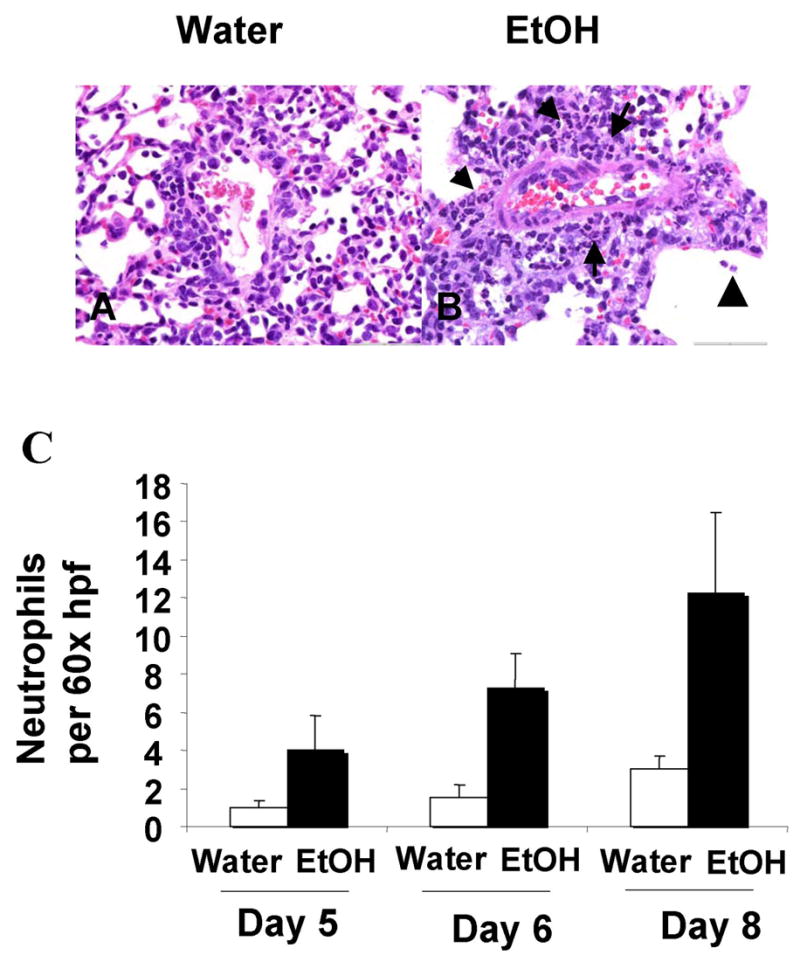
Chronic alcohol consumption increases neutrophil recruitment to the lungs. Lung, representative samples from 8-week water (A) and EtOH (B) conditioned C57Bl/6 mice eight days post influenza inoculation. Morphologically, the EtOH mice exhibited increased neutrophil (arrow) infiltration especially noted in a perivascular orientation with extension into alveolar air spaces (arrowhead) versus the typical mononuclear infiltration seen in water controls. (C) Quantification of neutrophils per high power field. Random foci in the lung were counted (60× hpf × 5), and the average neutrophils per high power field (hpf) tallied and the means for each group normalized to Day 5 water with a value of “1”. Water versus ethanol groups had significant differences in neutrophil infiltration (p<0.01, treatment effect, two way ANOVA) with significant posttest differences at Day 8 (p<0.05). 8 sections including 4 depths per lung were scored independently from 3 mice per group.
Discussion
Our results demonstrate that chronic EtOH consumption reduces the number of, and by 8 weeks of EtOH consumption, the effector ability of influenza-specific CD8 T cells. While the suppression of CD8 T cells numbers during chronic EtOH has been described elsewhere (17, 20, 35), herein we show for the first time that extended chronic EtOH consumption (i.e. 8 weeks+) further blocks the ability of virus-specific CD8 T cells to produce the effector molecule IFNγ (Fig 4D, G). A similar dysregulation of pulmonary CD8 T cell responses has been described during RSV infections of naive (i.e. water consuming) mice (38, 39). This loss in T cell effector function during RSV infections can be overcome by supplementation with IL-2 (39). Importantly, studies have shown reduced IL-2 production, rescue of defective T cell responses by exogenous IL-2 addition, and reduced T cell binding of IL-2 during acute alcohol consumption (40–42). To date, however we have found no difference in pulmonary IL-2 levels or in CD25, CD122, or CD132 expression on pulmonary influenza-tetramer+ CD8 cells between the water and chronic ethanol groups (data not shown). Instead this dysregulation may relate to differential APC populations in the lungs of influenza-infected water and ethanol-exposed mice. In other systems, peripheral DC populations have been shown to influence antigen specific T cell responses/dysregulation (43). Further, chronic ethanol is known to alter DC populations in the skin, spleen and liver (44–49). Our preliminary results suggest that chronic ethanol likewise alters the DC cell numbers and subsets available in the lungs (data not shown). However, what role these changes may play, if any, in the altered immune response and disease severity found during influenza infection of chronic ethanol consuming mice described above awaits subsequent study.
While the cellular and cytokine dependence of the EtOH suppression of IFNγ CD8 T cell effector function remains unknown, we do know that the dysregulation is influenza peptide specific as PMA and ionomycin stimulation of these cells drives an equivalent % of CD8 T cells to produce IFNγ (Fig 5). This result suggests that the 8-week EtOH mediated defect is upstream of Ca++ flux and PKC activation. Since we did not observe any differences in the intensity of tetramer staining on influenza-specific CD8 T cells (data not shown) from water and EtOH mice, it suggests that altered levels of cell surface TCR expression or reduced TCR affinity for influenza virus peptide:MHC complexes do not explain the inability of 8 week EtOH CD8 T cells to produce IFNγ. Recent studies have shown that EtOH reduces Toll-like receptor (TLR) signaling by altering recruitment and distribution of molecules in lipid rafts (50). Since recruitment and redistribution of molecules into lipid rafts is likewise an important facet of TCR mediated signaling (51, 52), these results suggest that the defect leading to EtOH mediated IFNγ dysregulation in T cells may ultimately lie in lipid rafts and signaling molecules downstream of TCR engagement but upstream of PKC and Ca++ flux.
Given the peptide-MHC specific nature of TCR dependent IFNγ production by CD8 T cells, the EtOH induced IFNγ defect would only be observed when directly examining antigen specific effector responses. Whether this EtOH induced dysregulation is unique to influenza-specific T cells or is more global and occurs with pulmonary T cells or CD8 T cell responses in general remains in question. Interestingly, a recent study using the same EtOH in drinking water model has shown a similar loss of IFNγ production by listeriolysin O (LLO) specific CD8 T cells in the spleen following Listeria monocytogenes infections (Gurung et al, manuscript in preparation). Together these results suggest that chronic EtOH may alter the effector abilities of CD8 T cells in many tissues possibly via alteration of TCR signaling or in APC subset availability.
The mechanism regulating the overall global decrease in T cells in chronic EtOH mice remains unknown at this time, but previous studies have demonstrated that T cells in alcoholics and animal models show increased levels of activation in the unimmunized state (17, 20, 53). In turn, this chronic activation of the T cell pool may alter the T cell’s ability to expand and respond to subsequent viral challenges (i.e. anergy), place the T cells under increased regulatory cell control, or lead to their elimination through activation induced cell death (AICD). Such mechanisms would decrease the overall numbers of T cells available to respond to the influenza virus challenge. Consistent with this idea we did observe reduced numbers of both total CD8 T cells and influenza-specific CD8 T cells in the lung draining lymph nodes on day 8 post infection (data not shown). This suggests that the reduced pulmonary response observed herein could relate to altered precursor frequency, activation or expansion of influenza-specific CD8 T cells within the lymph nodes. Our observation of decreased numbers of total CD8 T cells in the lungs following influenza virus infections is consistent with the results of Shellito and Olariu, who observed decreased numbers of CD8 T cells in the lungs of EtOH mice following Pneumocystis carinii infections (35). This loss of CD8 T cells from the lungs was not mirrored by a similar loss of these cells in the spleen, and therefore the differential CD8 T cell numbers were ascribed to be related to altered migration of the T cells into EtOH lungs (35). In contrast, we found that the decreased numbers of CD8 T cells in the lungs of influenza virus infected EtOH mice were paralleled by similar losses of these cells in the spleens and lymph nodes (data not shown) suggesting that in our case altered migration of the T cells from the lymphoid organs into the lungs may not explain the observed differences. At this time the relative contributions of these potential mechanisms to the observed defect in CD8 T cell recruitment and influenza-virus specific CD8 T cell immunity specifically in chronic EtOH vs. water environments awaits further study.
All together the results presented herein suggest that chronic EtOH exposure reduces adaptive immunity and thereby increases pulmonary viral titers, inflammation, and disease severity. Further, given the reduced levels of influenza-specific CD8 T cells and the known alterations that EtOH exposure has on pulmonary resident cells like alveolar macrophages (8, 54), it is likely that the overall inflammatory environment in the lungs of water and EtOH mice are distinct and contribute to the observed pathology. In this regard, highly lethal influenza infections are thought to be associated with high levels of inflammatory cytokines (37, 55). Moreover, recent studies have suggested that the increased neutrophilic infiltration associated with experimental 1918 influenza A infections may, in part, be responsible for the increased inflammatory cytokine and chemokine expression (25). Consistent with those studies, we have also observed increased neutrophilia and in a preliminary experiment marked increases in inflammatory cytokine mRNA, including type I IFNs and IL-17F, in the lungs of influenza-infected EtOH mice (data not shown). Therefore collectively the 1918 influenza virus studies together with our own data presented herein suggest that the increased pathology associated with influenza infection of EtOH mice could in part relate to the increased pulmonary neutrophilia. However, the benefits of blocking or reducing this neutrophilia in EtOH mice may be limited as removal of neutrophils from 1918 infected mice led to higher viral titers and similar lethality (25). Therefore the increased pulmonary neutrophilia observed herein and in other lethal influenza virus infections (25) may instead be indicative of a compensatory innate immune response geared at limiting viral infection during times of reduced influenza-specific CD8 T cell immunity.
In addition to the increased neutrophilia, our results show that the pulmonary lesions of influenza-infected EtOH consuming mice were routinely associated with marked edema (Fig 6G). The delicate fluid balance in the airways and alveolar epithelia is regulated largely by active electrolyte transport (56). Epithelial tight junctions additionally regulate paracellular permeability. Previous studies have demonstrated that chronic EtOH consumption results in loss of epithelial tight junction proteins and increased paracellular permeability. This leakage is related to a cascade of factors including the loss of glutathione, concomitant increases in oxidative stress, upregulation of TGF-β, and reduced GM-CSF receptor signaling (56). Furthermore influenza virus infection alone is known to inhibit amiloride-sensitive sodium channel activity and increase fluid retention in the lungs (57). Therefore the marked edema observed within the lungs of influenza-infected EtOH consuming mice may reflect both alcohol-induced increased epithelial permeability as well as viral-induced fluid retention.
Our observed increases in pulmonary viral loads, inflammation, edema, and neutrophilia as well as decreased numbers of dendritic cells in the lungs of influenza infected EtOH mice are consistent with the recent findings of Jerrells et al during pulmonary infections of EtOH mice with RSV (58). However unlike the influenza infection data presented herein, the neutrophilia in that work was more predominate during the first 24–48 hours post infection and dissipated at later times (i.e. day 5 post RSV infection). Further, while we have observed a decrease in total lymphocytes in the lungs of influenza-infected animals, Jerrells et al observed an increased number of pulmonary lymphocytes in EtOH mice on day 5 post RSV infection (58). It should be noted that the RSV studies described above were focused upon the bronchial alveolar lavage fluid (BALF) rather than the full lung environment (i.e. BALF and lung interstitium) examined herein. This difference in focus may in part explain the differences in kinetics and lymphocyte numbers between RSV and influenza virus infections of EtOH conditioned mice. Alternatively these differences could instead relate to potency of the various virus infections in mice as infectious RSV virions were cleared from the lungs during the first 48–72 hours of the infection (58), while influenza virus infections persist for 5–8 days in water control mice [Fig 2 and (24)]. Regardless, the similarities between the two models suggest that pulmonary infections of alcoholics with seasonal respiratory virus such as RSV and influenza would lead to increased disease severity that featured increased neutrophilia, inflammation, and edema as well as sustained viral titers and reduced pulmonary APC numbers.
In closing, we have shown that chronic EtOH consumption increases the risk for severe disease and death during influenza infections. This increased severity appears multifactoral and includes increased edema and neutrophilia and a loss of competent influenza-specific effector T cells. Given the distinct role CD8 T cells play in controlling influenza infections by eliminating infected cells, it suggests that strategies designed to boost CD8 T cell immunity to this important human pathogen in these individuals could reduce both the burden and severity of influenza infections and lead to more desirable outcomes.
Acknowledgments
We thank Drs. Thomas Waldschmidt, Annette Schlueter, Paul McCray, and Steven M. Varga for critical reading of this manuscript.
Footnotes
This work was supported by NIH AA-014405 to R.T.C. and Department of Pathology Start-Up Funds to K.L.L..
Abbreviations used in this paper: CAP, community acquired pneumonia; EtOH, ethanol; p.i., post infection; RSV, respiratory syncytial virus; AICD, activation induced cell death; NP, influenza nucleocapsid protein; HA, influenza hemagglutinin protein; PA, influenza acid polymerase protein; LLO, listeriolysin O; PMA, phorbol myristate acetate; PKC, protein kinase C
“This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (JI). The American Association of Immunologists, INC (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copy edited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.Jimmunol.org”
References
- 1.Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, Young SA, Chambers ST, Murdoch DR. Incidence and Characteristics of Viral Community-Acquired Pneumonia in Adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 2.Angeles Marcos M, Camps M, Pumarola T, Antonio Martinez J, Martinez E, Mensa J, Garcia E, Penarroja G, Dambrava P, Casas I, Jimenez de Anta MT, Torres A. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther. 2006;11:351–359. [PubMed] [Google Scholar]
- 3.Fauci AS. Race against time. Nature. 2005;435:423–424. doi: 10.1038/435423a. [DOI] [PubMed] [Google Scholar]
- 4.Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 5.LaForce FM, Nichol KL, Cox NJ. Influenza: virology, epidemiology, disease, and prevention. Am J Prev Med. 1994;10 Suppl:31–44. [PubMed] [Google Scholar]
- 6.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 7.Spaude KA, Abrutyn E, Kirchner C, Kim A, Daley J, Fisman DN. Influenza vaccination and risk of mortality among adults hospitalized with community-acquired pneumonia. Arch Intern Med. 2007;167:53–59. doi: 10.1001/archinte.167.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Bagby GJ, Happel KI, Summer WR, Nelson S. Pulmonary host defenses and alcohol. Front Biosci. 2002;7:d1314–1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]
- 10.Capps J, Colman G. Influence of alcohol on prognosis of pneumonia in Cook County Hospital. J A M A. 1923;80:750–752. [Google Scholar]
- 11.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 12.MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- 13.Rush B. An inquiry into the effects of ardent spirits upon the human body and mind. Q J Stud Alcohol. 1943;4:321–341. [Google Scholar]
- 14.Schmidt W, De Lint J. Causes of death of alcoholics. Q J Stud Alcohol. 1972;33:171–185. [PubMed] [Google Scholar]
- 15.NIAAA. Alcohol use and alcohol use disorders in the United States. US Alcohol Epidemiologic Data Reference Manual. 2006:9. [Google Scholar]
- 16.Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook RT, Zhu X, Coleman RA, Ballas ZK, Waldschmidt TJ, Ray NB, LaBrecque DR, Cook BL. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33:175–181. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 19.Pruett SB, Han YC, Wu WJ. A brief review of immunomodulation caused by acute administration of ethanol: involvement of neuroendocrine pathways. Alcohol Alcohol Suppl. 1994;2:431–437. [PubMed] [Google Scholar]
- 20.Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- 21.Spitzer JH, Meadows GG. Modulation of perforin, granzyme A, and granzyme B in murine natural killer (NK), IL2 stimulated NK, and lymphokine-activated killer cells by alcohol consumption. Cell Immunol. 1999;194:205–212. doi: 10.1006/cimm.1999.1511. [DOI] [PubMed] [Google Scholar]
- 22.Waldschmidt TJ, Cook RT, Kovacs EJ. Alcohol and inflammation and immune responses: summary of the 2005 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2006;38:121–125. doi: 10.1016/j.alcohol.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Waldschmidt TJ, Cook RT, Kovacs EJ. Alcohol and inflammation and immune responses: summary of the 2006 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2008;42:137–142. doi: 10.1016/j.alcohol.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160:322–327. [PubMed] [Google Scholar]
- 27.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 28.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996;206:1–14. doi: 10.1007/978-3-642-85208-4_1. [DOI] [PubMed] [Google Scholar]
- 30.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174:5332–5340. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 33.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 34.Lukacher AE, V, Braciale L, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shellito JE, Olariu R. Alcohol decreases T-lymphocyte migration into lung tissue in response to Pneumocystis carinii and depletes T-lymphocyte numbers in the spleens of mice. Alcohol Clin Exp Res. 1998;22:658–663. doi: 10.1111/j.1530-0277.1998.tb04308.x. [DOI] [PubMed] [Google Scholar]
- 36.Johnson TR, Varga SM, Braciale TJ, Graham BS. Vbeta14(+) T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J Virol. 2004;78:8753–8760. doi: 10.1128/JVI.78.16.8753-8760.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Hyun Kim J, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 38.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 39.Chang J, Choi SY, Jin HT, Sung YC, Braciale TJ. Improved effector activity and memory CD8 T cell development by IL-2 expression during experimental respiratory syncytial virus infection. J Immunol. 2004;172:503–508. doi: 10.4049/jimmunol.172.1.503. [DOI] [PubMed] [Google Scholar]
- 40.Laso FJ, Iglesias-Osma C, Ciudad J, Lopez A, Pastor I, Torres E, Orfao A. Alcoholic liver cirrhosis is associated with a decreased expression of the CD28 costimulatory molecule, a lower ability of T cells to bind exogenous IL-2, and increased soluble CD8 levels. Cytometry. 2000;42:290–295. [PubMed] [Google Scholar]
- 41.Choudhry MA, Messingham KA, Namak S, Colantoni A, Fontanilla CV, Duffner LA, Sayeed MM, Kovacs EJ. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21:239–243. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- 42.Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- 43.Fulton R, Varga SM. Functional impairment of CD8 T cells following acute virus infections. J Immunol. 2007;178:43.32. [Google Scholar]
- 44.Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- 45.Lau AH, Thomson AW, Colvin BL. Chronic ethanol exposure affects in vivo migration of hepatic dendritic cells to secondary lymphoid tissue. Hum Immunol. 2007;68:577–585. doi: 10.1016/j.humimm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Ness K, Schlueter A. Dynamic changes in murine Langerhans cell migration as a result of chronic ethanol consumption. J Immunol. 2006;176:S63. [Google Scholar]
- 47.Edsen M, Schlueter A. Effect of chronic ethanol exposure on murine dendriti cell numbers and function. F A S E B J. 2004;18:A426S. [Google Scholar]
- 48.Fan J, Schlueter A. Chronic ethanol ingestion disrupts cytokine production by dendritic cells. J Immunol. 2006;176:S62. [Google Scholar]
- 49.Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol Clin Exp Res. 2008;32:657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- 51.Van Laethem F, Leo O. Membrane lipid rafts: new targets for immunoregulation. Curr Mol Med. 2002;2:557–570. doi: 10.2174/1566524023362122. [DOI] [PubMed] [Google Scholar]
- 52.Werlen G, Palmer E. The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr Opin Immunol. 2002;14:299–305. doi: 10.1016/s0952-7915(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 53.Cook RT, Ballas ZK, Waldschmidt TJ, Vandersteen D, LaBrecque DR, Cook BL. Modulation of T-cell adhesion markers, and the CD45R and CD57 antigens in human alcoholics. Alcohol Clin Exp Res. 1995;19:555–563. doi: 10.1111/j.1530-0277.1995.tb01548.x. [DOI] [PubMed] [Google Scholar]
- 54.Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 56.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol. 2007;292:L813–823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- 57.Chen XJ, Seth S, Yue G, Kamat P, Compans RW, Guidot D, Brown LA, Eaton DC, Jain L. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2004;287:L366–373. doi: 10.1152/ajplung.00011.2004. [DOI] [PubMed] [Google Scholar]
- 58.Jerrells TR, Pavlik JA, DeVasure J, Vidlak D, Costello A, Strachota JM, Wyatt TA. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol. 2007;41:357–369. doi: 10.1016/j.alcohol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]