SUMMARY
The Rho-kinases are widely utilized downstream targets of the activated Rho GTPase that have been directly implicated in many aspects of Rho-dependent effects on F-actin assembly, acto-myosin contractility, and microtubule stability, and consequently play an essential role in regulating cell shape, migration, polarity, and division. We have determined that the single closely related Drosophila Rho-kinase ortholog, DRok, is required for several aspects of oogenesis, including maintaining the integrity of the oocyte cortex, actin-mediated tethering of nurse cell nuclei, “dumping” of nurse cell contents into the oocyte, establishment of oocyte polarity, and the trafficking of oocyte yolk granules. These defects are associated with abnormalities in DRok-dependent actin dynamics and appear to be mediated by multiple downstream effectors of activated DRok that have previously been implicated in oogenesis. DRok regulates at least one of these targets, the membrane-cytoskeletal cross-linker DMoesin, via a direct phosphorylation that is required to promote localization of DMoesin to the oocyte cortex. The collective oogenesis defects associated with DRok deficiency reveal its essential role in multiple aspects of proper oocyte formation, and suggest that DRok defines a novel class of oogenesis determinants that function as key regulators of several distinct actin-dependent processes required for proper tissue morphogenesis.
Keywords: Oogenesis, Rho-kinase, actin, Moesin, cytoskeleton, Drosophila
INTRODUCTION
The molecular pathways underlying the complex morphogenesis of tissues in developing multicellular organisms are beginning to be elucidated. At the cellular level, it is clear that numerous highly coordinated shape changes and movements are among the key regulatory processes required for proper tissue development, and these depend on dynamic cytoskeletal rearrangements that are stringently regulated by the activities of the Ras-like GTPases of the Rho sub-family (Hall, 1998; Nobes and Hall, 1995b; Ridley, 1996). These evolutionarily conserved proteins, which include the prototypical family members, Rho, Rac and Cdc42, have been implicated in a variety of cellular processes associated with cytoskeletal rearrangements, including cell shape change, cell migration, cell adhesion (Nobes and Hall, 1995a; Nobes and Hall, 1999; Raftopoulou and Hall, 2004), gene transcription (Hill et al., 1995; Sahai et al., 1998) and protein trafficking (Qualmann and Mellor, 2003; Symons and Rusk, 2003), among others.
The Rho proteins function as molecular switches, cycling between an inactive GDP-bound state and an active GTP-bound state (Symons and Settleman, 2000). In their active form, Rho GTPases transduce signals by binding various downstream effector targets to trigger cellular responses to extracellular stimuli (Burridge and Wennerberg, 2004; Van Aelst and D'Souza-Schorey, 1997). Among the key identified Rho effectors is Rho-kinase, a serine/threonine kinase that has been found to mediate Rho-directed cellular responses through direct phosphorylation of various protein substrates that participate in diverse biological processes, including smooth muscle contraction, cytokinesis, axon outgrowth, cell migration, and cell adhesion (Riento and Ridley, 2003). Extensive studies of Rho-kinase function in mammalian smooth muscle as well as non-muscle cells have implicated Rho-kinase in acto-myosin contractility: Upon stimulus-induced activation, GTP-bound Rho binds to and activates Rho-kinase which in turn phosphorylates myosin regulatory light chain and the myosin-binding subunit of myosin phosphatase. This dual phosphorylation results in a conformational change that allows myosin II to form filaments and increases its actin-activated ATPase activity, leading to the formation of actin stress fibers and focal adhesions in non-muscle cells (Kawano et al., 1999; Kimura et al., 1996), to smooth muscle contraction (Uehata et al., 1997), and to neurite retraction (Amano et al., 1998; Hirose et al., 1998).
Rho-kinase has also been shown to mediate Rho-induced effects on the actin cytoskeleton through phosphorylation of other actin-binding proteins, such as adducin, to regulate the organization of the subcortical spectrin-F-actin meshwork in MDCK epithelial cells (Fukata et al., 1999b), and the ERM (Ezrin-Radixin-Moesin) family proteins, to ensure the anchoring of F-actin to the plasma membrane and subsequent microvilli-like structure formation (Fukata et al., 1999a; Oshiro et al., 1998). In addition, Rho-kinase directly phosphorylates LIM-kinase, which regulates the turnover of actin filaments through an inactivating phosphorylation of the actin-capping protein, cofilin (Maekawa et al., 1999).
In light of its prominent role in mediating Rho-dependent cytoskeletal dynamics, Rho-kinase function has also been studied in the context of tissue morphogenesis in several multicellular model organisms where it has been implicated in various developmental processes, including organogenesis in higher vertebrates such as chicken and mouse (Wei et al., 2001), embryo elongation and cytokinesis in C. elegans (Piekny and Mains, 2002; Piekny et al., 2000; Wissmann et al., 1997), and gastrulation in zebrafish (Lai et al., 2005). Rho-kinase has also been shown to function downstream of the Wnt/planar cell polarity pathway to ensure convergent extension cell movements during vertebrate gastrulation in the Xenopus embryo (Kim and Han, 2005). In Drosophila, analysis of somatic clones of Drok2, a loss-of-function mutation of the single closely related Rho-kinase ortholog, Drok (Mizuno et al., 1999), revealed a role for DRok in the evolutionarily conserved Frizzled-Dishevelled pathway that controls planar cell polarity. Thus, Drok2 mutant clones exhibit tissue polarity defects resulting in an abnormal number of wing hairs and improper orientation of photoreceptor clusters in the eye (Winter et al., 2001). In this developmental context, DRok’s ability to regulate acto-myosin contractility appears to account largely for its biological function.
Zygotic Drok2 mutant animals die just prior to the third instar larval stage (Winter et al., 2001), and maternal contribution of Drok mRNA or DRok protein to the egg may preclude the identification of roles for DRok in earlier fly development. Therefore, to further explore DRok biological functions in early Drosophila development, we generated germline clones (GLCs) of DRok, using a null allele. We observed that DRok is required for proper oogenesis and that DRok deficiency results in loss of integrity of the oocyte cortex and in defective cytoplasmic transport, two developmental processes that depend on actin cytoskeletal organization (Polesello et al., 2002; Theurkauf and Hazelrigg, 1998). Many of the previously reported Rho-kinase-mediated developmental functions have been found to involve Rho-kinase signaling to nonmuscle myosin light chain and consequent acto-myosin contractility. Here we show that DRok is likely to control the oocyte cortex integrity through its regulation of the phosphorylation and localization of the cytoskeletal-membrane anchoring protein, DMoesin, at the oocyte cortex. We also demonstrate that DRok is involved in the establishment of egg polarity and the trafficking of yolk granules. We conclude that Drosophila oogenesis provides a complex developmental setting in which DRok functions as a spatio-temporal regulator of tissue morphogenesis in diverse compartments of the egg chamber through the engagement of multiple downstream effectors.
Materials and methods
Drosophila strains
Drosophila stocks were maintained at 25°C. To generate germline clones of Drok2, the following stocks were used: the y, w, rok2 P[neoFRT19A]/Fm7i Act-GFP mutant line, kindly provided by L Luo, from which the mutation was segregated from the FRT19A site and consequently recombined with P[neoFRT18E] to generate the y, w, rok2 P[neoFR18E]/Fm7i, Act-GFP line; ovoD2 P[neoFRT18E]/Fm6;P{ hs-Flp}38 flies. Third instar larval progeny of the genotype y, w, rok2 P[neoFR18E]/ ovoD2 P[neoFRT18E];P{ hs-Flp}38/+ were incubated at 37°C for 2 hours each day for 3 days (Chou and Perrimon, 1996). Females with Drok2 homozygous GLCs (non-FM6 females) were allowed to lay eggs and tested for sterility. Germline clones of Drok1 (Bloomington Stock Center) were generated similarly. Other stocks utilized include w1118 and w1118; P[nosVP16-GAL4] P[UAS-αTub84B-GFP] (Bloomington Stock Center).
RNA in situ hybridization
In situ mRNA hybridization to adult ovaries of the wild-type genotype was performed using a full-length Drok cDNA. oskar and bicoid mRNA in situ hybridizations to adult ovaries of the wild-type and Drok2 germline clone genotypes were carried out using PCR fragments from wild-type fly genomic DNA, corresponding to amplified sequences from oskar or bicoid mRNA regions. 5’-ACGTTCTAGACAAAAATGCCAGTACCCATCA-3’ and 5’-CATGGGATCCCCCTTTCGTTGATTAGACAGGA-3’, and 5’-ACGTTCTAGACACCACTTTTACCAGCTCTCAA-3’ and 5’-CATGGGATCCCTGTAGCGTCGTCTTCTTGCT-3’ were used as left and right primers for oskar and bicoid, respectively, with the introduction of the Xba1 and BamH1 restriction sites (in bold type) for subsequent subcloning into the Xba1 and BamH1 linearized pBSK vector. Ovaries from 2–4-day old well-fed female flies were dissected in EBR (13 mM NaCl, 0.47 mM KCl, 0.19 mM CaCl2, 1 mM HEPES, pH 6.9), fixed in 1.6 mls of fixing solution (0.1M HEPES, pH 6.9, 2 mM MgSO4, 1 mM EGTA), 0.4 ml of 20% paraformaldehyde and 8 ml of Heptane, for 20’ with vigorous shaking. The following steps were performed according to a standard protocol for in situ hybridization, with the indicated modifications (O'Neill and Bier, 1994). Proteinase K treatment was performed for 10 min. Hybridization with a digoxygenin-labeled RNA probe, prepared as suggested by the manufacturer (Boehringer Mannheim), was performed at 50°C overnight and followed by washes in hybridization solution, 1:1 mixture of hybridization solution and PBT (PBS, 0.1% Tween-20), and PBT at 50°C. The stained ovaries were mounted in 50% glycerol in PBS.
Immunohistochemistry
Ovaries were dissected and fixed as described (Cant et al., 1994). The following antibodies were used: rabbit anti-Phospho-ERM (1:50, Cell Signaling Technology), mouse anti-Hu-li-tai-shao-RC (1:10, anti-Hts-RC), kindly provided by Lynn Cooley, anti-Gurken (1D12, 1:10, Developmental Studies Hybridoma Bank), mouse anti-β tubulin (1:500, mouse ascites E7, Developmental Studies Hybridoma Bank). Immunostaining using rabbit anti-Moesin antiserum (1:5000, D. Kiehart, Duke University) was performed as described. The secondary antibodies Cy3-conjugated or Cy2-conjugated goat anti-rabbit (Jackson Labs), Cy3-conjugated or Alexa 488-conjugated goat anti-mouse (Jackson ImmunoResearch Laboratories), were used at a dilution of 1:200. F-actin was visualized after staining with TRITC-Phalloidin (Sigma, 1:200). Nuclei were stained with Alexa 488-conjugated Wheat Germ Agglutinin (WGA) (1:1000, Molecular Probes). Lectin staining was performed according to Jankovics et al. (2002). Ovaries were mounted in anti-fade solution (50% glycerol containing 0.05% propyl gallate) and fluorescent images were recorded using a Zeiss LSM510 confocal microscope.
In vitro kinase assays
Reactions of 30 µl containing 50 mU of purified recombinant mRok (Upstate), 2 µg of GST fusion protein, 0.5 µl of 32P-ATP (10 mM HEPES [pH 7.5], 150 mM NaCl, 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT and 25 mM β-glycerol phosphate) were incubated for 20 minutes at 30°C and the reaction products were subjected to SDS-PAGE electrophoresis. Gels were stained with Coomassie solution to reveal protein content, dried, and subjected to autoradiography.
Timelapse confocal imaging
Female flies were fattened for two days on yeast-cornmeal-molasses with supplemental dried active baker’s yeast at 25°C. Females were injected in the abdomen with 0.4% trypan blue in normal saline. Two hours after injection, ovaries were dissected into ovarioles in halocarbon 700 oil on glass-bottom culture dishes (Bioptechs). For injection of C3 transferase, wildtype egg chambers were prepared as above, and were injected with C3 transferase (approximately 100 nM) following dissection into halocarbon oil using a microinjection apparatus. As controls, egg chambers were injected with GST protein alone (in the same buffer) or buffer alone. In both cases, there was no effect on ovaries (no premature swirling) - even with higher concentrations of GST (up to 500nM) injected compared to GST-C3. Timelapse movies were recorded by taking images of a single 1–2 micron central section of the oocyte every 10 seconds on a Zeiss LSM 510 META confocal microscope. Excitation was achieved with a 543nm laser and detection was through a 560 long pass filter. Projected images represent six consecutive time points.
Immunofluorescence imaging of microtubules
Females were fattened as for timelapse, and dissected into Robb’s medium (55mM KOAc, 40mM NaOAc, 100mM sucrose, 10mM glucose, 1.2mM MgCl2, 1.0mM CaCl2, 100mM HEPES, pH 7.4) at room temperature for a maximum of eight minutes, and were fixed and stained immediately thereafter according to the method of Cha et al. (Cha et al., 2001), using a FITC-labeled anti-α-tubulin monoclonal antibody DM1A (Sigma). Visualization was by confocal microscopy using a Zeiss LSM 510 META with excitation at 488nm and collecting emission using a BP-505–560 filter. A Plan-Apochromat 40x 1.3 Oil objective was used in all cases for imaging.
Recombinant protein production
The Rho-inhibitory C3 transferase coding sequence, in a pGEX vector (GE/Amersham), was transformed into Rosetta-Gami-B Bl21(DE3) pLysS (Novagen). Cultures were inoculated and grown overnight at 37°C, diluted tenfold, and grown to OD600=1.0. Expression was induced with 50 µM IPTG, cultures were transferred to 18°C, and grown overnight (at least 18 hours). Cells were lysed by sonication in lysis buffer (50 mM Tris pH 7.6, 150 mM NaCl, 5% glycerol, 5 mM DTT) with Complete protease inhibitor tablets (Roche). Triton X-100 was added to 1% and lysates were incubated at 4°C for 30 minutes. Lysates were centrifuged at 10,000 rpm in a JA-17 or SS-34 rotor and the supernatants were coupled to Glutathione-sepharose 4B (GE/Amersham) by 90 minute nutation at 4°C. The matrix was washed three times with lysis buffer (without protease inhibitors) and eluted two times one hour with elution buffer (50 mM Tris pH 8.0, 150 mM NaCl, 5% glycerol, 5 mM DTT, 20 mM reduced glutathione). Elutions were pooled, dialyzed into injection buffer (5 mM Kcl, 0.1 mM NaPO4 pH 6.8), and stored at 4°C.
GST-DMoe and GST-DMoeT559A C-terminal protein fusions were generated by PCR and cloned into the pGEX20 vector. The oligonucleotides 5’-ACGTGGATCCAAGAATATGGAGGCCGTCGAG-3’ and 5’-CATGTCTAGATTACATGTTCTCAAACTGATCGACGC-3’ were used as left and right primers containing BamHI and XbaI restriction sites (in bold type), respectively, to amplify the DMoe C-terminal fragment containing the putative Rho-kinase phosphorylation site. Primers 5’-CGTGACAAGTACAAGGCGCTCCGCGAGATTCGT-3’ and 5’-CTTACGAATCTCGCGGAGCGCCTTGTACTTGTC-3’ were used to generate the threonine to alanine mutation at position 559 in the same DMoe fragment. GST fusion proteins were produced as described (Jiang et al., 2005). In vitro kinase assays were performed with purified rat active mRok (Upstate) and either GST, GST fused to the DMoe C-terminal fragment, or to the DMoe Cterminal fragment with threonine 559 mutated to alanine, eliminating the putative Rho-kinase phosphorylation site.
RESULTS
DRok is required for normal oogenesis
The developmental function of DRok has, thus far, only been examined in the context of tissue polarity and axon outgrowth using zygotic loss-of-function mutants (Ng and Luo, 2004; Winter et al., 2001). Therefore, to examine a potential requirement for DRok in early Drosophila development, we generated germline clones (GLCs) of a Drok null allele. This strategy relies upon the Flp-FRT recombination system to eliminate maternal contribution of Drok mRNA, and thereby provides an opportunity to examine potential roles for DRok in developing eggs or early embryos. Using this strategy, we observed that females carrying Drok2 GLCs are sterile and lay a relatively small number of eggs, all of which are abnormally shaped. Unlike wild-type eggs, in which the dorsal appendages adopt a tubular morphology (Fig. 1A), the DRok-deficient eggs, which invariably fail to hatch, exhibit fused flat dorsal appendages (100% of the clones) (Fig. 1B, and Table 1). In addition, whereas in wild-type egg chambers at stage 13 nurse cells have transferred most of their cytoplasm to the oocyte (Fig. 1C), in Drok2 mutant egg chambers, nurse cells largely retain their cytoplasm, and about half of the mutant oocytes are reduced in size (Fig. 1D, and Table 1). Similar phenotypes were found in Drok1 germline clones, where Drok1 is an independently derived loss-of-function allele of Drok, suggesting that the observed defects reflect the genetic disruption of Drok and are not due to a non-specific secondary mutation (Fig. 1E, and Table1). These findings indicate that DRok is essential for normal oogenesis.
Fig. 1. Drok2 and Drok1 germline clones produce abnormal eggs with dorsal appendage defects.
(A, B) Phase contrast images of dorsal views of a wild-type stage 14 egg chamber (A) and a Drok2 germline mutant stage 14 egg chamber (B). Drok2 germline clones produce eggs with chorion patterning defects such as fused and flat dorsal appendages. (C–E) Phase contrast images of a wild type (C), a Drok2 mutant (D), and a Drok1 mutant (E) stage 13 egg chambers. Drok2 and Drok1 mutant egg chambers exhibit dumpless-like nurse cells, resulting in eggs smaller than their wild-type counterparts. The arrows point to the newly forming dorsal appendages, which marks the developmental stage of these eggs. (F) In situ hybridization to Drok mRNA in wild-type stage 6 (st 6), stage 10a (st 10a) and stage 13 (st 13) egg chambers. Drok is mainly expressed in nurse cells, along with a diffuse expression in the oocyte throughout oogenesis. Sense mRNA was used as a negative control.
Anterior is to the left and dorsal is to the top.
Table1.
Drok2 and Drok1 GLCs exhibit multiple oogenesis defects
| genotype | Oocytes with fused/flat dorsal appendages(%) | Egg chambers with dumpless Nurse cells(%) | Small eggs(%) | Oocytes with actin clumps in ooplasm(%) | Oocytes with altered plasma membrane(%) |
|---|---|---|---|---|---|
| Drok2/Drok2 | 100 (N=152) | 97 (N=127) | 50 (N=152) | 95 (N=180) | 82 (N=180) |
| Drok1Drok1 | 100 (N=30) | 96 (N=45) | 58 (N=40) | 75 (N=92) | 62 (N=92) |
| wt | 0 (N=100) | 0 (N=100) | 3 (N=100) | 0 (N=100) | 0 (N=100) |
| genotype | Egg chambers with defective NC actin cytoskeleton(%) | Oocytes with reduced/diffused Phospho-DMoesin at the cortex(%) | Oocytes with phosphor-DMoesin clumps in ooplasm(%) | Egg chambers with misshapen ring canals(%) | |
|---|---|---|---|---|---|
| Drok2/Drok2 | 86 (N=64) | 70 (N=133) | 22 (N=133) | 93 (N=77) | |
| Drok1Drok1 | 72 (N=92) | NA | NA | NA | |
| wt | 0 (N=50) | 8 (N=120) | 0 (N=120) | 0 (N=55) | |
N equals the total number of egg chambers
NC=Nurse Cell
NA=Not Available
We used in situ hybridization to confirm that Drok mRNA is normally present in developing egg chambers. Drok mRNA is expressed somewhat uniformly throughout the cytoplasm of nurse cells (Fig. 1F) and diffuse mRNA expression is detected in the oocyte, throughout oogenesis, suggesting that DRok is in fact maternally provided to the oocyte.
DRok is required for establishing normal oocyte polarity
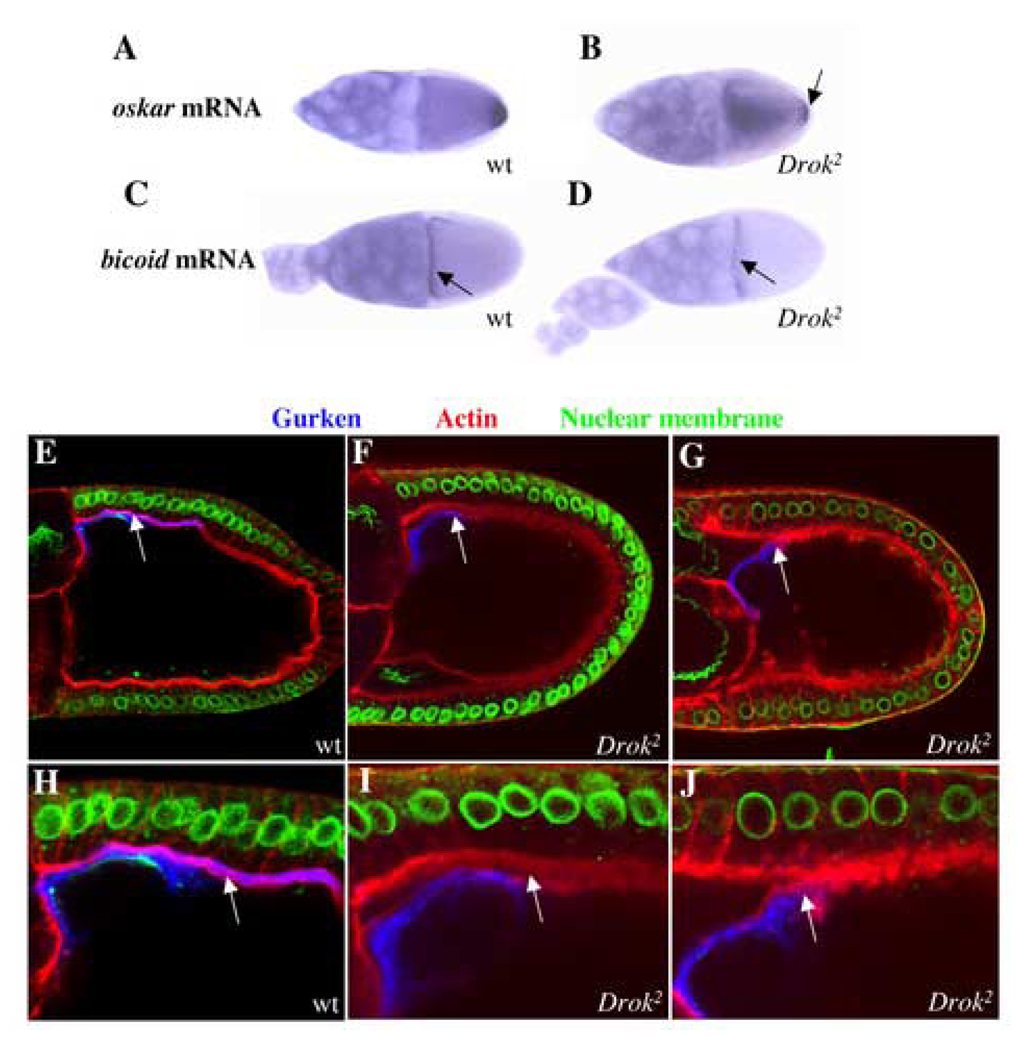
Our observation that stage 13–14 Drok2 mutant oocytes harbor incompletely formed or fused dorsal appendages, a defect that is often associated with abnormal egg polarity (Neuman-Silberberg and Schupbach, 1994; Neuman-Silberberg and Schupbach, 1996), prompted us to investigate the polarity of Drok2 mutant oocytes. By examining oskar mRNA localization as a marker of antero-posterior polarity (Kim-Ha et al., 1991) and the localization of bicoid mRNA as a marker of the anterior pole (van Eeden and St Johnston, 1999), we observed that, whereas oskar mRNA is restricted to the posterior tip of wild-type oocytes (Fig. 2A), its localization is altered in Drok2 mutant oocytes, where oskar mRNA is detected throughout the ooplasm (Fig. 2B). The loss of posterior localization of oskar mRNA in Drok2 GLCs is also observed in early oocytes (stage 8/9) (data not shown). We note that, as has been reported in GLCs of Dmoesin (Dmoe GLCs), a putative effector target of DRok (Polesello et al., 2002), a small fraction of correctly positioned oskar mRNA can be detected in Drok2 GLCs (Fig. 2B), indicating that the absence of either DRok or DMoesin does not entirely abolish the localization of oskar mRNA. On the other hand, similar to Dmoe GLCs, bicoid mRNA localizes properly at the anterior margin of wild-type and Drok2 mutant oocytes (Fig. 2C,D). Together, these results indicate that DRok, like DMoesin, is not required for the formation of the anterior pole, but is required for formation of the posterior pole.
Fig. 2. Drok2 GLCs exhibit polarity defects.
(A–D) In situ hybridizations to oskar mRNA (A, B) or bicoid mRNA (C, D) in wild-type (A,C) or Drok2 GLC mutant (B,D) egg chambers. oskar mRNA is normally restricted to the posterior side of wild-type oocytes (A) but this localization is disrupted in Drok2 mutant oocytes (B). The arrow denotes some oskar mRNA still anchored at the posterior tip of mutant oocytes. bicoid mRNA anterior margin distribution is unaltered in Drok2 mutant oocytes (C, D).
(E–G) Triple anti-Gurken, TRITC-Phalloidin and nuclear staining of a wild-type (E and close-up H) or Drok2 mutant (F and close-up I, G and close-up J) oocytes. In both the wild-type and mutant oocytes, Gurken accumulates in close proximity to the oocyte nucleus, and distributes normally to the antero-dorsal region of the oocyte. However, unlike in wild-type oocytes, Gurken is not being secreted in the extracellular space between the oocyte membrane and the neighboring follicle cell apical membranes, in Drok2 mutant oocytes (arrows in H, I and J).
We also investigated dorso-ventral polarity of oocytes by examining the subcellular localization of the Gurken product, a TGFα-like ligand for the Drosophila EGFR. The antero-dorsal localization of gurken mRNA and the localized activity of its product are responsible for the formation of the dorso-ventral axis (Neuman-Silberberg and Schupbach, 1993; Neuman-Silberberg and Schupbach, 1994; Neuman-Silberberg and Schupbach, 1996). We determined that the Gurken protein is present near the oocyte nucleus, and is correctly positioned to the antero-dorsal region of both wild-type and Drok2 mutant oocytes (Fig. 2E,F), even in Drok2 mutant oocytes with a severely affected morphology (Fig. 2G). This indicates that DRok is not required for proper localization of Gurken in the establishment of dorso-ventral polarity.
Gurken regulates the activity of the EGFR, which is expressed in the surrounding follicle cells, and during oogenesis, intercellular communication events, which involve the Gurken/EGFR pathway, take place between the germline and the follicle cells (Nilson and Schupbach, 1999). Previous studies indicate that Gurken is cleaved at the oocyte membrane and its soluble extracellular domain is then secreted for subsequent activation of the EGFR on the plasma membrane of neighboring follicle cells (Ghiglione et al., 2002). Therefore, we examined Gurken secretion in Drok2 GLCs. In wild-type oocytes, we observed that Gurken is secreted into the intercellular space between the oocyte plasma membrane and the adjacent follicle cells, which are revealed by F-actin staining (Fig. 2E,H). However, Gurken fails to be secreted from the majority of Drok2 GLC oocytes (80% of the Drok2 GLCs) and remains within the oocyte, either adjacent to the plasma membrane or apparently “stuck” within the plasma membrane (Fig. 2F,G,I,J). Taken together, these results suggest that DRok is not required for the proper localization of Gurken, but is necessary for the proper distribution of Gurken between the oocyte and adjacent follicle cell plasma membranes. This potentially reflects a role for DRok in the normal processing of Gurken and its routing through the secretory pathway, or its role in maintaining oocyte plasma membrane integrity, as described below.
DRho1 and DRok are required for yolk granule distribution
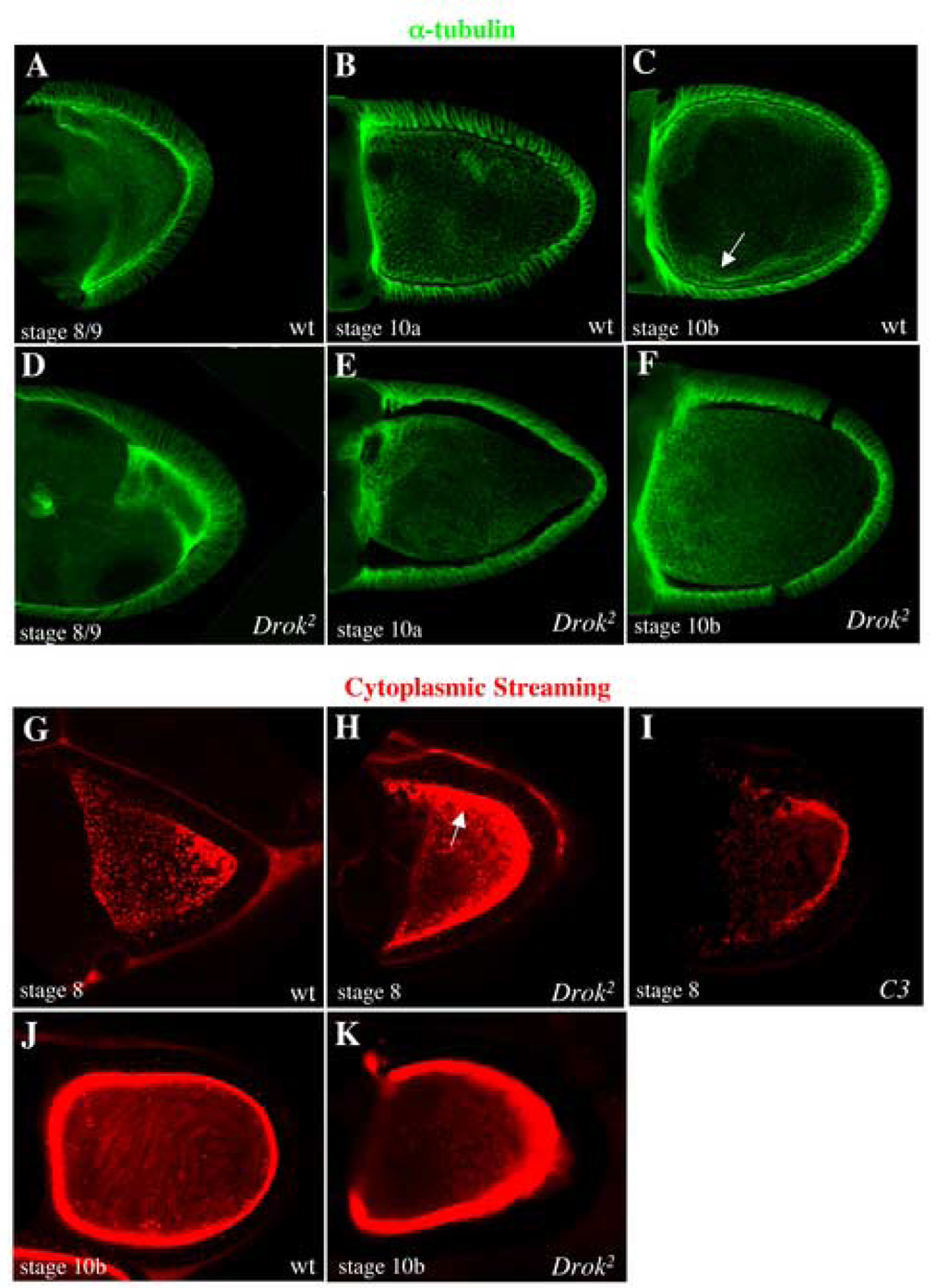
A polarized microtubule cytoskeleton is required for the localization of polarity determinants within the oocyte, and Rho GTPase signaling has been directly implicated in microtubule dynamics in several experimental systems (Theurkauf et al., 1992). Therefore, we determined whether microtubules are affected in Drok2 oocytes. Anti-α tubulin staining of wild-type oocytes reveals a subcortical array of microtubules at stage 8 (Fig. 3A) followed by a gradient of bundled microtubules from anterior to posterior from stage 10a (Fig. 3B) and a more lateral bundling of microtubules from stage 11 (Fig. 3C). This microtubule cytoskeleton arrangement appears to be normal in Drok2 mutant oocytes where microtubules seem to be well organized (Fig. 3D–F).
Fig. 3. DRok does not perturb the bundling of microtubules but is involved in yolk granule trafficking.
(A–F) Visualization of wild-type (A–C) and Drok2 mutant oocytes (D–F), all expressing the Tubulin-GFP fusion protein to reveal microtubules. As expected in wild-type oocytes, early stages (stage 8/9) are marked by an array of subcortical microtubules around the oocyte (A). Starting from stage 10a, microtubules are bundling from the anterior margin of the oocyte and this creates an anterior to posterior gradient of microtubules in the oocyte (B). Later stages are characterized by anterior and more lateral bundling of microtubules that extend more in the ooplasm, as they are ready for cytoplasmic streaming (C). This microtubule distribution is not perturbed in Drok2 mutant oocytes and microtubules bundle quite normally from early to late stages (D–F).
(G–I) Visualization of auto-fluorescent yolk granules by time-lapse microscopy in wild-type (G, J), Drok2 GLCs (H, K) and C3-treated wild-type egg chambers (I). While yolk granules are uniformly present throughout stage 8 wild-type oocytes (G), they aggregate and accumulate at the cortex of same stage Drok2 mutant oocytes (H). This phenotype has also been detected in C3-treated oocytes (I). While cytoplasmic streaming can be observed in stage 10b wild-type oocytes as swirling arrays corresponding to temporal projections of yolk granules movements (J), visualization of streaming is not possible in stage 10b Drok2 mutant oocytes as most yolk granules have accumulated at the cortex (K).
Both actin and microtubule cytoskeletons have been functionally linked in the establishment of oocyte polarity during Drosophila oogenesis (Theurkauf et al., 1992). The fact that disruption of the actin cytoskeleton either with cytochalasin D or with mutants in genes such as chickadee, which encodes the actin-binding protein Profilin, results in microtubule-based premature streaming in the oocyte (Manseau et al., 1996) prompted us to examine microtubule cytoskeleton dynamics in Drok2 mutant oocytes. This was assessed by comparing the movement of autofluorescent yolk granules during ooplasmic streaming in wild-type and Drok2 mutant oocytes. Ooplasmic streaming is a process by which microtubules move within the oocyte to mix the oocyte cytoplasm with the cytoplasm being rapidly added from the nurse cells, during the microfilament-dependent dumping from the nurse cells into the oocyte, starting at stage 10b (Gutzeit, 1986). While we readily observe the movement of yolk granules throughout the ooplasm of wild-type oocytes starting at stage 10b (Fig. 3J and Supplementary Material, movie 1), there is no detectable movement of yolk granules at similar stages in Drok2 GLC oocytes (Fig. 3K and Supplementary Material, movie 2). In fact, unlike in wild-type oocytes, most of the yolk granules in Drok2 GLCs accumulate and remain at the oocyte cortex beginning as early as stage 8 as seen by the more intense red staining along the oocyte cortex (Fig. 3G,H, Supplementary Material, movie 3, Supplementary Material, movie 4). This prevented the visualization of microtubule movement and ooplasmic streaming in later stages (Fig. 3K). There is also some cortical accumulation of yolk granules in wild-type stage 10b oocytes, but this is far less than that seen in Drok2 mutant oocytes. Interestingly, the observed accumulation of yolk granules at the oocyte cortex has not been previously described in other Drosophila mutants that exhibit oogenesis defects.
To determine whether this role of DRok in the oocyte reflects a downstream activity of the Rho GTPase, we injected wild-type egg chambers with the Rho-specific bacterial inhibitory toxin, C3. Significantly, the C3-injected egg chambers exhibit the same yolk phenotype as Drok2 mutant oocytes (Fig. 3I). Together, these results suggest that a DRho1-DRok pathway is required prior to the onset of ooplasmic streaming to regulate the trafficking of the yolk granules within the ooplasm, and that such trafficking is independent of the microtubule cytoskeleton.
Subcortical F-actin organization and plasma membrane integrity are disrupted in DRok-deficient oocytes
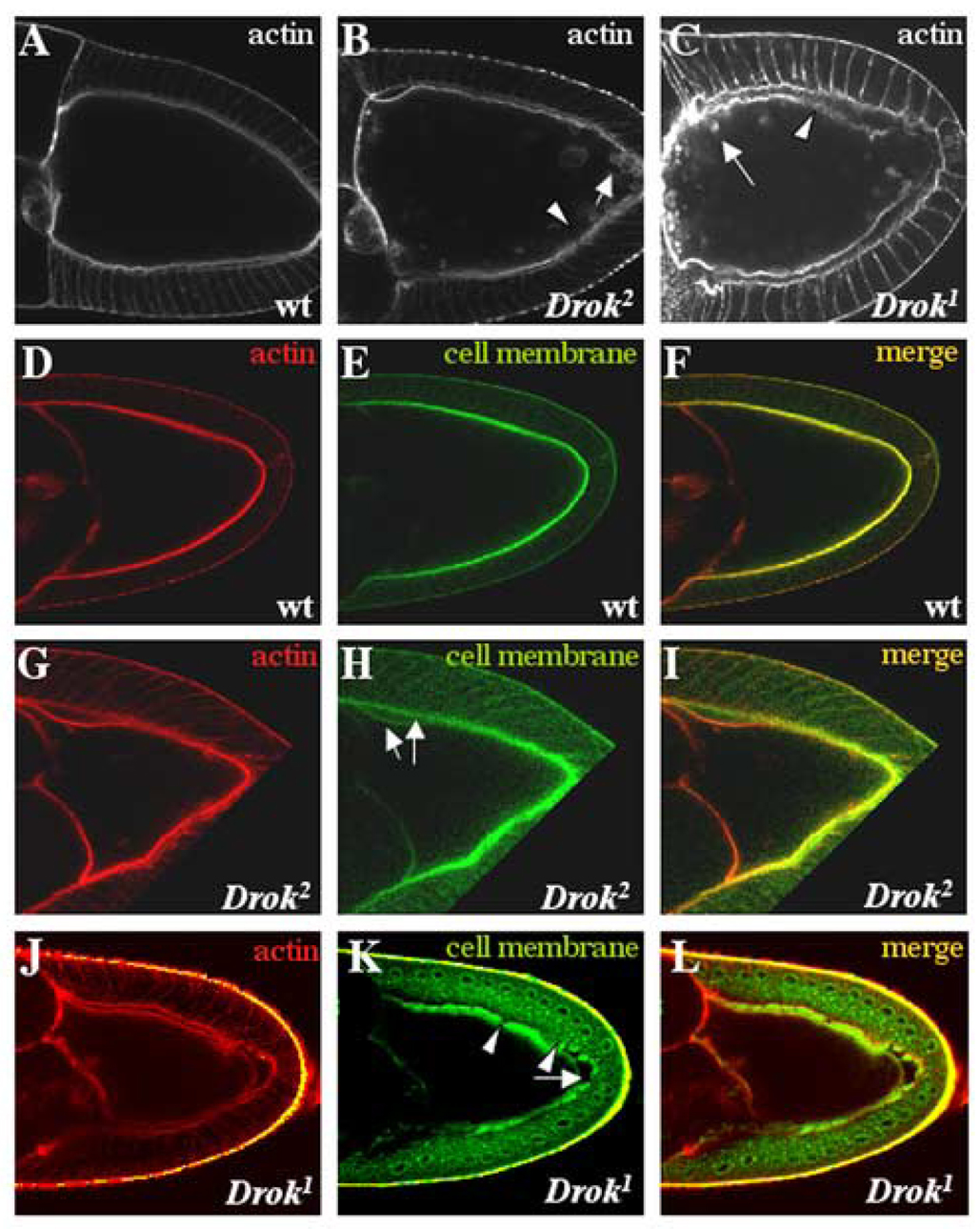
Since Rho-Rho-kinase signaling plays an important role in regulating F-actin assembly in numerous experimental models, we examined the actin cytoskeleton in Drok2 mutant eggs. Dissection of Drok2 GLC egg chambers followed by phalloidin staining to detect F-actin revealed that subcortical F-actin is disorganized in most mutant oocytes (Table 1), and is characterized by the presence of actin clumps within the oocyte and along the length of the oocyte cortex (Fig. 4B,C). Moreover, the cortical F-actin in Drok2 mutant oocytes exhibits a somewhat diffuse organization along the oocyte membrane (Fig. 4B, arrowhead). This is in striking contrast to the “tight” subcortical F-actin distribution seen in a wild-type oocyte (Fig. 4A), and suggests that DRok contributes to the maintenance of cortical actin integrity in Drosophila oocytes. Significantly, genetic disruption of the DRho1 GTPase in oocytes results in similar effects on F-actin organization along the cortex (Magie et al., 1999), consistent with a role for DRho1-DRok signaling in the regulation of cortical F-actin at the oocyte membrane.
Fig. 4. Subcortical F-actin organization and plasma membrane integrity are disrupted in DRok-deficient oocytes.
(A–C) Phalloidin staining of a wild type (A), a Drok2 mutant (B) and a Drok1 mutant (C) stage 10a oocytes. Drok2 and Drok1 mutants display disorganized F-actin at the cortex of the oocyte (B and C, arrowheads pointing to discontinuous F-actin staining), along with the presence of actin clumps near the cortex or within the ooplasm (B, C, arrows). (D–L) Visualization of the F-actin network (D, G and J), using phalloidin, and cell membranes (E, H and K), using a fluorescein-conjugated lectin. (F, I and L) are the merged images of actin and membrane stainings. In the wild type stage 9 oocyte, the lectin co-localizes with F-actin and outlines juxtaposed membranes between the oocyte and the surrounding follicle cells (F). Mutant stage 9 or 10 oocytes display co-localization of F-actin and lectin (I), but exhibit an abnormal lectin distribution, associated with an apparent detachment of the oocyte membrane from the follicle cell layer (H, arrows), as well as a defect in the oocyte shape itself. This lectin defect was also observed in Drok1 mutant oocytes (K, arrow). Anterior is to the left and dorsal is to the top.
In addition to the actin defects described above, we frequently observe a significant deformation along localized regions of the cortical membrane in Drok2 GLC oocytes, resulting in protrusions into the oocyte cytoplasm in a majority of stage 9 and later stage oocytes (Table1). To determine whether these deformations result from an abnormally shaped oocyte or reflect a detachment of subcortical actin from an otherwise normal cell membrane, we used a cell membrane-specific marker (fluorescently-labeled lectin) that co-localizes with F-actin and outlines juxtaposed membranes between the oocyte and the surrounding follicle cell layer in a wild-type egg chamber (Fig. 4D–F). In Drok2 GLCs, the lectin marker co-localizes normally with F-actin, revealing an abnormal oocyte cell shape and a significant detachment of the oocyte membrane from the apical membrane of follicle cells (Fig. 4G–I). The expressivity of that phenotype ranged from mild (stage 9 egg chambers) to very severe (stage10 and 11 egg chambers) (data not shown), suggesting that the defect progresses during oocyte development. In severe cases, the oocyte membrane seems to lose its integrity, as indicated by the appearance of diffuse lectin staining between the oocyte and the follicle cell layer (data not shown). Moreover, strong phenotypes are associated with discontinuous lectin staining of the oocyte membrane (Fig. 4K, arrowheads in Drok1 mutant oocytes). These results suggest that DRok is required for the integrity of the oocyte plasma membrane, and is consequently required to maintain the proper shape of the oocyte. Similarly, Drok1 germline clones are characterized by a disorganized subcortical F-actin network associated with the presence of actin clumps and a reduction in plasma membrane integrity (Fig. 4C, J–L and Table 1), confirming that Drok2 germline clone-generated defects are due specifically to the genetic disruption of the Drok gene. These results suggest that DRok is required for the integrity of the oocyte plasma membrane, and is consequently required to maintain the proper shape of the oocyte.
Drok2 mutant nurse cells exhibit a “dumpless-like” phenotype
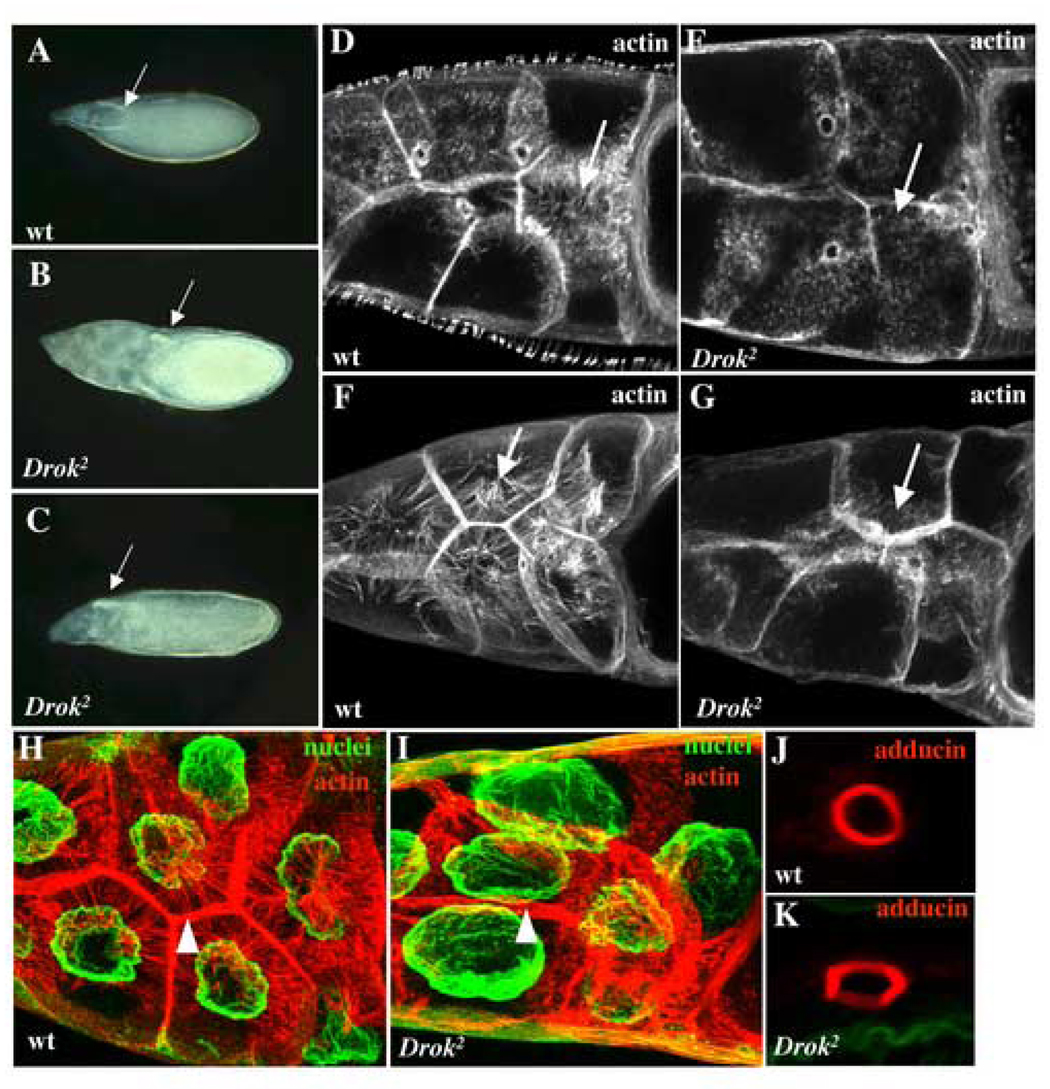
During oogenesis, the 15 polyploid nurse cells of the egg chamber continually provide the oocyte with nutrients, proteins, and maternal RNAs to support its growth through actin-rich structures called rings canals. At stage 11 of oogenesis, the entire cytoplasmic content of the nurse cells is rapidly transferred to the oocyte within 30 minutes, in a process known as “dumping” (see Spradling, 1993 for a review of oogenesis). Nurse cell dumping was previously shown to depend upon an intact actin cytoskeleton (Cant et al., 1994; Verheyen and Cooley, 1994; Xue and Cooley, 1993). In wild-type stage 13 egg chambers, the nurse cells retain a cluster of nuclei after they have dumped all of their cytoplasm into the oocyte. In contrast, in similarly-staged Drok2 (or Drok1) GLC egg chambers, the nurse cells have retained most of their cytoplasm (Fig. 5A–C). This dumpless-like nurse cell phenotype was observed in most late stage Drok2 (or Drok1) mutant egg chambers and results in the generation of either small eggs (50%) (Fig. 5B, Table 1) or normal size eggs (50%) that appear “deflated”, and presumably lack much of their normal cytoplasmic content (Fig. 5C).
Fig. 5. DRok is required for cytoplasmic transport during oogenesis.
(A–C) Phase contrast images of wild-type (A) and Drok2 GLC (B, C) stage 13 egg chambers. The arrows denote the newly forming dorsal appendages, a marker of stage 13 egg chambers. In (A), the nurse cells have dumped their entire cytoplasmic contents into the oocyte and are no longer visible at the anterior region of the egg chamber, whereas DRok-deficient nurse cells retain most of their cytoplasm, resulting in a much smaller egg (B). (C) An example of a less severe dumpless-like phenotype where the mutant oocyte is about the size of a wild-type oocyte, but appears somewhat “deflated”.
(D–G) Phalloidin staining of wild-type stage 10b (D) and stage 11 (F), and Drok2 mutant stage 10b (E) and stage 11 (G) nurse cells. The rapid phase of cytoplasmic transport takes place from stage 10b until the end of stage 11. In wild-type nurse cells, thick transverse F-actin filaments initially form around the nurse cell cortex at stage 10b (D, arrow) and assemble in bundles all around the nucleus, extending into the nurse cell cytoplasm between the nucleus and the plasma membrane at stage 11 (F, arrow). By contrast, in DRok-deficient nurse cells, F-actin filaments are much thinner and shorter when they do form, and they fail to extend from the plasma membrane to the center of the cells (E, G, arrows).
(H, I) Double F-actin and nuclear staining of wild-type and Drok2 mutant stage11 nurse cells. In wild-type nurse cells, the nuclei remain anchored at the center of each cell by the surrounding F-actin filament bundles. In mutant nurse cells, the nuclei, which appear larger, are clearly displaced from their normal centered localization within the nurse cells (I). The arrowheads point to the subcortical F-actin network which appears more diffuse and disorganized in the mutant nurse cells. (J, K) High magnification images of a ring canal from a wild-type (J) or a Drok2 mutant (K) nurse cell stained with an anti-adducin antibody (anti-Hts-RC). These donut-shaped actin and actin-binding protein-rich structures of wild-type nurse cells are somewhat abnormally shaped and are more “squared” in Drok2 mutant nurse cells, but their size and number do not vary between the two genotypes. Anterior is to the left and dorsal to the top.
As has been previously observed in other dumpless mutants, including chickadee, quail or singed, failure of cytoplasmic transport is often due to the obstruction of the ring canals by nurse cell nuclei that fail to be properly tethered by actin filaments (Cant et al., 1994; Mahajan-Miklos and Cooley, 1994; Verheyen and Cooley, 1994). Therefore, we determined whether Drok2 egg chambers exhibit an abnormal actin cytoskeleton and whether ring canal obstruction potentially accounts for the dumpless-like phenotype. The Anti-Hts-RC (Hu li tai shao-Ring Canals) antibody was used to detect the adducin cytoskeletal protein, a specific actin-binding component of the ring canals (Yue and Spradling, 1992). In contrast to anti-Hts-RC staining in wild-type, Drok2 GLCs at stage 10b or 11 revealed a somewhat irregular and square shape of the ring canals in most GLCs (93%) with between one and four abnormally shaped canals per egg chamber, but no major difference in the overall adducin distribution and number of ring canals (Fig. 5J,K, Table 1). Phalloidin staining to reveal F-actin in ring canals yielded similar findings (Fig. 5D,E). These findings indicate that DRok is not required for the formation of ring canals, but appears to play some role in either establishing or maintaining their normal morphology.
Additional analysis of F-actin organization within the nurse cells revealed that while wild-type egg chambers typically exhibit thick F-actin filaments extending from the plasma membranes towards the nurse cell nuclei in order to anchor them, the F-actin network, although present in some clones, is perturbed in nurse cells within the vast majority of Drok2 or Drok1 GLCs (86% and 72%, respectively) (Table1). Specifically F-actin filaments are generally much thinner and shorter in those rare cells where they do form (Fig. 5D,E), and do not span the nurse cell cytoplasm from the plasma membrane to the nucleus (Fig. 5F,G). This suggests two potential defects. On the one hand, the acto-myosin contractile apparatus may be affected, which would prevent nurse cells from contracting properly, thereby altering cytoplasmic transport. On the other hand, actin-mediated anchoring of the nuclei may be defective, which could obstruct the ring canals, consequently preventing cytoplasmic transport. To address the latter possibility, we used double-staining of F-actin and nuclear membranes to reveal the F-actin network which normally tethers nuclei to the center of each nurse cell (Fig. 5H). In Drok2 GLCs, staining clearly reveals the lack of functional F-actin filaments within nurse cells, as well as the presence of nuclei of increased size (about 30% increased volume compared to wild-type nuclei) that are not properly localized near the center of the nurse cells. Significantly, while these enlarged nuclei are in close proximity to the ring canals, they do not appear to obstruct them (Fig. 5I). DRok-deficient nurse cells (in Drok2 or Drok1 GLCs) also exhibit a less structured and “looser” subcortical F-actin organization (Fig. 5I) when compared to wild-type nurse cells (Fig. 5H). Taken together, these results indicate that while DRok is not required for the formation of ring canals, it is essential for cytoplasmic transport. Moreover, lack of cytoplasmic transport in Drok2 GLCs does not result from obstruction of the ring canals by untethered nurse cell nuclei, which clearly distinguishes Drok from the “chickadee, singed or quail” class of dumpless mutants. In addition, DRok is required for maintaining the plasma membrane integrity of nurse cells.
DMoesin membrane localization is disrupted in DRok-deficient oocytes
In mammalian cells, Rho-kinase has been shown to directly phosphorylate the ERM (Ezrin-Radixin-Moesin) family protein Moesin in vitro (Matsui et al., 1998; Oshiro et al., 1998). In vivo, Rho-kinase-dependent phosphorylation of ERM proteins has been reported to vary according to cell type and it is presently unclear as to whether Rho-kinase acts directly or indirectly to modify ERM phosphorylation (Ivetic and Ridley, 2004; Jeon et al., 2002; Matsui et al., 1999; Oshiro et al., 1998). In Drosophila, genetic disruption of the closely related ortholog, Dmoesin, results in a phenotype in developing oocytes that resembles the F-actin defects seen in Drok2 GLCs, including the accumulation of F-actin clumps within the oocyte cytoplasm (Polesello et al., 2002). ERM proteins have been implicated in several biological processes, including cell-cell adhesion, maintenance of cell shape, and cell motility. Upon carboxy-terminal threonine phosphorylation they are recruited to the plasma membrane, where they function as cross-linkers between the cell membrane and the actin cytoskeleton (Bretscher, 1999; Mangeat et al., 1999). DMoesin is required for anchoring F-actin filaments to the plasma membrane in the oocyte, and this function requires phosphorylation of T559 in DMoesin, which is analogous to a Thr site in mammalian Moesin (Fig. 6D) that can be directly phosphorylated by Rho-kinases in vitro (Jankovics et al., 2002; Matsui et al., 1998; Oshiro et al., 1998; Polesello et al., 2002). Therefore, we tested the possibility that membrane localization of DMoesin is disrupted in the oocytes of Drok2 GLCs.
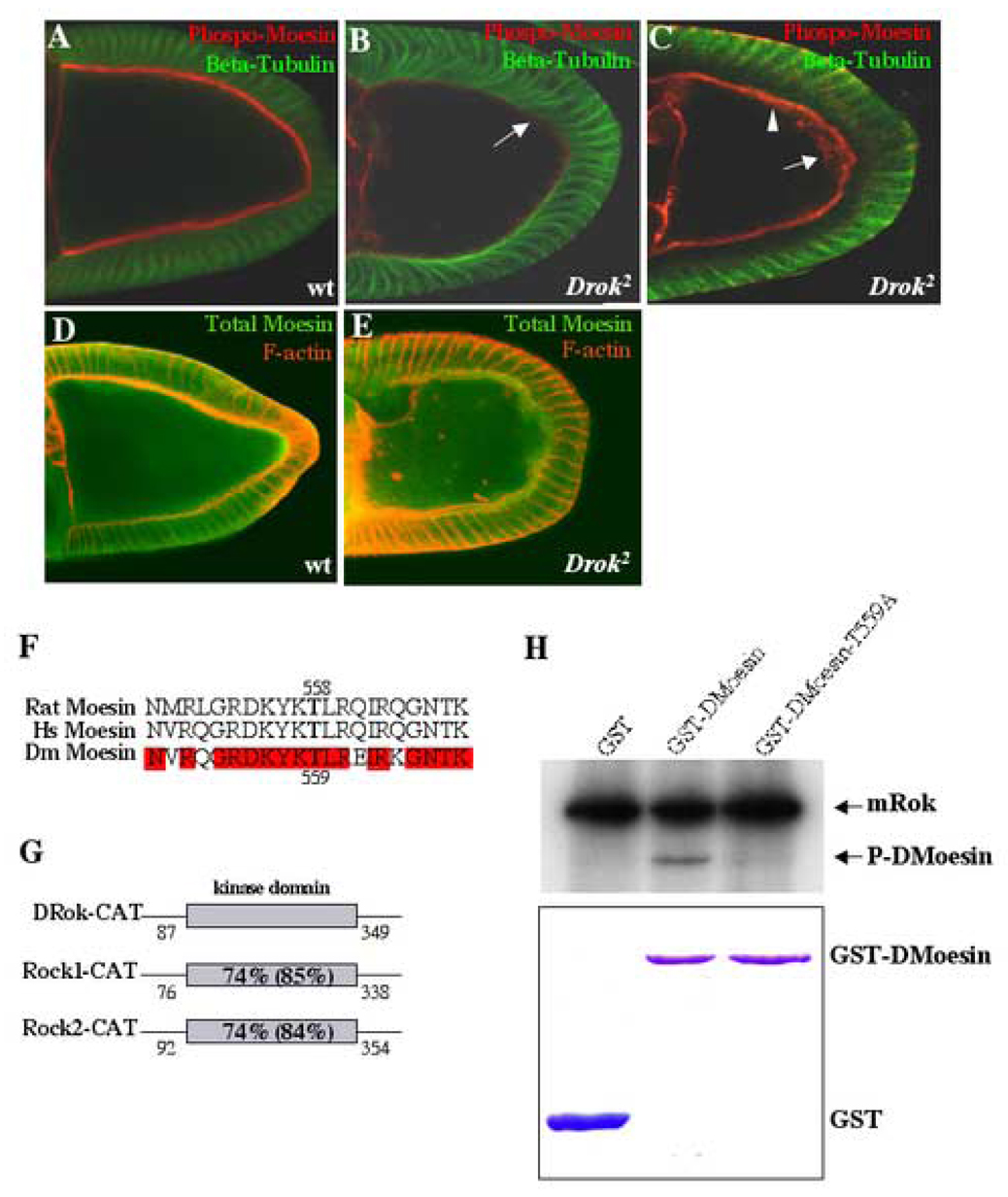
Fig. 6. DMoesin membrane localization is disrupted in DRok-deficient oocytes.
(A–C) Double immunostaining of wild-type (A) and Drok2 mutant stage 10 oocytes (B, C), using an antibody specific for phospho-T558 mammalian Moesin and an antibody against β-tubulin. Phospho-DMoesin localizes to the oocyte plasma membrane in the wild-type oocyte (A). In Drok2 GLCs, phospho-DMoesin is significantly decreased at the oocyte cortex (B, arrow). In most clones, the cortex still retains some phospho-DMoesin staining, but it appears more diffuse (C, arrowhead). About 20% of the Drok2 mutant oocytes also exhibit phospho-DMoesin patches in their ooplasm (C, arrow).
(D, E) Double immunostaining of wild-type (D) and Drok2 mutant stage 10 oocytes (E), using an antibody against total DMoesin and phalloidin. Both wild-type and Drok2 mutant oocytes exhibit a similar diffuse pattern of total DMoesin throughout the ooplasm with no significant difference in DMoesin expression levels between the two genotypes. Anterior is to the left and dorsal is to the top.
(F) Alignment of the amino acid sequences surrounding the specific threonine residue (in bold type) defined at position 558 in human and rat Moesin and position 559 in Drosophila Moesin. Note the significant identity between all three sequences (identical residues are shaded in red).
(G) Schematic representation of the catalytic domains of DRok (DRok-CAT) and mammalian Rho-kinases, mRok1 and mRok2 (mRok1-CAT, mRok2-CAT) with the numbered start and ending amino acids for each domain. The bold type numbers within the kinase domains correspond to the percentage identity and similarity (in parentheses) between DRok-CAT and either mRok1-CAT or mRok2-CAT.
(H) In vitro kinase assay, using the commercially available purified kinase domain of mammalian Rho-kinase (mRok) and GST recombinant proteins. Kinase reactions included 32P-ATP, and the indicated bands correspond to radio-labeled proteins visualized by autoradiography following protein resolution by SDS-PAGE. As expected, autophosphorylation of mRok is detected in all reactions. In the presence of GST-DMoesin, mRok directly phosphorylates DMoesin, and the phosphorylation is abolished when using a GST-DMoesin-T559A mutant (threonine 559 is replaced by an alanine residue) (upper panel). Recombinant proteins were separately assessed after separate SDS-PAGE and Coomassie Blue staining to confirm that equal amounts of protein were used in the assays (lower panel).
Using an antibody directed against the conserved T558 phospho-peptide site in Moesin, we observe that phospho-DMoesin localizes to the oocyte plasma membrane in a wild-type egg chamber (Fig. 6A). However, in Drok2 GLCs, there is a significant reduction in phospho-DMoesin at the oocyte cortex (Fig. 6B, Table 1). In most clones, the cortex still retains some phospho-DMoesin staining which appears more diffuse relative to the staining seen in wild-type oocytes (Fig. 6C). In addition, in ~20% of the GLCs, phospho-DMoesin is also found mislocalized in patches within the ooplasm (Fig. 6C). Figures B and C represent relatively strong and weak phenotypes, respectively, with regard to decreased phospho-DMoesin at the oocyte plasma membrane. However, the overall distribution and expression level of total DMoesin is unchanged in Drok2 mutant oocytes, exhibiting a diffuse staining pattern throughout the ooplasm that is indistinguishable from the staining seen in wild-type oocytes (Fig. 6D,E). Taken together, these observations suggest that proper membrane localization of DMoesin requires DRok activity.
We then determined whether Rho-kinase can directly phosphorylate DMoesin on the T559 residue using an in vitro kinase assay with purified recombinant proteins. In this experiment, we used the commercially available purified kinase domain corresponding to mammalian Rho-kinase (mRok), which is highly conserved with the kinase domain of DRok (Fig. 6E). In the in vitro assay, mRok undergoes autophosphorylation, as expected, and additionally, is able to directly phosphorylate DMoesin (Fig. 6F). The observed phosphorylation of DMoesin is virtually abolished when using a GST-DMoesin-TA mutant in which the putative phosphorylation site was substituted with an alanine residue (Fig. 6F). Taken together, these findings suggest that direct phosphorylation of DMoesin by DRok on a single site facilitates the association of DMoesin with the cortical oocyte membrane during oogenesis to maintain its proper integrity.
Overall, the similarity of antero-posterior polarity, microtubule cytoskeleton integrity and F-actin distribution phenotypes between Drok2 and Dmoe GLCs, along with the cell biology and biochemical phosphorylation data suggests that DRok and its putative effector protein DMoesin interact in the developing oocyte and that DRok mediates some, but not all, of its biological effects through DMoesin. However, Drok2 and Dmoe GLCs are not identical. For example, Drok2 GLCs exhibit a yolk granule transport phenotype, whereas Dmoe GLCs do not. Moreover, the oocyte plasma membrane integrity is more severely affected in Drok2 GLCs than in Dmoe GLCs. These differences imply that DRok must signal through effector proteins other than DMoesin to exert additional distinct effects during oogenesis.
Discussion
In this study, we have determined that the single closely related Drosophila ortholog of the mammalian Rho-kinases, DRok, is required for oogenesis and participates in several distinct aspects of this complex developmental processes, including organization of the oocyte cortex, cytoplasmic transport from nurse cells, and germline-soma cross-signaling necessary for establishment of the antero-posterior and dorso-ventral axis of the resulting mature egg. In addition, we found that DRok is required for a developmental process around stage 7–8 of oogenesis, which enables the yolk granules to move freely in the ooplasm after they have been internalized or transferred to the oocyte and before cytoplasmic streaming can take place (stage 10b and higher). These findings, when considered in the context of a variety of other previously reported mutants that exhibit oogenesis phenotypes, suggest that DRok represents a novel class of oogenesis regulators.
DRok has been previously implicated as an effector of the DRho1 GTPase in the regulation of planar cell polarity in the eye and in the wing, downstream of Frizzled/Dishevelled signals (Winter et al., 2001). In germline cells, DRok and DRho1 mutants exhibit some overlapping actin defects; e.g., the oocyte cortex exhibits a more diffuse F-actin distribution in both Drok2 GLCs and rho1 loss-of-function egg chambers in which Rho1 levels have been reduced in a heterozygous mutant rho1 and wimp background (rho1 GLCs are not viable) (Magie et al., 1999). In addition, wild-type oocytes injected with the Rho-inhibitory C3 toxin exhibit the same ooplasmic streaming defects as Drok2 mutant oocytes, as discussed below, strongly suggesting that the germline clone phenotypes reflect the disruption of DRho1-DRok signaling in germ cells.
DRok regulates oocyte polarity
Previous analysis of the polarity of Dmoe GLC oocytes indicated that DMoesin is specifically required for the localization of posterior determinants such as oskar mRNA and Oskar protein but not the formation of the dorso-ventral axis nor the anterior pole. DMoesin appears to function in maintaining posterior polarity by anchoring actin to the membrane cortex which in turn anchors microtubule-delivered oskar mRNA and its protein product Oskar to the posterior pole (Polesello et al., 2002). Similarly, in Drok2 GLCs, the localization of anterior (bicoid) or dorso-ventral determinants (gurken) is not altered although most oskar mRNA is found mislocalized within the ooplasm starting at stage 9. While the establishment of oocyte polarity generally depends upon microtubule cytoskeleton organization (Theurkauf et al., 1992), it has been reported that Dmoe null mutations do not disrupt the microtubule cytoskeleton and do not perturb its polarity (Polesello et al., 2002). Similarly, we observed that microtubules in Drok2 mutant oocytes appear normal. Taken together with the fact that some oskar mRNA remains anchored at the posterior tip of the oocyte plasma membrane in Drok2 GLCs, as is seen in Dmoe GLCs, this indicates that oskar mislocalization, and consequently, the alteration of posterior polarity in Drok2 GLCs is not due to an abnormally organized microtubule cytoskeleton. Moreover, unlike other germline clone mutants with oocyte polarity defects, such as chic or capu, the Drok2 and Dmoe polarity defects most likely reflect the incapacity of the disorganized subcortical actin cytoskeleton to properly anchor oskar at the posterior membrane of the oocyte.
The similarity between the oskar polarity phenotype of Drok2 and Dmoe GLCs is also consistent with a likely role for DMoesin as an essential DRok substrate that mediates its effects on the formation of posterior polarity, and further supports the functional significance of a signaling pathway from DRok to DMoesin to the actin cytoskeleton in oocyte development. Moreover, the proper localization of Gurken, as defined by the position of the oocyte nucleus, which migrates in a microtubule-dependent manner from the posterior to the anterior and then to the antero-dorsal side of the oocyte starting at stage 8, is consistent with the presence of a grossly normal appearing microtubule cytoskeleton in DRok-deficient oocytes.
DRok plays a role in germline-soma cross-signaling
A majority of mutations resulting in egg chambers with dorso-ventral axis patterning defects, such as dorsal appendages aberrations, have been associated with genes encoding components of the Gurken-EGFR signaling pathway (Neuman-Silberberg and Schupbach, 1994; Neuman-Silberberg and Schupbach, 1996). Cross-signaling between the oocyte and the surrounding follicle cells at the antero-dorsal side of the oocyte has been extensively studied and involves the binding of the secreted Gurken ligand to the EGFR present on the apical membrane of follicle cells and subsequent activation of downstream signaling to control the formation of follicle cell-derived dorsal structures (Nilson and Schupbach, 1999). Although Gurken localization is correct in Drok2 mutant oocytes, loss of Gurken secretion (in 80% of the Drok2 mutant oocytes) in the intercellular space between the oocyte membrane and the follicle apical membranes indicates the likelihood of altered communication between the oocyte and surrounding follicle cells, possibly resulting in a disruption of the EGFR signaling pathway leading to dorsal appendage defects. The observed requirement for DRok in Gurken secretion may reflect a well established role of Rho signaling in the control of vesicular trafficking and secretion (Symons and Rusk, 2003). However, it remains possible that the apparent absence of Gurken secretion into the intercellular space reflects a consequence of the observed disruption of oocyte plasma membrane integrity.
DRok and the trafficking of yolk granules in the early oocyte
The unexpected observation in our time-lapse confocal microscopy studies that most autofluorescent yolk granules in Drok2 mutant oocytes or C3-treated wild-type egg chambers accumulate at the oocyte membrane suggests a role for DRho1 and DRok in early vitellogenesis. Vitellogenesis is a process that begins around stage 8 and is defined by the co-secretion of vitelline membrane and yolk material by the surrounding follicle cells leading to the eventual formation of chorionic structures of the egg and normal oocyte growth, respectively. After their secretion, yolk proteins are internalized into the oocyte through endocytosis and are swirled around the ooplasm at later stages, when microtubule-dependent streaming occurs (Spradling, 1993). The high concentration of yolk granules at the oocyte membrane from early vitellogenesis underlies a possible defect in endocytosis of the yolk granules. Together with the fact that C3-treated egg chambers and Drok2 GLCs exhibit an identical yolk granule phenotype, this suggests that DRok mediates Rho1’s role in the trafficking of yolk granules at the oocyte plasma membrane. In addition, nurse cells also normally accumulate yolk material and transfer it to the oocyte. The detection of yolk granules moving to the plasma membrane of Drok2 mutant oocytes or oocytes in C3-treated egg chambers after they are deposited by the nurse cells is an intriguing phenotype that has not been previously reported and may reflect a trafficking defect in the ooplasm. Further studies to examine molecular components of the endocytic machinery will be required to develop a better understanding of the roles of Rho1 and DRok in yolk granule trafficking within the ooplasm. Notably, it is also conceivable that alteration of oocyte plasma membrane integrity through disruption of actin cytoskeleton organization in most Drok2 GLCs, as we have observed, could exert a secondary effect on the endocytosis of yolk granules.
Because of the yolk granule phenotype in Drok2 GLCs in early oogenesis, it is not possible to visualize microtubule cytoskeleton dynamics at later stages in time-lapse confocal microscopy. Thus, it is difficult to determine whether Drok2 mutant oocytes would undergo normal or premature ooplasmic streaming at stage 10b-11. As a functional relationship between actin and microtubule cytoskeletons has been suggested based on findings with several mutants with oogenesis defects, it is quite conceivable that the abnormalities of the actin cytoskeleton in Drok2 mutant oocytes could affect microtubule cytoskeleton dynamics. Indeed, it has been demonstrated that some aspect of the actin cytoskeleton normally represses microtubule-based streaming within the oocyte (Manseau et al., 1996). Thus, it is possible that the accumulation of yolk granules near the plasma membrane of Drok2 mutant oocytes reflects a combination of trafficking/endocytosis defects and actin-cytoskeleton perturbation-induced alteration of microtubule cytoskeleton dynamics in the ooplasm during early oogenesis.
DRok is required in the nurse cells to ensure their contractility during nurse cell dumping
The oocyte volume in Drok2 GLCs is frequently smaller than that seen in wild-type oocytes, before the rapid phase of cytoplasmic transport takes place. This suggests a possible defect in the slow phase of cytoplasmic transport. It has been previously reported that transport of some particles towards the oocyte during stages 7–10A depends upon a proper acto-myosin network. In addition, sqhAX3 GLCs exhibit a similar oocyte size defect. sqhAX3 is a loss-of-function mutation in the sqh locus which codes for the Drosophila ortholog of myosin light chain of myosin II (Jordan and Karess, 1997). Taken together with the fact that DRok has been shown to phosphorylate Sqh in vivo (Amano et al., 1996; Winter et al., 2001), this data suggests that DRok mediates, via regulation of Sqh, some aspects of the acto-myosin contractility involved in cytoplasmic transport from early stages of oogenesis.
The observation of dumpless-like oversized nurse cells in most of Drok2 GLCs also supports a role for DRok in the rapid phase of cytoplasmic transport at stage 10B-11 of oogenesis. Unlike other classes of dumpless mutants including chickadee, singed or quail, failure of rapid cytoplasmic transport from the Drok2 mutant nurse cells to the oocyte does not result from the obstruction of the ring canals by unanchored nurse cell nuclei, suggesting that Drok constitutes a distinct class of dumpless-like mutants. In addition, in sqhAX3 GLCs, dumpless nurse cells are associated with a lack of acto-myosin contractility by nurse cells, as revealed by mislocalization of myosin II and by absence of the perinuclear organization of actin filaments bundles in the nurse cells. Therefore, sqhAX3 mutant nurse cells cannot contract properly to expulse their cytoplasm through otherwise weakly damaged ring canals. Drok2 and sqhAX3 mutant nurse cells do not share the same actin filament phenotype, as Drok2 mutant nurse cells exhibit a more dramatic phenotype associated with absence of radial filaments and disorganization of cortical actin. It is, however, likely that DRok and Sqh are part of the same signaling pathway that regulates acto-myosin contractility in nurse cells, as it has already been shown that DRok phosphorylates Sqh in Drosophila development. Moreover, the severity of the Drok2 mutant F-actin phenotypes may reflect DRok’s potential to engage multiple distinct downstream substrates, of which Sqh is only one. Significantly, the actin-binding protein, adducin, is also reportedly a direct substrate for mammalian Rho-kinases (Fukata et al., 1999b), and the Drosophila Adducin ortholog, Hts, is a major component of ring canals (Yue and Spradling, 1992). Thus, it is possible that the observed defects in ring canal morphology in Drok2 GLCs involve abnormal regulation of adducin by DRok. However, it is difficult to determine whether this ring canal phenotype contributes to the dumpless-like nurse cell phenotype observed in Drok2 GLCs.
The observation that nurse cell nuclei are substantially increased in size in Drok2 GLCs suggests a possible involvement of DRok in increased endoreplication of the nurse cells. The Rho-related Rac and Cdc42 GTPases have previously been associated with endoreplication in porcine aortic endothelial (PAE) cells, although Rho has not been implicated thus far (Muris et al., 2002). Interestingly, this nurse cell nuclei phenotype has not been observed in other previously described GLC mutants of other actin cytoskeleton-regulating signaling components that exhibit oogenesis defects. Thus, chic as well as sqhAX3 GLCs reveal cytokinesis defects associated with the presence of multinucleated nurse cells. In addition, the majority of sqhAX3 mutant egg chambers harbor less than 15 nurse cells (64% of sqhAX3 mutant egg chambers have less than 7 nurse cells), a phenotype that is not shared by Drok2 mutant nurse cells (Jordan and Karess, 1997; Manseau et al., 1996). These findings also suggest that Drok2 defines a new category of oogenesis mutants that affect the actin cytoskeleton.
DRok is required for oocyte plasma membrane integrity
Both Dmoe and Drok2 GLCs exhibit similar actin defects in the oocyte, associated with a loose uneven cortical actin distribution and the presence of actin clumps in the ooplasm and near the cortex. Moreover, phospho-DMoesin levels are decreased at the cortex or mislocalized within the ooplasm of Drok2 GLCs and the conserved kinase domain of Rho-kinase phosphorylates DMoesin on threonine 559 in vitro. A potential mechanism for the DRok-DMoesin signal in this setting is that DRok controls actin reorganization through phosphorylation of DMoesin, which has been previously shown to cross-link actin to the plasma membrane when phosphorylated on T559 at the oocyte cortex (Polesello et al., 2002). However, the detection of some phospho-DMoesin in the Drok2 GLCs indicates that the critical T559 residue can be phosphorylated by other kinases in the oocyte. Indeed, direct phosphorylation of T559 of mammalian Moesin by protein kinase C (PKC)-θ has been shown in vitro (Pietromonaco et al., 1998). In addition, mammalian Rho-kinase and PAK have been reported to both phosphorylate the very conserved T508 residue of LIM-kinase in vitro (Edwards et al., 1999; Ohashi et al., 2000). Therefore, phosphorylation of the conserved T559 residue of Moesin by additional kinases might also occur in Drosophila, highlighting the complexity of cross-talk within developmental signaling pathways.
The observation that Drok2 mutant oocytes are morphologically more affected than Dmoe mutant oocytes with regard to the deformed plasma membrane (Fig. 4; 82% of the GLCs) also suggests that to exert its functions at the oocyte cortex, DRok is not only signaling to DMoesin but probably also to additional downstream targets that cooperate with DMoesin in the maintenance of the cortical actin cytoskeleton. The strong phenotype associated with the deformed oocyte plasma membrane, which separates dramatically from the apical plasma membranes of the follicle cell layer in most Drok2 GLCs (82%, Table1), raises an intriguing question about DRok’s apparent role in an adhesive process. That specific phenotype has not been previously reported in studies of other oogenesis mutants associated with defective adhesion between the oocyte and the surrounding follicle cells. Previous reports regarding such adhesion largely address cross-signaling between the apical Notch receptor and the germline-derived putative secreted and transmembrane proteins, Brainiac and Egghead, respectively, in which germline loss of either Brainiac or Egghead results in loss of epithelial apico-basal polarity and accumulation of follicular epithelial cells in multiple layers around the oocyte, but does not lead to a physical separation between the oocyte and the follicle cells membranes (Goode et al., 1996). The unique phenotype of Drok2 GLCs could reflect a role for DRok in mediating a distinct signaling pathway from the oocyte to regulate its shape and its adherence to the surrounding follicle cells. Alternatively, the aberrant morphology of the nurse cells, which appear to “push” against the oocyte without contracting, might produce a mechanical stress on the oocyte itself that prevents it from remaining apposed to the follicle cell layer. Notably, we have also found that the follicle cells themselves also appear to require DRok function for the maintenance of their shape, and it is possible that their ability to signal to the oocyte is also affected by DRok deficiency.
In summary, we have determined that the single closely related Drosophila Rho-kinase ortholog, DRok, is required for several aspects of oogenesis, including maintaining the integrity of the oocyte cortex, actin-dependent tethering of nurse cell nuclei, “dumping” of nurse cell contents into the oocyte, establishment of oocyte polarity, and the trafficking of oocyte yolk granules. It is likely that several previously identified direct phosphorylation targets of DRok, including DMoesin, Sqh (myosin light chain), and Hts (adducin), which have each been implicated in various aspects of oogenesis, mediate at least some of the functions of DRok in developing egg chambers. These findings indicate an essential role for Rho-DRok signaling via multiple DRok effectors in several distinct aspects of oogenesis.
Supplementary Material
Acknowledgements
We are grateful to Laurel Raftery, Andi McClatchey, Lynn Cooley, and members of the Cooley and Settleman laboratories for helpful discussions. We thank Alexey Veraksa for assistance with confocal microscopy and Martha Betson for critical comments on the manuscript. Reagents were generously provided by Liqun Luo, Daniel Kiehart and Lynn Cooley. Some Drosophila stocks and antibodies were obtained from the Bloomington Stock Center and Developmental Studies Hybridoma Bank, respectively. This work was supported by NIH grants RO1 GM60466 to J.S. and RO1 GM066847 to S.M.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr Opin Cell Biol. 1999;11:109–116. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha BJ, Koppetsch BS, Theurkauf WE. In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell. 2001;106:35–46. doi: 10.1016/s0092-8674(01)00419-6. [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kaibuchi K. Activation of moesin and adducin by Rho-kinase downstream of Rho. Biophys Chem. 1999a;82:139–147. doi: 10.1016/s0301-4622(99)00113-1. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999b;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglione C, Bach EA, Paraiso Y, Carraway KL, 3rd, Noselli S, Perrimon N. Mechanism of activation of the Drosophila EGF Receptor by the TGFalpha ligand Gurken during oogenesis. Development. 2002;129:175–186. doi: 10.1242/dev.129.1.175. [DOI] [PubMed] [Google Scholar]
- Goode S, Melnick M, Chou TB, Perrimon N. The neurogenic genes egghead and brainiac define a novel signaling pathway essential for epithelial morphogenesis during Drosophila oogenesis. Development. 1996;122:3863–3879. doi: 10.1242/dev.122.12.3863. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO. The role of microfilaments in cytoplasmic streaming in Drosophila follicles. J Cell Sci. 1986;80:159–169. doi: 10.1242/jcs.80.1.159. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovics F, Sinka R, Lukacsovich T, Erdelyi M. MOESIN crosslinks actin and cell membrane in Drosophila oocytes and is required for OSKAR anchoring. Curr Biol. 2002;12:2060–2065. doi: 10.1016/s0960-9822(02)01256-3. [DOI] [PubMed] [Google Scholar]
- Jeon S, Kim S, Park JB, Suh PG, Kim YS, Bae CD, Park J. RhoA and Rho kinase-dependent phosphorylation of moesin at Thr-558 in hippocampal neuronal cells by glutamate. J Biol Chem. 2002;277:16576–16584. doi: 10.1074/jbc.M110380200. [DOI] [PubMed] [Google Scholar]
- Jiang W, Sordella R, Chen GC, Hakre S, Roy AL, Settleman J. An FF domain-dependent protein interaction mediates a signaling pathway for growth factor-induced gene expression. Mol Cell. 2005;17:23–35. doi: 10.1016/j.molcel.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Jordan P, Karess R. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J Cell Biol. 1997;139:1805–1819. doi: 10.1083/jcb.139.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Lai SL, Chang CN, Wang PJ, Lee SJ. Rho mediates cytokinesis and epiboly via ROCK in zebrafish. Mol Reprod Dev. 2005;71:186–196. doi: 10.1002/mrd.20290. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 1999;126:5353–5364. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos S, Cooley L. The villin-like protein encoded by the Drosophila quail gene is required for actin bundle assembly during oogenesis. Cell. 1994;78:291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999;9:187–192. doi: 10.1016/s0962-8924(99)01544-5. [DOI] [PubMed] [Google Scholar]
- Manseau L, Calley J, Phan H. Profilin is required for posterior patterning of the Drosophila oocyte. Development. 1996;122:2109–2116. doi: 10.1242/dev.122.7.2109. [DOI] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Yonemura S, Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol. 1999;9:1259–1262. doi: 10.1016/s0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Amano M, Kaibuchi K, Nishida Y. Identification and characterization of Drosophila homolog of Rho-kinase. Gene. 1999;238:437–444. doi: 10.1016/s0378-1119(99)00351-0. [DOI] [PubMed] [Google Scholar]
- Muris DF, Verschoor T, Divecha N, Michalides RJ. Constitutive active GTPases Rac and Cdc42 are associated with endoreplication in PAE cells. Eur J Cancer. 2002;38:1775–1782. doi: 10.1016/s0959-8049(02)00100-4. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. Dorsoventral axis formation in Drosophila depends on the correct dosage of the gene gurken. Development. 1994;120:2457–2763. doi: 10.1242/dev.120.9.2457. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. The Drosophila TGF-alpha-like protein Gurken: expression and cellular localization during Drosophila oogenesis. Mech Dev. 1996;59:105–113. doi: 10.1016/0925-4773(96)00567-9. [DOI] [PubMed] [Google Scholar]
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Nilson LA, Schupbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. doi: 10.1016/s0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995a;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995b;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JW, Bier E. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques. 1994;17:874–875. 870. [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of moesin by rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem. 1998;273:34663–34666. doi: 10.1074/jbc.273.52.34663. [DOI] [PubMed] [Google Scholar]
- Piekny AJ, Mains PE. Rho-binding kinase (LET-502) and myosin phosphatase (MEL-11) regulate cytokinesis in the early Caenorhabditis elegans embryo. J Cell Sci. 2002;115:2271–2282. doi: 10.1242/jcs.115.11.2271. [DOI] [PubMed] [Google Scholar]
- Piekny AJ, Wissmann A, Mains PE. Embryonic morphogenesis in Caenorhabditis elegans integrates the activity of LET-502 Rho-binding kinase, MEL-11 myosin phosphatase, DAF-2 insulin receptor and FEM-2 PP2c phosphatase. Genetics. 2000;156:1671–1689. doi: 10.1093/genetics/156.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco SF, Simons PC, Altman A, Elias L. Protein kinase C-theta phosphorylation of moesin in the actin-binding sequence. J Biol Chem. 1998;273:7594–7603. doi: 10.1074/jbc.273.13.7594. [DOI] [PubMed] [Google Scholar]
- Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Mellor H. Regulation of endocytic traffic by Rho GTPases. Biochem J. 2003;371:233–241. doi: 10.1042/BJ20030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. Embo J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC. Germline cysts: communes that work. Cell. 1993;72:649–651. doi: 10.1016/0092-8674(93)90393-5. [DOI] [PubMed] [Google Scholar]
- Symons M, Rusk N. Control of vesicular trafficking by Rho GTPases. Curr Biol. 2003;13:R409–R418. doi: 10.1016/s0960-9822(03)00324-5. [DOI] [PubMed] [Google Scholar]
- Symons M, Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 2000;10:415–419. doi: 10.1016/s0962-8924(00)01832-8. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Hazelrigg TI. In vivo analyses of cytoplasmic transport and cytoskeletal organization during Drosophila oogenesis: characterization of a multi-step anterior localization pathway. Development. 1998;125:3655–3666. doi: 10.1242/dev.125.18.3655. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 1992;115:923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- van Eeden F, St Johnston D. The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis. Curr Opin Genet Dev. 1999;9:396–404. doi: 10.1016/S0959-437X(99)80060-4. [DOI] [PubMed] [Google Scholar]
- Verheyen EM, Cooley L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development. 1994;120:717–728. doi: 10.1242/dev.120.4.717. [DOI] [PubMed] [Google Scholar]
- Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z, Rivkees SA, Schwartz RJ, Imanaka-Yoshida K. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128:2953–2962. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Wissmann A, Ingles J, McGhee JD, Mains PE. Caenorhabditis elegans LET-502 is related to Rho-binding kinases and human myotonic dystrophy kinase and interacts genetically with a homolog of the regulatory subunit of smooth muscle myosin phosphatase to affect cell shape. Genes Dev. 1997;11:409–422. doi: 10.1101/gad.11.4.409. [DOI] [PubMed] [Google Scholar]
- Xue F, Cooley L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72:681–693. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- Yue L, Spradling AC. hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 1992;6:2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.