Abstract
A high-performance liquid chromatographic assay with tandem mass spectrometric detection was developed and validated for quantitation of the broad spectrum kinase inhibitor, flavopiridol, in human plasma. Sample preparation conditions included liquid-liquid extraction in acetonitrile (ACN), drying, and reconstitution in 20/80 water/ACN. Flavopiridol and the internal standard (IS), genistein, were separated by reversed phase chromatography using a C-18 column and a gradient of water with 25 mM ammonium formate and ACN. Electrospray ionization and detection of flavopiridol and genistein were accomplished with single reaction monitoring of m/z 402.09 > 341.02 and 271.09 > 152.90, respectively in positive-ion mode [M+H]+ on a triple quadrupole mass spectrometer. Recovery was greater than 90% throughout the linear range of 3 nM to 1,000 nM. Replicate sample analysis indicated within- and between-run accuracy and precision to be less than 13% throughout the linear range. This method has the lowest LLOQ reported to date for flavopiridol, and it allows for more accurate determination of terminal phase concentrations and improved pharmacokinetic parameter estimation in patients receiving an active dosing schedule of flavopiridol.
Introduction
Flavopiridol (FP) is a semi-synthetic flavonoid initially identified as a potent inhibitor of cyclin-dependent kinases (CDKs) [1–3]. FP has broad biological activity through inhibition of phosphokinases eliciting tumor cell growth inhibition and apoptosis through p-53 independent pathways [4–6], down-regulation of anti-apoptotic proteins, Mcl-1 and XIAP [7] and inhibition of RNA polymerase II [8].
With promising preclinical activity, FP is credited as the first CDK inhibitor to enter human clinical trials [9]. Numerous phase I and phase II trials in adults and children with various hematologic and solid tumor malignancies were completed since then [10–17]. Unfortunately, minimal responses were observed in these trials.
An additional component of each of the reported clinical studies was the evaluation of FP pharmacokinetics (PK) in order to better understand drug disposition in the respective patient populations. The utility of PK information depends heavily on the quality of the data used for parameter estimation. A validated analytical method with sensitivity adequate for accurately measuring FP concentrations beyond two or three biological half-lives is necessary if the PK data will be used for predictive purposes. Several analytical methods have been published throughout the preclinical and clinical development of FP. These assays utilize high performance liquid chromatography (HPLC) for separation and either ultraviolet (UV) light absorption [18–23], electrochemical (EC) [24], or mass spectrometry (MS) [11,23,25] detection methods. UV methods provide modest sensitivities with lower limits of quantitation (LLOQ) between 37 and 90 nM, while EC and MS methods are significantly more sensitive with LLOQ reported at 10 (EC) and 6 to 11.5 nM (MS), respectively.
In support of multiple phase I and II clinical trials, we sought to develop and validate a highly sensitive method for FP quantitation in human plasma. Using standard sample preparation procedures, liquid chromatography separation and mass spectrometry detection, we established a rapid and simple assay for FP quantitation in patient plasma samples. This analytical method achieves the highest sensitivity (3 nM LLOQ) of any method reported to date, enabling improved characterization of FP pharmacokinetics and more complete determination of FP disposition. Herein, we report the full validation of this method for use in National Cancer Institute (NCI) sponsored clinical trials.
Materials
Human plasma was obtained from the American Red Cross (Columbus, OH). FP (NSC 649890, HMR 1275, Alvocidib, (−)cis-5,7-dihydroxy-2-(2-chlorophenyl)-8-(4R-(3S-hydroxy-1-methyl) piperidinyl)-4H-1-benzopyran-4-one, C21H20O5NCl, MW 401.84) was obtained from the NCI as a hydrochloride salt with MW 438.29. Ammonium formate, HPLC grade water and acetonitrile (ACN) were obtained from Thermo Fisher Scientific (Waltham, MA). The internal standard (IS), genistein (MW 270.24), and all other chemicals were obtained from Sigma (St. Louis, MO) unless otherwise noted.
Preparation of Stock Solutions and Calibration Samples
Stock solutions of FP and genistein were produced in dimethylsulfoxide (DMSO) at a concentration of 1 mM and stored in polypropylene centrifuge tubes (Life Science Products, Rochester, NY) at −20°C for up to 2 months. ACN containing 200 nM genistein was produced from IS stock solution and stored at −20°C for up to 2 months. The stock solution of FP was used to produce 10X solutions in DMSO. These were subsequently diluted 1:10 in blank human plasma to produce standard samples (500 μl total volume) at varying concentrations between 3 nM and 1 μM in polypropylene centrifuge tubes. Blank and zero samples were produced by adding DMSO (without drug) to plasma and extracting with ACN (without IS) or ACN with 200 nM IS, respectively.
Sample Processing
ACN containing IS (1.0 ml) was added to each sample (500 μl) to precipitate proteins and extract FP. After vortexing (15 s) and centrifugation (16,000 × g, 4°C, 10 min.) the supernatant was transferred to a clean centrifuge tube and dried in a refrigerated speed-vac system (Thermo Fisher). Samples were reconstituted in 150 μl 20/80 water/ACN, vortexed, centrifuged at 16,100 × g at 4°C for 10 min., and 120 μl was loaded into polypropylene inserts (Thermo Fisher) in autosampler vials for analysis.
Sample Analysis
Reconstituted samples were analyzed on an Agilent (Santa Clara, CA) 1100 HPLC system connected to a ThermoFisher TSQ Quantum Discovery Max mass spectrometer operated by LCQuan software. The HPLC system comprised a dual pump with static mixer, degasser, heated column compartment and well-plate autosampler. Samples (20 μl injections) were separated on a reversed phase Zorbax (Agilent) C-18 column (3.5 μm, 2.1 × 50 mm) with a Metaguard (Varian, Walnut Creek, CA) C-18 guard column (5 μm, 2 × 10 mm). Mobile phases were 95/5 water/ACN with 25 mM ammonium acetate (A) and 95/5 ACN/water (B). Initial mobile phase composition was 10% B with a gradient to 100% B from 0.3 to 1.3 min. This was held for 2.9 min. followed by a 0.1 min. gradient return to initial conditions for equilibration until the end of the 8.7 min. run. The flow rate remained constant at 0.4 ml/min throughout the run.
FP and IS were ionized via electrospray ionization (ESI) and fragmented with collision gas for analysis using single reaction monitoring (SRM) in positive ion mode. Parameters were adjusted to optimize fragment ion intensities, and proposed reaction mechanisms and fragment ion structures were generated with Mass Frontier software (Thermo Fisher, v. 2.0). Final TSQ parameter settings were as follows: collision energy, 28 V; scan time, .05 s; scan width, .002 m/z; Q1 and Q3 peak widths, 0.7 full width at half maximum m/z; chrom filter, 8 s; collision gas pressure, 1.5 mTorr. HPLC flow was directed to the ion source from 2.4 to 4.4 min. and diverted to waste at all other times. Mass transitions monitored were 402.09 > 341.02 (FP) and 271.09 > 152.90 (IS), [M + H]+ (see Figure 1). Peak areas were integrated using the Interactive Chemical Integration System (ICIS) algorithm, and least squares regression was employed with 1/x weighting to fit a straight line to the peak area ratio (FP/IS) vs. concentration data. No blank or zero samples were used for curve fitting, and the line was not forced through zero.
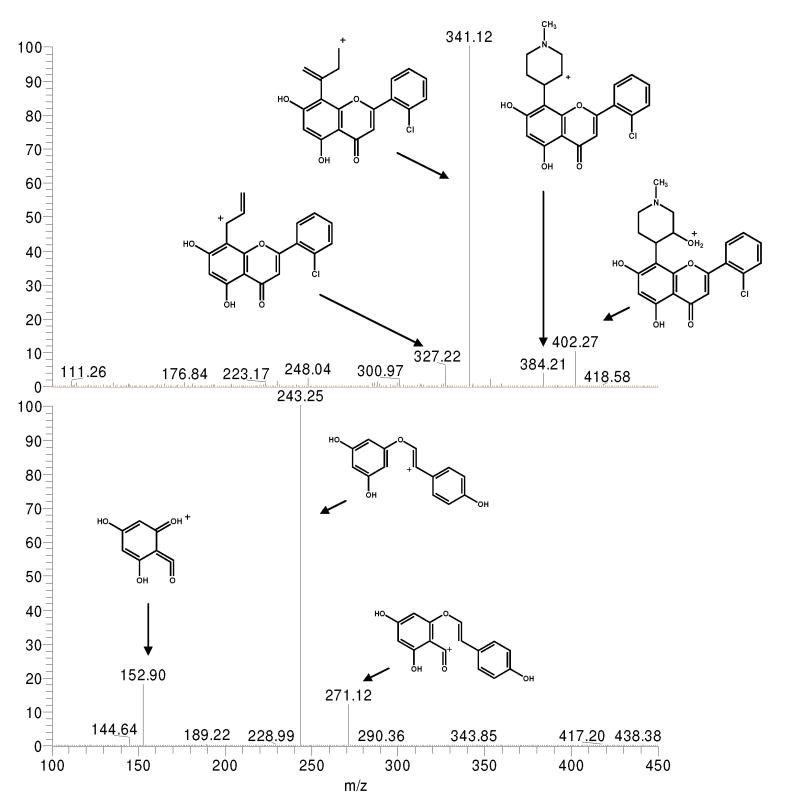
Figure 1.
Product ion scans and proposed structures for prominent fragment ions of FP (panel A) and IS (panel B). Samples were infused directly via a syringe pump and mixed with LC flow. LCMS parameters were set as indicated in the text.
Method Validation
Method validation for FP was conducted according to the Food and Drug Administration guidelines [26]. Calibration standards were prepared for each analysis at concentrations of 3, 10, 30, 100, 300 and 1000 nM. Quality control (QC) validation samples were prepared at 6, 60 and 600 nM concentrations. Validation runs included blank (no analyte or IS) and zero (IS only) samples for selectivity assessment in plasma from 8 volunteers. Stock solution stability was evaluated by producing replicate QCs (6, 60, 600 and 1000 nM) with freshly made stock and stock that had been stored at −20°C for 2 months. Replicate plasma QC samples were aliquoted (500 μl) and stored at −70°C for freeze-thaw and long-term stability. Sets of QC samples were removed, thawed then placed back into the freezer for a minimum of 24 hours. This was repeated for a total of three freeze-thaws, and samples were analyzed on the day of the final thaw within 2 weeks after initial freezing. Other sets of QC samples were analyzed after 2 months, and an additional replicate set of samples at 1000 nM was analyzed after 9 months at −70° C. Short-term room-temperature stability was evaluated by producing QC samples and allowing them to remain at room temperature for 8 hours before processing. Post-preparative autosampler stability was determined by reinjection of samples 28 hours following initial injection. To evaluate the validity of sample dilution, samples were produced at 1 and 3 μM and diluted in plasma 1:5 and 1:10 before extraction and addition of IS. Recovery was assessed by comparison of chromatographic peak areas and peak area ratios (FP/IS) in neat solution (20/80 water/ACN) vs. extracted plasma. Ion suppression via matrix effect was evaluated by adding FP with and without IS to dried plasma during the reconstitution step and comparing FP peak areas to neat solution samples.
Results
Assay Conditions
The choice of genistein as a suitable internal standard was based on structural similarity to FP and a commercially available supply. Liquid-liquid sample preparation methods were initially evaluated and found to provide excellent recovery from plasma with greater than 90% recovered throughout the linear range.
The responses of FP and IS were evaluated with electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), both in positive and negative modes. Positive mode ESI was chosen because of superior response and sensitivity over APCI and negative mode ESI under the described method conditions. Although no carry-over from previous samples was observed for FP and IS after low concentration injections, minimal residual FP signal was evident in blanks injected after high concentrations (e.g. 1,000 nM). To minimize residual signal and to avoid inaccuracies in patient sample analysis, a 10 second needle wash with 50% ACN was applied with every injection, and all patient sample injections proceeded with presumed lower before higher concentrations (i.e. pre-dose samples followed by reverse time order for post-dose PK samples; low, medium and high QCs placed early, middle or late, respectively, within a patient set of samples). Additionally, two blank solvent injections were placed before each set of individual patient samples. Gradient conditions were established to elute FP and IS at 2.90 and 2.98 minutes, respectively. Although a previously reported method suggested the requirement of mobile phase pH below 3 or above 11 for minimal tailing and acceptable peak shape [24], we found a pH of 4.2 with ammonium acetate (50 mM) to be adequate. However, initial methods with ammonium acetate were abandoned due to salt build-up in the ion source housing. Evaluation of ammounium formate indicated no salt build-up and minimal tailing, and it was therefore chosen for the final method.
Product ion scans indicated several fragments originating from the FP and IS parent ions. Spectra illustrating mass fragmentation patterns and proposed positively charged product ions are shown in Figure 1. The proposed fragment ions for FP arise from restructuring of the piperidinyl group, similar to recently reported fragmentation pathways for the glycosyl group of the isoflavone glycoside, puerarin [27]. Proposed fragment ions for IS are also similar to previously reported results with bond breakage in the central ring of genistein [28]. Product ions with high intensity and maximum signal-to-noise ratio (SN) were chosen for quantitation with SRM. Transitions selected for FP and IS were 402.09 > 341.02 and 271.09 > 152.90, respectively.
Recovery, Selectivity and Sensitivity
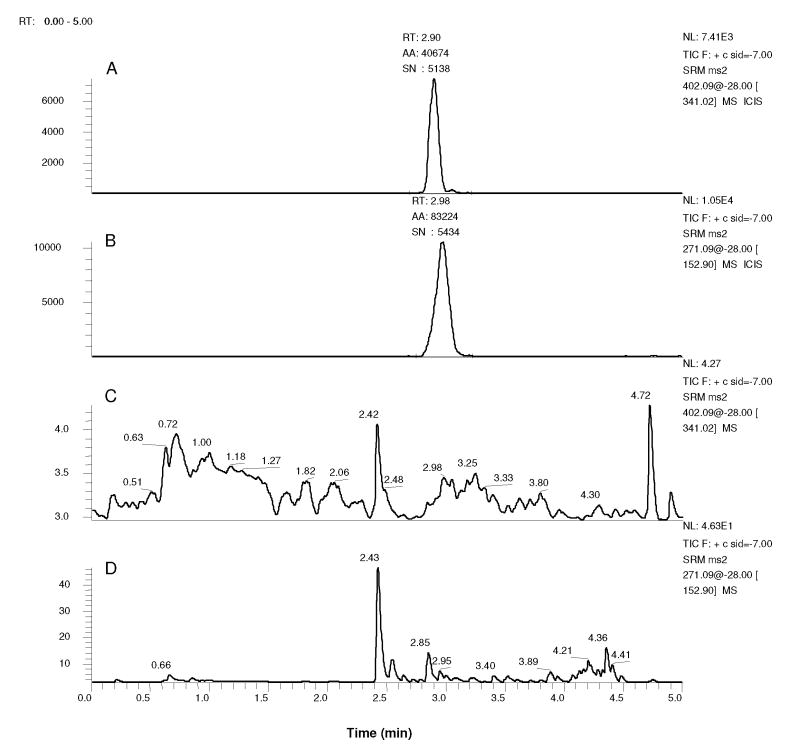
No interfering peaks were observed in blank extracted plasma samples from 8 volunteers using the established method conditions. Additionally, no FP signals have been observed in more than 120 pre-dose patient samples evaluated to date. Figure 2 displays representative chromatograms of blank plasma and plasma spiked with 3 nM FP. Blank plasma chromatograms show only background signal with absolute intensities less than 5 or 50 in the FP and IS filters, respectively (chromatograms C and D). Representative of the 3 nM FP spiked plasma samples from the 8 volunteers, an FP peak, with SN of 5,138 and intensity of 7,410, is observed in chromatogram A of Figure 2. Although the lower limit of detection (LLOD) was not determined in this study, SN levels at 3 nM suggest picomolar concentrations of FP would be detectable. The 3 nM LLOQ is reflective of nonlinearity as opposed to a lack of sensitivity. FP peak areas from quintuplicate spiked plasma QC samples at 6, 60 and 600 nM were 109.5%, 90.3% and 99.0% of the mean from neat solution FP peak areas at the same respective concentrations indicating greater than 90% recovery at all concentrations. Similarly, matrix samples were 106.8%, 98.5% and 94.3% compared to mean neat peak areas. Together, this data indicates recovery greater than 90% and negligible matrix effect from plasma. Peak areas from quintuplicate samples spiked with 3 nM FP with and without IS were 81,054 ± 9189 and 79,800 ± 5572, respectively indicating no measureable differences.
Figure 2.
Mass chromatograms of 450 μl plasma spiked with 50 μl 30 nM FP in DMSO (A and B, final FP concentration is 3 nM) or DMSO alone (C and D). Samples were processed with 1 ml ACN either containing 200 nM IS (A and B) or not (C and D). Chromatograms indicate signal with mass filter m/z 402.09 > 341.02 (A and C) or 271.09 > 152.90 (B and D). Peak detection was applied to chromatograms A and B with the ICIS algorithm. Peaks are labeled with retention times (RT), integrated peak areas (AA) and SN.
Accuracy and Precision
Validation runs consisted of spiked standard plasma samples at 3, 10, 30, 100, 300 and 1000 nM with quintuplicate QCs prepared at 6, 60 and 600 nM. Linearity was achieved with R2 values of 0.998 or greater using 1/X weighting. Table 1 lists mean calculated FP concentrations from 5 separate runs. Within-run and between-run accuracy and precision values calculated from quintuplicate QCs are displayed in Table 2. Between-run accuracy and precision was determined from three validation runs using grand means and standard deviations of calculated QC concentrations (n=15). Accuracy and precision values meet the acceptable FDA criteria with 11% or less variation throughout the linear range.
Dilution of plasma samples will be required with anticipated FP concentrations in the 1 to 5 μM range using clinically acceptable dosing regimens. To evaluate the effects of dilution, quintuplicte plasma samples spiked with 1 and 3 μM FP were diluted 1:5 and 1:10 in blank plasma. After processing as described above and applying appropriate dilution factors, FP accuracy and precision were within 12% as indicated in Table 2. This data supports validity for sample dilution.
Stability
The FP stock solution was stable after 2-months in storage at −20°C with an undetectable loss of compound at the 3 QC levels (mean 98.3 ± 9.5%, n=15) after 2 months. Autosampler stability was determined by re-injecting samples 28 hours after an initial injection. Results indicated QC concentrations from later injections were 93.6 ± 7.9% of the original concentrations (N = 15). Short-term (100.1 ± 9.3%) and long-term storage (94.6 ± 11.2%) and freeze-thaw (94.2 ± 8.6%) stability data were similar with minimal or no detectable degradation.
Flavopiridol Pharmacokinetics
Application of this method is underway for analysis of clinical samples from ongoing phase I and II trials in hematologic and solid tumor cancers (NCI-5745, NCI-7204, NCI-5746, NCI-6947, NCI-7000, NCI-7002). Figure 4 displays FP concentration vs. time data from two patients with chronic lymphocytic leukemia (CLL) treated in NCI-5746. As members of the fourth cohort in this trial, these patients received 30-minute infusions of 30 mg/m2 followed by 4-hour infusions of 30 or 50 mg/m2 for totals of 60 and 80 mg/m2 on days 1 and 8, respectively. Three of the concentrations displayed on the plot are between 3 and 5 nM, and these occur 24 to 48 hours after start of drug infusion. The LLOQ of 3 nM achieved in this method enables accurate quantitation at these later time points and consequently enables terminal-phase PK parameter estimation with improved accuracy compared to the previously published methods with lower reported sensitivities. With a response rate of 50% as a single agent in patients with CLL [29], this dosing schedule is under investigation in the trials mentioned above. Accurate PK parameter estimations in the patient populations evaluated in these trials will therefore rely on a validated assay with sensitivity comparable to the method reported here.
Discussion
The quality of pharmacokinetic and drug disposition data is dependent on the accuracy, precision and sensitivity of the analytical methods used to measure the drug and/or its metabolites. Several assays for quantitation of FP have been reported, and many of these have been used for clinical pharmacokinetic parameter estimation [11,12,18–25]. Interestingly, half-lives in the 5-hour range were typically calculated with analytical methods using UV detection, whereas the longer half-lives were determined with more sensitive methods using MS or EC detection. Most of the UV detection methods were capable of accurate quantitation of FP levels through 12 to 24 hours after end of infusion, often relying on portions of the distribution phase for elimination rate constant estimation. One exception to this is the study by Bible and colleagues, whereby concentrations were reported at 48 hours after end of infusion with a UV detection method. Mean non-compartmental elimination half-lives of 20 to 24 hours were calculated from their data [18]. However, the 48 and 72-hour plasma FP concentrations (24 and 48 hours after the end of the 24-hour continuous infusion) presumably used in their calculations appeared to be at or near their LLOQ of 50 nM (.02 μg/ml). These longer estimated half-lives are potentially attributable to overestimated terminal concentrations. Even with LC/MS assays, sensitivity has been inadequate for accurate quantitation during the terminal phase. In a study by Schwartz and colleagues, several terminal phase sample concentrations could not be determined as they were below their LLOQ of 11.5 nM [12].
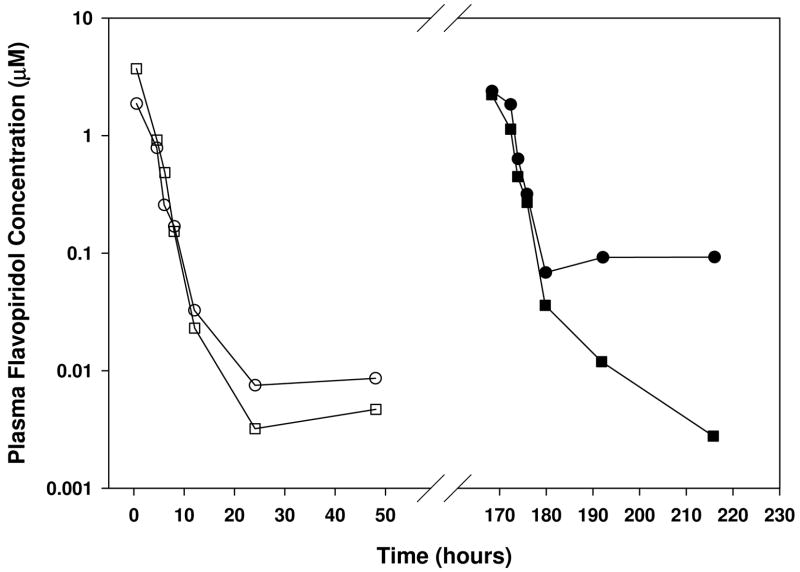
Accurate determination of flavopiridol concentrations beyond 24 hours is essential for improved PK parameter estimation. The most sensitive of previously reported assays indicated an LLOQ of approximately 6 nM using only 250 μl of plasma [13]. Herein, we report an assay that achieves an LLOQ of 3nM. Importantly, this assay has enabled quantitation of plasma FP through 48 hours with the most active dosing schedule reported in clinical trials [29]. Non-compartmental PK analysis of this data estimated FP half-lives at 12 to 14 hours with the 30-minute/4-hour bolus/infusion dosing schedule, indicating coverage up to nearly 4 half-lives. Although this assay requires more plasma (500 μl) compared to some of the previously reported methods, the additional sensitivity obtained is critical as 48-hour concentrations sometimes measure between 3 and 6 nM (examples are shown in Figure 3).
Figure 3.
Flavopiridol plasma concentration vs. time plots for two patients (circles and squares) receiving an initial 60 mg/m2 dose (open symbols) and an escalated second dose of 80 mg/m2 (filled symbols) on treatment days one and eight, respectively.
Dosing schemes targeting plasma concentrations similar to active preclinical in vitro concentrations have failed to produce significant responses in phase I and II clinical studies. Although most of these studies reported only few or no responses, recent reports with modified dosing schedules indicate significant activity in patients with chronic lymphocytic leukemia (CLL) [29]. With the development of active dosing schedules in CLL, clinical FP evaluation is underway in various other malignancies, including multiple myloma, acute lymphoblastic leukemia, acute myeloid leukemia, Non-Hodgkin’s lymphoma, and solid tumors. In our experience, plasma levels have been quantifiable at all time points throughout these patient populations with the use of this method. Reports of these results are forthcoming as trials are completed.
Acknowledgments
This work was supported by grants from the National Cancer Institute U01 CA76576 (MRG) and CA016058, the Leukemia and Lymphoma Society Specialized Center of Research (JCB, MP, JD, MG, TL), R21 CA112947-01A1 (TL, MP, JD, MG, and JB), and the College of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Losiewicz MD, Carlson BA, Kaur G, Sausville EA, Worland PJ. Biochem Biophys Res Commun. 1994;201:589. doi: 10.1006/bbrc.1994.1742. [DOI] [PubMed] [Google Scholar]
- 2.Worland PJ, Kaur G, Stetler-Stevenson M, Sebers S, Sartor O, Sausville EA. Biochem Pharmacol. 1993;46:1831. doi: 10.1016/0006-2952(93)90590-s. [DOI] [PubMed] [Google Scholar]
- 3.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Cancer Res. 1996;56:2973. [PubMed] [Google Scholar]
- 4.Byrd JC, Shinn C, Waselenko JK, Fuchs EJ, Lehman TA, Nguyen PL, Flinn IW, Diehl LF, Sausville E, Grever MR. Blood. 1998;92:3804. [PubMed] [Google Scholar]
- 5.Pepper C, Thomas A, Fegan C, Hoy T, Bentley P. Leuk Lymphoma. 2003;44:337. doi: 10.1080/1042819021000029984. [DOI] [PubMed] [Google Scholar]
- 6.Sausville EA, Johnson J, Alley M, Zaharevitz D, Senderowicz AM. Ann N Y Acad Sci. 2000;910:207. doi: 10.1111/j.1749-6632.2000.tb06710.x. [DOI] [PubMed] [Google Scholar]
- 7.Kitada S, Zapata JM, Andreeff M, Reed JC. Blood. 2000;96:393. [PubMed] [Google Scholar]
- 8.Chao SH, Price DH. J Biol Chem. 2001;276:31793. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 9.Senderowicz AM. Invest New Drugs. 1999;17:313. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- 10.Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, Hill K, Steinberg SM, Figg WD, Tompkins A, Arbuck SG, Sausville EA. J Clin Oncol. 1998;16:2986. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GK, Ilson D, Saltz L, O'Reilly E, Tong W, Maslak P, Werner J, Perkins P, Stoltz M, Kelsen D. J Clin Oncol. 2001;19:1985. doi: 10.1200/JCO.2001.19.7.1985. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GK, O'Reilly E, Ilson D, Saltz L, Sharma S, Tong W, Maslak P, Stoltz M, Eden L, Perkins P, Endres S, Barazzoul J, Spriggs D, Kelsen D. J Clin Oncol. 2002;20:2157. doi: 10.1200/JCO.2002.08.080. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro GI, Supko JG, Patterson A, Lynch C, Lucca J, Zacarola PF, Muzikansky A, Wright JJ, Lynch TJ, Jr, Rollins BJ. Clin Cancer Res. 2001;7:1590. [PubMed] [Google Scholar]
- 14.Stadler WM, Vogelzang NJ, Amato R, Sosman J, Taber D, Liebowitz D, Vokes EE. J Clin Oncol. 2000;18:371. doi: 10.1200/JCO.2000.18.2.371. [DOI] [PubMed] [Google Scholar]
- 15.Lin TS, Howard OM, Neuberg DS, Kim HH, Shipp MA. Leuk Lymphoma. 2002;43:793. doi: 10.1080/10428190290016908. [DOI] [PubMed] [Google Scholar]
- 16.Flinn IW, Byrd JC, Bartlett N, Kipps T, Gribben J, Thomas D, Larson RA, Rai K, Petric R, Ramon-Suerez J, Gabrilove J, Grever MR. Leuk Res. 2005;29:1253. doi: 10.1016/j.leukres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Peterson BL, Gabrilove J, Odenike OM, Grever MR, Rai K, Larson RA. Clin Cancer Res. 2005;11:4176. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 18.Bible KC, Lensing JL, Nelson SA, Lee YK, Reid JM, Ames MM, Isham CR, Piens J, Rubin SL, Rubin J, Kaufmann SH, Atherton PJ, Sloan JA, Daiss MK, Adjei AA, Erlichman C. Clin Cancer Res. 2005;11:5935. doi: 10.1158/1078-0432.CCR-04-2566. [DOI] [PubMed] [Google Scholar]
- 19.Innocenti F, Stadler WM, Iyer L, Ramirez J, Vokes EE, Ratain MJ. Clin Cancer Res. 2000;6:3400. [PubMed] [Google Scholar]
- 20.Tan AR, Headlee D, Messmann R, Sausville EA, Arbuck SG, Murgo AJ, Melillo G, Zhai S, Figg WD, Swain SM, Senderowicz AM. J Clin Oncol. 2002;20:4074. doi: 10.1200/JCO.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 21.Whitlock JA, Krailo M, Reid JM, Ruben SL, Ames MM, Owen W, Reaman G. J Clin Oncol. 2005;23:9179. doi: 10.1200/JCO.2004.01.0660. [DOI] [PubMed] [Google Scholar]
- 22.Zhai SP, Sausville E, Figg WD. Biomedical Chromatography. 2002;16:379. doi: 10.1002/bmc.166. [DOI] [PubMed] [Google Scholar]
- 23.Karp JE, Passaniti A, Gojo I, Kaufmann S, Bible K, Garimella TS, Greer J, Briel J, Smith BD, Gore SD, Tidwell ML, Ross DD, Wright JJ, Colevas AD, Bauer KS. Clin Cancer Res. 2005;11:8403. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 24.Stinson SF, Hill K, Siford TJ, Phillips LR, Daw TW. Cancer Chemother Pharmacol. 1998;42:261. doi: 10.1007/s002800050815. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro GI. Clin Cancer Res. 2004;10:4270s. doi: 10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Guidance for Industry, Bioanalytical Method Validation, 2001.
- 27.Li H, Wan L, Hashi Y, Chen S. Rapid Commun Mass Spectrom. 2007;21:2497. doi: 10.1002/rcm.3087. [DOI] [PubMed] [Google Scholar]
- 28.March RE, Miao XS, Metcalfe CD, Stobiecki M, Marczak L. International Journal of Mass Spectrometry. 2004;232:171. [Google Scholar]
- 29.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, Moran M, Blum KA, Rovin B, Brooker-McEldowney M, Broering S, Schaaf LJ, Johnson AJ, Lucas DM, Heerema NA, Lozanski G, Young DC, Suarez JR, Colevas AD, Grever MR. Blood. 2007;109:399. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]