Abstract
Purpose
To determine the role of amifostine as a protectant against cisplatin-induced ototoxicity in patients with average risk (AR) medulloblastoma treated with craniospinal radiotherapy and 4 cycles of cisplatin-based dose-intense chemotherapy and stem cell rescue.
Patients and Methods
The primary objective was to determine whether, in patients with AR medulloblastoma (n=62), amifostine would decrease the need for hearing aids (defined as ≥ grade 3 ototoxicity in one ear) compared to a control group (n=35), one year from initiating treatment. (Figure 1) Ninety-seven patients received CSI (23.4 Gy) followed by 55.8 Gy to the primary tumor bed, using 3-D conformal technique and 4 cycles of high-dose cyclophosphamide (4000 mg/m2 per cycle), cisplatin (75 mg/m2 per cycle), and vincristine (two 1.5 mg/m2 doses per cycle) and stem cell rescue. When used, amifostine (600 mg/m2 per dose) was given as a bolus immediately prior to and 3 hours into the cisplatin infusion.
Results
The median age of the 97 patients was 8.7 years (range, 3.2–20.2 years). The study and control groups were similar in age and sex distribution. Amifostine was well-tolerated. One year after treatment initiation, 13 (37.1%) of the control-group versus 9 (14.5%; p=0.005 Chi-Square one-sided test) of the amifostine-treated patients had ≥ grade 3 ototoxicity, requiring hearing aid in at least one ear.
Conclusion
Amifostine administered prior to and during the cisplatin infusion can significantly reduce the risk of severe ototoxicity in patients with AR medulloblastoma receiving dose-intense chemotherapy.
Keywords: amifostine, ototoxicity, cisplatin
INTRODUCTION
Medulloblastoma is the most common malignant brain tumor of childhood. The addition of platinum-based chemotherapy to post-operative craniospinal irradiation has increased cure rates for patients with localized, resected medulloblastoma to over 80% and permitted reductions in the dose of craniospinal radiation.1,2 However, 23–50% of patients experience cisplatin-associated ototoxicity. 2–4
When administered before chemotherapy or radiation, amifostine, provides broad-spectrum cytoprotection of hematologic, renal, neural, and mucosal tissues without attenuating antitumor effect.5,6 Amifostine is rapidly converted by alkaline phosphatase to its active metabolite WR-1065.7,8 The much greater concentration of membrane-bound alkaline phosphatase in normal than in neoplastic tissue accounts for the differential protection. 9
The recommended adult single daily amifostine dose is 910 mg/m2 with hypotension as the dose-limiting toxicity.10–12 Evidence suggests that multiple daily doses of amifostine may improve its cytoprotective effects, especially with drugs such as cisplatin.13–15 A phase I pediatric study established the recommended pediatric dose of amifostine for a twice daily-dose regimen as 600 mg/m2 per dose.16
Studies of amifostine’s protective effect against cisplatin-induced ototoxicity have yielded conflicting preclinical 17,18 and clinical 5,19–25 results. The use of other agents including sodium thiosulfate26, 27, sodium salicylate28 and n-acetylcysteine27 have also been explored to protect against cisplatin-induced ototoxicity.
We prospectively investigated whether amifostine, administered prior to and during cisplatin infusion, would protect children with average risk (AR) medulloblastoma, who received craniospinal (CSI) irradiation followed by 4 cycles of platinum-based dose-intense chemotherapy, from developing grade 3 or 4 ototoxicity (requiring a hearing aid in at least one ear) one year after initiation of therapy.
PATIENTS AND METHODS
Patients
Between October 1996 and May 2005, 113 patients aged ≥3 and ≤21 years with newly diagnosed, previously untreated AR medulloblastoma received protocol–prescribed therapy at participating institutions. AR was classified according to a modified Chang staging system,29 and as previously described.1 Eligibility criteria for enrollment on this treatment protocol have been previously described.1 The protocol was reviewed and approved by the Institutional Review Board of all participating institutions and informed consent for treatment was obtained from all patients, parents or legal guardian, as appropriate.
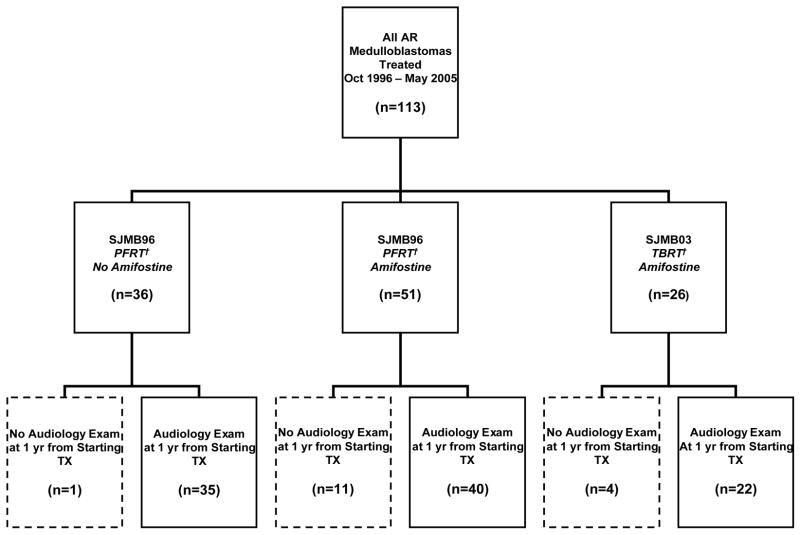
Figure 1 summarizes the treatment groups. The control arm comprised of 36 patients with medulloblastoma treated on a prospective trial (SJMB 96) described in the treatment section. One patient died within a year of starting treatment. Hence, 35 patients with audiology exams approximately one year from starting therapy were included in the control group. In August 1999, the protocol was amended to include amifostine. Fifty-one patients with AR medulloblastoma were enrolled on the amended study (SJMB 96). The follow-up study (SJMB03) expanded the biology components into primary objectives while retaining the same dose-intense cisplatin-based chemotherapy regimen with amifostine. The first 26 evaluable patients enrolled on SJMB03 were also included in the amifostine-treatment cohort.
Figure 1.
Distribution of AR Medulloblastoma Patients by Amifostine Administration and Treatment Protocol
† PFRT=Posterior Fossa Irradiation, TBRT=Tumor Bed Irradiation
Among the 77 patients with AR medulloblastoma included in the amifostine-treatment group, 15 were excluded from the analysis for the following reasons: off-study within one year due to toxicity (not amifostine-related) (n=5), off study at parents’ request (n=1), progressive disease (n=2), death (n=3), no amifostine given (physician preference) (n=1), no audiology exams within the appropriate time frame from study enrollment (n=2), or positive pregnancy test precluding use of high-dose chemotherapy (n=1). Thus, the amifostine-treatment group described and analyzed on this study comprised of 62 consecutive, evaluable patients treated with amifostine who had audiology assessments approximately one year from starting therapy.
After completing the initial analysis, we noted that 6 of the amifostine-treated patients who did not receive all 4 courses of cisplatin, did not experience severe ototoxicity. To ensure that the inclusion of these patients did not compromise the study findings, we reanalyzed the data after replacing these patients with 6 consecutive patients on the ongoing SJMB03 protocol who had received all courses of cisplatin and amifostine and had one year audiology follow-up.
Treatment
Maximal resection of the tumor was attempted in all patients. All patients received 23.4 Gy CSI and 55.8 Gy to the primary tumor bed, using 3-D conformal (3D-CRT) technique. Seventy-five patients on SJMB96 (35 without and 40 with amifostine, respectively) received an initial 12.6 Gy 3D-CRT boost to the posterior fossa (cumulative 36 Gy), followed by primary site irradiation to 55.8 Gy using a 2 cm clinical target volume margin; 22 patients enrolled on the SJMB03 study (all treated with amifostine) received primary site irradiation to 55.8 Gy using a 1 cm clinical target margin. After a 6-week rest, all patients began 4 cycles of high-dose chemotherapy as previously described.1,30
When given, amifostine, 600 mg/m2/dose, was administered intravenously over 1 minute, immediately prior to and 3 hours into the cisplatin infusion. Supportive care guidelines for amifostine included 1) adequate pre-hydration; 2) holding antihypertensive medication for 24 hours prior to infusion, 3) placing patients supine and monitoring blood pressure every 5–10 minutes for 25 minutes after amifostine infusion, administering a 20 ml/kg normal saline bolus over 1 hour with decreases of ≥ 20 mm systolic; monitoring of serum total and ionized calcium for 24 hours after amifostine administration and, if ionized calcium < 1.0 mmol/l, administration of intravenous calcium chloride (10–20 mg/kg/dose).
After observing mild to moderate hypocalcemia in several patients, we began prophylactic administration of continuous intravenous calcium chloride infusion (20 mg/kg) concurrently with the 6-hour cisplatin infusion and then again 6 hours after the completion of the cisplatin infusion, while monitoring ionized serum calcium for 24 hours. Additional supportive care and stem-cell collection procedures have been described previously.1,30
Patient monitoring and follow-up
During protocol therapy, patients’ disease status and toxicities (graded using the National Cancer Institute’s common toxicity criteria) were monitored with appropriate laboratory assessments and imaging studies. After completion of therapy, follow-up examinations and imaging were performed every 3 months for the first 18 months, every 6 months until 5 years, and yearly thereafter.
Hearing Evaluations
Audiograms were obtained at diagnosis, after radiotherapy completion, after each cycle of chemotherapy, and 6 weeks, 6 months, one year and thereafter annually after the completion of all therapy. Evaluation of hearing depended on the patient’s age, development, and cooperation. Conventional audiometry was generally performed on patients ≥ 5 years: the patient would sit in a sound-booth and indicate when a pure tone stimulus was heard by raising his or hand or by pressing a button. Conditioned play was typically performed on children aged 3–5 years: patients would sit in a sound-booth and indicate when a pure tone stimulus was heard by playing a simple game (i.e., throwing blocks into a bucket). Conventional pure-tone audiometry and conditioned play audiometry were obtained using a GSI-61 audiometer (Grason-Stadler, Inc., USA) with ER-3A/5A insert earphones and TDH-50 headphones. Air conduction thresholds were measured at 2.5, 5, 1, 2, 3, 4, 6, and 8 kHz.
The ototoxicity criteria were similar to criteria used in the recently completed phase III intergroup average-risk medulloblastoma protocol (A9961) (Table 1). Any patients with ≥ grade 3 ototoxicity (> 25 dB HL loss at 2000 Hz) required hearing aids. We obtained the thresholds from 2000–8000 HZ and the audiologist-determined toxicity grades for all exams. The audiology exam obtained closest to one year from study enrollment was selected for analysis. Grades were assigned by audiologists who performed exams as part of their routine clinical responsibilities. Grades were confirmed by plotting the thresholds and grades over time for each patient, ensuring that the thresholds were consistent with the grade assigned by the audiologist.
Table 1.
Ototoxicity Grading Criteria
| GRADE 1: | 40 dB HL loss at 6000 and/or 8000 Hz |
| GRADE 2: | > 25 dB HL loss at 3000 and/or 4000 Hz |
| GRADE 3*: | > 25 dB HL loss at 2000 Hz |
| GRADE 4*: | ≥ 40 dB HL loss at 2000 Hz |
A loss is defined as a change from baseline at any one frequency
Patient requires hearing aids
Eighty percent of patients had at least 1 audiology exam between 9 and 15 months; 19 patients (8 in the control group and 11 in the amifostine group) did not. For these patients we deduced the occurrence of ototoxicity at one year from study enrollment using thresholds and grades of the exam obtained prior to the one year date (median 7 months after study enrollment [range: 4.8–8.6 months]) and of the first exam after the one year date (median 1.6 years after study enrollment [1.3–2.5 years]).
The left and right cochlea were defined systematically on the treatment planning CT and the mean radiation dose for each cochlea was computed.
Dose Modifications for Ototoxicity
If patients experienced grade 3 ototoxicity, the cisplatin dose was reduced by 50%. For grade 4 ototoxicity, cisplatin was held and not restarted unless follow-up audiograms demonstrated improved hearing.
STATISTICAL DESIGN
The study objective was to investigate amifostine’s ability to protect children receiving a dose-intense cisplatin-based regimen from severe ototoxicity, defined as ≥grade 3 ototoxicity requiring hearing aids in at least one ear, one year after treatment initiation. We estimated31 that 62 patients receiving amifostine would be required for 80% power (α = 0.05, one-sided chi-square test) to detect a 20% reduction from the observed rate of 37% in the 35 AR patients treated on the protocol without amifostine. Two interim analyses32 were performed, after 24 patients and 32 patients; both resulting in continuation of the trial.
To investigate the effect of cochlear RT dose and treatment with amifostine on the occurrence of a grade 3 or 4 ototoxicity at one year from treatment initiation, a method utilizing generalized estimating equations (GEE),33 as implemented in PROC GENMOD of the SAS/STAT® software was used. PROC MIXED of the SAS/STAT software was used to investigate differences in the RT dose to the cochlea between the control group and those receiving amifostine. These repeated measures models analyze the cochlear doses from each ear while taking into consideration that the outcomes within a subject may be correlated.
RESULTS
Characteristics for the control (n=35) and amifostine (n=62) groups are summarized in Table 2.
Table 2.
Patient Characteristics
| Patient Characteristics | SJMB96 – PF*RT – No Amifostine (n=35) | SJMB96 – PF* RT Amifostine (n=40) | SJMB03 – TBRT* Amifostine (n=22) |
|---|---|---|---|
| AGE AT STUDY ENROLLMENT | |||
| Median | 7.81 | 9.23 | 8.43 |
| Min | 3.18 | 4.09 | 3.43 |
| Max | 17.16 | 20.16 | 17.70 |
| SEX [N (percent)] | |||
| F | 16 (45.7) | 14 (35.0) | 9 (40.9) |
| M | 19 (54.3) | 26 (65.0) | 13 (59.1) |
| RACE [N (percent)] | |||
| Asian | 1 (2.9) | 0 | 1 (4.6) |
| Black | 4 (11.4) | 6 (15.0) | 2 (9.1) |
| Hispanic | 5 (14.3) | 3 (7.5) | 0 |
| Other | 0 | 4 (10.0) | 3 (13.6) |
| White | 25 (71.4) | 27 (67.5) | 16 (72.7) |
| Received Four Courses of Cisplatin [N (percent)] | 31 (88.6) | 35 (87.5) | 19 (86.4) |
| Cumulative Dose of Cisplatin per m2 [Median (range)] | 301.1 (76.7 – 308.9) | 299.9 (79.0 – 306.0) | 299.6 (186.9 – 304.4) |
| TOTAL | 35 | 40 | 22 |
PFRT = Posterior Fossa Irradiation, TBRT= Tumor Bed Irradiation
Among the 35 patients in the control group, 4 (11%) did not receive all 4 courses of cisplatin because of development of grade 3 ototoxicity prior to completing 4 courses (n=3), and grade 1 ototoxicity in a blind patient (n=1; at physician’s discretion).
Eight of the 62 patients treated with amifostine (13%) did not receive all 4 courses of cisplatin. One received only one course of chemotherapy because of overwhelming sepsis; 7 received only 3 courses of cisplatin for the following reasons: grade 3 ototoxicity after course 3 (n=2), grade 2 ototoxicity with pre-existing grade 4 ototoxicity in the other ear (n=1), bilateral grade 2 ototoxicity with previous history of meningitis (n=1), decreased GFR (n=1), persistent thrombocytopenia (n=1) and osteomyelitis (n=1).
Hearing Loss
One year from study enrollment, 9 of 62 patients in the amifostine-group (14.5%) had grade 3 or 4 ototoxicity requiring hearing aids, compared to 13 of 35 (37.1%) in the control group (p=0.005, Table 3).
Table 3.
Frequency of Grade 3 or 4 Ototoxicity by Amifostine Administration and Study Cohort
| SJMB96 PFRT†– No Amifostine | SJMB96 PFRT†– Amifostine | SJMB03 TBRT†- Amifostine | |
|---|---|---|---|
| Grade 3 or 4 Ototoxicity | 13 (37.1) | 6 (15.0) | 3 (13.6) |
| No Grade 3 or4 Ototoxicity | 22 (62.9) | 34 (85.0) | 19 (86.4) |
| Total | 35 | 40 | 22 |
PFRT = Posterior Fossa Irradiation, TBRT= Tumor Bed Irradiation
Among amifostine-treated patients, the proportion of patients with severe ototoxicity was similar among the 40 who received a posterior-fossa boost (13.6%) and the 22 who did not (15.0%). If we restrict the comparison to include only those patients in the SJMB96 protocol (all of whom received the posterior-fossa boost), the proportion of patients with severe ototoxicity remains significantly less in the amifostine (n=40) compared to the control group (n=35, p=0.014).
Among the 8 amifostine-treated patients who received fewer than 4 courses of cisplatin, 6 did not experience severe ototoxicity one year after treatment initiation. Thus, we reanalyzed the data replacing those 6 patients in the amifostine group with 6 consecutive patients on the ongoing SJMB03 study, who received all four courses of cisplatin. Even with this new cohort, amifostine significantly decreased the percentage of patients experiencing severe ototoxicity: 10 of 62 (16%) vs.13 of 35 (37.1 %) (p=.010).
Cochlear radiation doses were available in 56 patients. Fourteen patients had ≥ grade 3 ototoxicity in at least one ear. The mean cochlear radiation dose with ≥ grade 3 ototoxicity was 49.4Gy (34.6–47.5Gy) compared to 49Gy (31.0–60Gy) in ears with < grade 3 ototoxicity (p=0.94).
In a univariate GEE model, no amifostine use was the only factor significantly associated with severe(≥ grade 3) ototoxicity (p=0.042); cochlear radiation dose was not (p=0.80). In a multi-variate GEE model including both cochlear dose and amifostine, only the absence of amifostine remained significantly associated with severe ototoxicity (p=.047).
Eighty-two patients had hearing assessments 2 years after treatment initiation. The incidence of severe ototoxicity in the control group (n=34) was 35% compared to 17% in the amifostine group (n=48, p=0.048). Although the number of amifostine-treated patients with 3-year follow-up was too small for adequate statistical analysis, amifostine continued to demonstrate a protective trend (data not shown).
Toxicities Related to Amifostine
Given the short half-life and toxicity profile of amifostine, we reviewed all adverse events that occurred within 48 hours of the amifostine infusion for all cycles. Table 4 includes grade 3 or 4 adverse events at least possibly attributable to amifostine.
Table 4.
Grade 3 and 4 Adverse Events Occurring Within 2 Days of Amifostine Administration and Considered At Least Possibly Attributable to Amifostine
| Adverse Event | No. of Occurrences (No. of Patients)† |
|---|---|
| Hypocalcemia | 3 (2) |
| Hypotension | 2 (2) |
| Nausea or Vomiting | 11 (8) |
Reported is the frequency observed in 62 patients receiving amifostine over 235 courses of treatment.
Progression-Free Survival
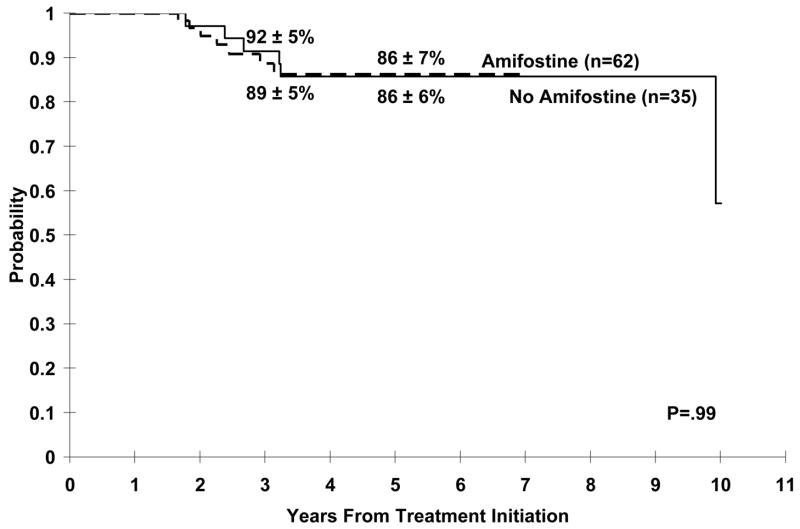
There was no difference in the progression-free survival (PFS) distributions between the control and amifostine groups (p=0.99) (figure 2); the median follow-up for those surviving in the control group was 8.1 years compared with 3.8 years in the amifostine group. 84% and 100% of the surviving patients in the control and amifostine groups, respectively, had follow-up within one year of data analysis.
Figure 2.
Event Free Survival With or Without Amifostine
DISCUSSION
This study demonstrates that amifostine, administered as a bolus infusion, before and during cisplatin (cumulative dose 300 mg/m2) infusion at 600 mg/m2/dose, significantly reduces the incidence of grade 3 or 4 ototoxicity in patients with AR medulloblastoma (p=0.005), without altering patients’ outcome.
Cisplatin ototoxicity, typically bilateral and high frequency in nature, is caused by damage to the outer hair cells in the organ of corti,34,35 the spiral ganglion and stria vascularis.36,37 The mechanism of cisplatin ototoxicity involves the formation of reactive oxygen species, generated in cells by cellular metabolism, inflammation and chemotherapy31. Amifostine’s active metabolite, WR1065, has been reported to attenuate cisplatin-induced toxicity by acting as a scavenger of oxygen free radicals38 and binding to the active species of platinum agents, to prevent 38,39 and reverse DNA platination.40
Few prospective studies have documented amifostine protection against cisplatin-induced ototoxicity. Although Kemp et al’s5 randomized phase III trial reported that pretreatment with amifostine resulted in a 43% reduction in the incidence of ototoxicity, other adult22,41 and pediatric trials23–25 have failed to show any protection.
Marina et al. reported the lack of protection of amifostine against cisplatin ototoxicity in children with extracranial, extragonadal germ cell tumors.23 Twenty-five children (15 evaluable for ototoxicity) received amifostine, 825 mg/m2 as a 15-minute infusion 30 minutes prior to cisplatin 40 mg/m2/day on days 1–5; 75% of patients had grade 2–4 ototoxicity, similar to historical controls. The lack of activity in Marina et al.’s study may reflect the schedule of amifostine and cisplatin administration, the higher total cisplatin dose, and the small sample size, which only had 80% statistical power to detect a 35% reduction in ototoxicity (74% versus 39%), had all 25 patients been evaluable for ototoxicity. Given the very short half-life of amifostine and its metabolite, WR1065, a single 15-minute infusion of amifostine, 30 minutes before cisplatin infusion may not be optimal. In the current non-randomized study, amifostine was given as a bolus over 1 minute, immediately prior to the cisplatin infusion and again 3 hours into the cisplatin infusion, based on evidence that WR1065 can reverse DNA platination and that multiple daily dosing may improve cytoprotection.13 14,15
Katzenstein et al reported the results of an unplanned interim analysis of amifostine’s effect on cisplatin-induced ototoxicity in a randomized trial of patients with hepatoblastoma. Patients received cisplatin 100 mg/m2 on day 1 with or without amifostine 740 mg/m2 over 15 minutes, prior to cisplatin. Analysis of 82 patients revealed significant hearing loss in 14% (5/37) of patients receiving amifostine versus 9% (4/45) of controls (p=0.72 ). Younger age, different dose, schedule and length of infusion of amifostine and cisplatin, may have contributed to the lack of protection reported. Further studies are needed to delineate the importance of these factors for amifostine-related protection against cisplatin-induced ototoxicity. However, this abstract reports on only 82 randomized patients, approximately 33% of the planned sample size. Futhermore, only 40% of the 203 eligible patients had sufficient data to be included in the analysis25.
Fisher et al. reported that 7 of 9 evaluable patients with newly diagnosed high risk and AR medulloblastoma or primitive neuroectodermal tumor treated with radiotherapy, cisplatin-based chemotherapy and amifostine, developed grade 2–3 hearing loss 1–3 years after treatment.24 Amifostine, 1000 mg/m2 was administered over 15 minutes prior to cisplatin and 4 hours into the 8 hour cisplatin infusion. However, study limitations include the small cohort, the inclusion of average and high risk patients, who received significantly different doses of CSI, combining patients with posterior fossa and supratentorial disease types, and the variability of the time points at which hearing was evaluated.
Amifostine was well-tolerated in the current study. Although mild to severe hypocalcemia has been reported in the literature in up to 80% of patients, our supportive care guidelines minimized the frequency and severity of hypocalcemia.
Long-term hearing deterioration after platinum therapy has been reported in the literature.4,43,44 We have also reported late, often unilateral hearing loss as radiation-related ototoxicity in up to 27% of patients after > 50Gy to the cochlea by the fifth year after radiotherapy,42 with a lower but dose-related incidence after 3D-CRT irradiation as used in the current report.43 A suggested dose threshold of 32 Gy in patients receiving chemotherapy was noted in the latter setting. In a report of cisplatin-treated patients followed for a median of 20.6 months after therapy, only those receiving CSI experienced mild progression of ototoxicity4. In contrast, Bertolini et al reported continued deterioration of hearing in platinum-treated patients followed for more than 2 years.44 To address the sustainability of amifostine’s protection against cisplatin ototoxicity over time, we demonstrated a continued trend towards otoprotection by amifostine at 2 and 3 years after initiation of therapy.
Amifostine did not confer tumor protection as evidenced by similar PFS in the control and amifostine-treated patients. Although the median follow-up for the amifostine cohort was shorter (3.4 years) than controls (7.6 years), median time to progression was comparable (2.7 years [controls] vs. 2.9 years [amifostine group]).
In summary, in patients with AR medulloblastoma treated prospectively with cisplatin-based, dose-intense chemotherapy, twice-daily doses of amifostine, 600 mg/m2/dose prior to and during the cisplatin infusion significantly decreased the need for hearing aids.
Acknowledgments
Supported in part by grants P30 CA21765 from the National Cancer Institute, The Pediatric Brain Tumor Foundation, The Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), The Ryan McGhee Foundation and by the American Lebanese Syrian Associated Charities (ALSAC)
References
- 1.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 4.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 5.Kemp G, Rose P, Lurain J, et al. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol. 1996;14:2101–2112. doi: 10.1200/JCO.1996.14.7.2101. [DOI] [PubMed] [Google Scholar]
- 6.Glover D, Glick JH, Weiler C, et al. WR-2721 protects against the hematologic toxicity of cyclophosphamide: a controlled phase II trial. J Clin Oncol. 1986;4:584–588. doi: 10.1200/JCO.1986.4.4.584. [DOI] [PubMed] [Google Scholar]
- 7.Romanul FCA, Bannister RG. Histochemistry:localized areas of high alkaline phosphatase activity in endothelium of arteries. Nature. 1962;195:611–612. doi: 10.1038/195611a0. (abstr) [DOI] [PubMed] [Google Scholar]
- 8.Calabro-Jones PM, Aguilera JA, Ward JF, et al. Uptake of WR-2721 derivatives by cells in culture: identification of the transported form of the drug. Cancer Res. 1988;48:3634–3640. [PubMed] [Google Scholar]
- 9.Yang JL, fernandez DJ, Speicher L. Biochemical determinants of the cytoprotective effects of amifostine. Proc Am Asoc Cancer Research. 1995;36:290. (abstr) [Google Scholar]
- 10.Kligerman MM, Shaw MT, Slavik M, et al. Phase I clinical studies with WR-2721. Cancer Clin Trials. 1980;3:217–221. [PubMed] [Google Scholar]
- 11.Turrisi AT, Glover DJ, Hurwitz S, et al. Final report of the phase I trial of single-dose WR-2721 [S-2-(3- aminopropylamino)ethylphosphorothioic acid] Cancer Treat Rep. 1986;70:1389–1393. [PubMed] [Google Scholar]
- 12.Glover D, Glick JH, Weiler C, et al. Phase I trials of WR-2721 and cis-platinum. Int J Radiat Oncol Biol Phys. 1984;10:1781–1784. doi: 10.1016/0360-3016(84)90549-2. [DOI] [PubMed] [Google Scholar]
- 13.Green D, Bensely D, Schein P. Preclinical evaluation of WR-151327: an orally active chemotherapy protector. Cancer Res. 1994;54:738–741. [PubMed] [Google Scholar]
- 14.Betticher DC, Anderson H, Ranson M, et al. Carboplatin combined with amifostine, a bone marrow protectant, in the treatment of non-small-cell lung cancer: a randomised phase II study. Br J Cancer. 1995;72:1551–1555. doi: 10.1038/bjc.1995.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budd GT, Lorenzi V, Ganapathi R, et al. Amifostine: potential for clinically useful cytoprotection. Support Care Cancer. 1994;2:380–384. doi: 10.1007/BF00344052. [DOI] [PubMed] [Google Scholar]
- 16.Fouladi M, Stempak D, Gammon J, et al. Phase I trial of a twice-daily regimen of amifostine with ifosfamide, carboplatin, and etoposide chemotherapy in children with refractory carcinoma. Cancer. 2001;92:914–923. doi: 10.1002/1097-0142(20010815)92:4<914::aid-cncr1401>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Church MW, Kaltenbach JA, Blakley BW, et al. The comparative effects of sodium thiosulfate, diethyldithiocarbamate, fosfomycin and WR-2721 on ameliorating cisplatin-induced ototoxicity. Hear Res. 1995;86:195–203. doi: 10.1016/0378-5955(95)00066-d. [DOI] [PubMed] [Google Scholar]
- 18.Hyppolito MA, Oliveira AA, Lessa RM, et al. Amifostine otoprotection to cisplatin ototoxicity: a guinea pig study using otoacoustic emission distortion products (DPOEA) and scanning electron microscopy. Rev Bras Otorrinolaringol (Engl Ed) 2005;71:268–273. doi: 10.1016/S1808-8694(15)31322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin JS, Wadler S, Beitler JJ, et al. Audiological findings in a Phase I protocol investigating the effect of WR 2721, high-dose cisplatin and radiation therapy in patients with locally advanced cervical carcinoma. J Laryngol Otol. 1995;109:744–747. doi: 10.1017/s0022215100131202. [DOI] [PubMed] [Google Scholar]
- 20.Mollman JE, Glover DJ, Hogan WM, et al. Cisplatin neuropathy. Risk factors, prognosis, and protection by WR- 2721. Cancer. 1988;61:2192–2195. doi: 10.1002/1097-0142(19880601)61:11<2192::aid-cncr2820611110>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Kaltenbach JA, Church MW, Blakley BW, et al. Comparison of five agents in protecting the cochlea against the ototoxic effects of cisplatin in the hamster. Otolaryngol Head Neck Surg. 1997;117:493–500. doi: 10.1016/S0194-59989770020-2. [DOI] [PubMed] [Google Scholar]
- 22.Ekborn A, Hansson J, Ehrsson H, et al. High-dose Cisplatin with amifostine: ototoxicity and pharmacokinetics. Laryngoscope. 2004;114:1660–1667. doi: 10.1097/00005537-200409000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Marina N, Chang KW, Malogolowkin M, et al. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors: a Children’s Oncology Group study. Cancer. 2005;104:841–847. doi: 10.1002/cncr.21218. [DOI] [PubMed] [Google Scholar]
- 24.Fisher MJ, Lange BJ, Needle MN, et al. Amifostine for children with medulloblastoma treated with cisplatin-based chemotherapy. Pediatr Blood Cancer. 2004;43:780–784. doi: 10.1002/pbc.20132. [DOI] [PubMed] [Google Scholar]
- 25.Katzenstein HM, Chang K, Krailo M, et al. A randomized study of platinum-based chemotherapy with or without amifostine for the treatment of children with hepatoblastoma: A report of the Intergroup Hepatoblastoma Study P9645. Proc Am Soc Clin Oncol. 2004;22:8518. [Google Scholar]
- 26.Muldoon LL, Pagel MA, Kroll RA, et al. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6:309–315. [PubMed] [Google Scholar]
- 27.Dickey DT, Wu YJ, Muldoon LL, et al. protection against cisplatin-induced toxicities by N-acetylcystein and sodium thiosulfate as assessed at the molecular, cellular and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–8. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 28.Hyppolito MA, de Oliveira JA, Rossato M. Cisplatin otoprotection with sodium salicylate. Eur Arch Otorhinolaryngol. 2006;263:798–803. doi: 10.1007/s00405-006-0070-6. [DOI] [PubMed] [Google Scholar]
- 29.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 30.Strother D, Ashley D, Kellie SJ, et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem- cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiotherapy: results of a collaborative study. J Clin Oncol. 2001;19:2696–2704. doi: 10.1200/JCO.2001.19.10.2696. [DOI] [PubMed] [Google Scholar]
- 31.Makuch R, Simon R. Sample size requirements for evaluating a conservative therapy. Cancer Treat Rep. 1978;62:1037–1040. [PubMed] [Google Scholar]
- 32.Xiong X, Tan M, Boyett J. A sequential procedure for monitoring clinical trials against historical controls. Stat Med. 2007;26:1497–1511. doi: 10.1002/sim.2635. [DOI] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 34.van Ruijven MW, de Groot JC, Smoorenburg GF. Time sequence of degeneration pattern in the guinea pig cochlea during cisplatin administration. A quantitative histological study. Hear Res. 2004;197:44–54. doi: 10.1016/j.heares.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg JH, Beijnen JH, Balm AJ, et al. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat Rev. 2006;32:390–397. doi: 10.1016/j.ctrv.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 36.van Ruijven MW, de Groot JC, Klis SF, et al. The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear Res. 2005;205:241–248. doi: 10.1016/j.heares.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Capizzi RL. The preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine. Semin Oncol. 1999;26:3–21. [PubMed] [Google Scholar]
- 39.Treskes M, van der Vijgh WJ. WR2721 as a modulator of cispl. Cancer Chemother Pharmacol. 1993;33:93–106. doi: 10.1007/BF00685326. [DOI] [PubMed] [Google Scholar]
- 40.Treskes M, Nijtmans L, Fichtinger-Schepman AM, et al. Cytostatic activity of cisplatin in the presence of WR2721 and its thiol metabolite WR1065 in OVCAR-3 human ovarian cancer cells as compared to V79 fibroblasts. Anticancer Res. 1992;12:2261–2265. [PubMed] [Google Scholar]
- 41.Planting AS, Catimel G, de Mulder PH, et al. Randomized study of a short course of weekly cisplatin with or without amifostine in advanced head and neck cancer. EORTC Head and Neck Cooperative Group. Ann Oncol. 1999;10:693–700. doi: 10.1023/a:1008353505916. [DOI] [PubMed] [Google Scholar]
- 42.Williams GB, Kun LE, Thompson JW, et al. Hearing loss as a late complication of radiotherapy in children with brain tumors. Ann Otol Rhinol Laryngol. 2005;114:328–331. doi: 10.1177/000348940511400413. [DOI] [PubMed] [Google Scholar]
- 43.Merchant TE, Gould CJ, Xiong X, et al. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int J Radiat Oncol Biol Phys. 2004;58:1194–1207. doi: 10.1016/j.ijrobp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]