Abstract
Diabetes mellitus is one of the major risk factors for cardiovascular disease which is the leading cause of death in the U.S. Increasing prevalence of diabetes and diabetic atherosclerosis makes identification of molecular mechanisms by which diabetes promotes atherogenesis an important task. Targeting common pathways may ameliorate both diseases. This review focuses on well known as well as newly discovered mechanisms which may represent promising therapeutic targets.
Introduction
Diabetes mellitus is one of the major risk factors for atherosclerosis, an inflammatory disease of the arterial wall, in which leukocytes and oxidized lipoproteins accumulate leading to formation of fatty streaks and atherosclerotic plaques [1] Atherosclerosis accounts for more than 600,000 deaths annually in the U.S. mainly due to myocardial infarction and stroke [2]. The majority of diabetic patients (>90%) suffer from type 2 diabetes [3], a progressive insulin secretory defect on the background of insulin resistance [4]. Type 1 diabetes is an autoimmune disease associated with progressive and often complete β cell destruction [3], lacking insulin production due to autoimmune destruction of pancreatic β-cells [4]. Type 1 diabetics require treatment with insulin [4]. Therapy of type 2 diabetes includes life style changes, drugs that reduce intestinal glucose uptake and hepatic gluconeogenesis (e.g. metformin) [4], drugs that increase insulin secretion from the pancreas (e.g. sulfonylurea, glucagon like peptide I agonists or dipeptidylpeptidase 4 inhibitors) [4], drugs that increase insulin sensitivity in peripheral organs (e.g. thiazolidinediones (TZD)) and insulin [4]. This review will specifically focus on molecular mechanisms by which diabetes promotes atherosclerosis. The first part will cover mechanisms related to hyperglycemia common of type 1 and 2 diabetes, the second part will focus on mechanisms related to the metabolic syndrome preceding type 2 diabetes.
Pro-atherogenic mechanisms associated with hyperglycemia
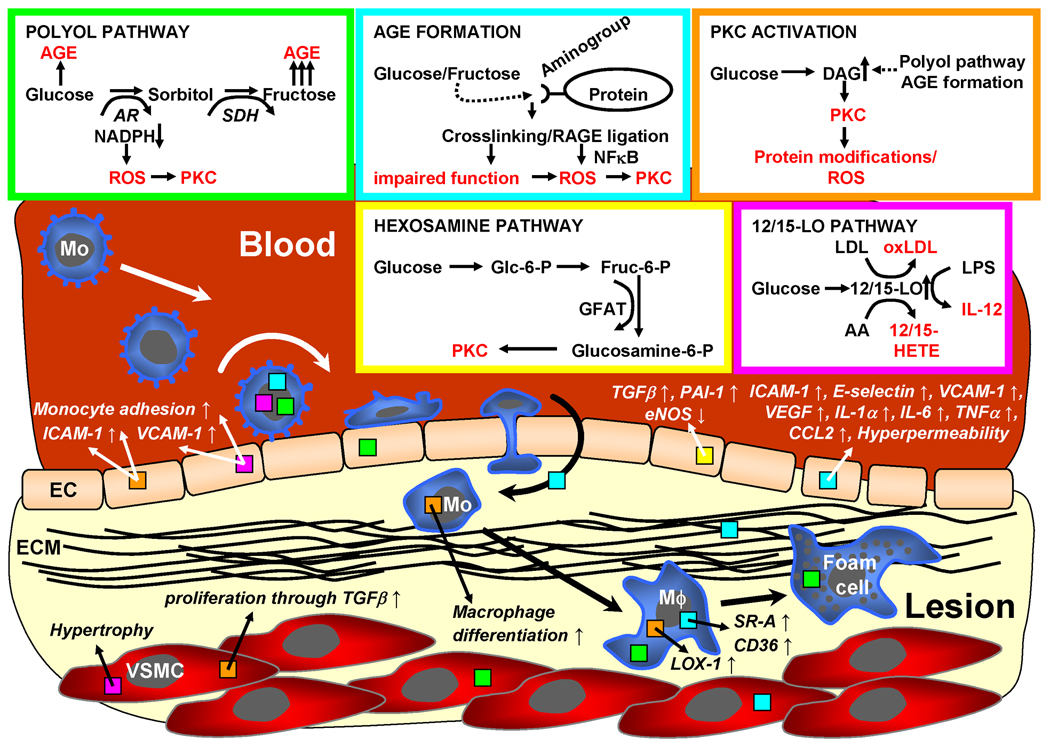
A key feature common to all end-stage forms of diabetes is hyperglycemia, which may in part account for macrovascular complications even though it may not be the only mechanism as discussed below. Different molecular mechanisms associated with hyperglycemia have been identified including 1. increased glucose flux through the polyol pathway [5], 2. formation of advanced glycation end products (AGE) [5], (3) activation of protein kinase C (PKC) [5,6], 4. increased glucose flux through the hexosamine pathway [5], and activation of the 12/15-lipoxygenase (12/15-LO) pathway [7,8]. All these mechanisms finally lead to increased superoxide formation [5] (Figure 1).
Figure 1. Pro-atherogenic mechanisms of diabetes associated with hyperglycemia.
Four hyperglycemia-related mechanisms may promote diabetic atherosclerosis: 1. The polyol pathway (green), 2. formation of advanced glycation end products (AGE, blue), 3. activation of protein kinase C (PKC) isoforms (orange), 4. the 12/15-lipoxyenase pathway (pink) and 5. the hexosamine pathway (yellow). All four mechanisms result in increased formation of reactive oxygen species (ROS) and promote diabetic atherosclerosis by various mechanisms as depicted in the figure. Colors of boxes in arrows, cells and ECM indicate relevant pathway.
12/15-LO = 12-/15-Lipoxygenase, AR = aldose reductase, EC = endothelial cell, ECM = extracellular matrix, Fruc = fructose, GFAT = glutamine-fructose-6-phopshate amidotransferase, Glc = glucose, Mo = monocyte, Mϕ = macrophage, RAGE = receptor for advanced glycation end products, SDH = sorbitol dehydroganse, VSMC = vascular smooth muscle cell, other abbreviations are explained in the text.
Polyol pathway
The polyol pathway consists of the rate-limiting enzyme aldose reductase (AR) and sorbitol dehydrogenase (SDH). Under normoglycemic conditions, AR reduces toxic aldehydes to inactive alcohols, thereby protecting cells [9]. AR has a low affinity for glucose and normally does not metabolize significant amounts of glucose. However, under hyperglycemic conditions, AR catalysis of glucose results in sorbitol production, which is subsequently metabolized by sorbitol dehydrogenase to fructose [10]. This may lead to increased osmotic stress due to intracellular sorbitol accumulation [5] and increased oxidative stress due to consumption of NADPH which is needed to regenerate glutathione [5]. Furthermore, increased fructose levels may promote increased AGE formation (see below) [5].
Recently, the glucose flux through the polyol pathway has been linked to atherosclerosis in LDL receptor (LDLR) deficient mice [11]. Overexpression of human AR in these mice (which physiologically display only low AR expression) resulted in increased atherosclerotic lesion size if the mice were made diabetic by injection of streptozotocin (STZ), which specifically destroys pancreatic β-cells and thereby mimics type 1 diabetes. Interestingly, atherosclerotic lesions in normoglycemic LDLR−/− did not differ significantly between AR overexpressing mice and mice with normal AR expression. AR overexpression also had no effect on atherosclerotic lesions in LDLR−/− mice with only mild diabetes [12]. These results suggest a pathological role for the polyol pathway depending on hyperglycemic conditions. However, the exact mechanism by which AR promotes atherosclerosis remains still to be elucidated. Our own data suggest that AR is upregulated during foam cell formation from human macrophages increasing oxidative stress in macrophages (Gleissner at al., submitted).
Clinically, AR inhibitors (ARI) have been used for many years to prevent and treat diabetic neuropathy [13]. However, ARI were not successful for treatment of other microvascular diabetic complications, but different AR inhibitors were used in different trials, which complicates interpretation [14]. Furthermore, AR polymorphisms in different individuals may be responsible for different susceptibility for ARI therapy [15]. Hyperglycemia is known to alter AR activity [16], thus the dosage of ARI may not have been equally efficient in all individuals tested. Currently, there is no clinical experience with AR inhibition in atherosclerotic patients. Even though we do not yet completely understand the pathophysiological role of the polyol pathway in diabetic atherosclerosis, targeting AR as a pro-inflammatory and pro-atherogenic mechanism in diabetic patients may be a suitable approach.
Advanced glycation end products (AGE)
Intracellular hyperglycemia leads to generation of reactive dicarbonyls, namely glyoxal, methylglyoxal and 3-deoxyglucosone, which react with amino groups of proteins and form advanced glycation end products (AGE) [5]. Modification of extracellular and intracellular proteins due to AGE formation may alter their function either by cross-linking molecules of the extracellular matrix (ECM) or by binding to the receptor for AGE (RAGE) expressed on several cell types relevant in atherogenesis, e.g. endothelial cells, macrophages and vascular smooth muscle cells [17,18]. AGE also bind to other receptors like galectin-3, macrophage scavenger receptors or others, however, no intracellular signal has been observed after engagement of these receptors [17]. Ligation of RAGE leads to translocation of NFκB into the nucleus and increased transcription of adhesion molecules like ICAM-1, E-selectin, VCAM-1, and pro-inflammatory factors like tissue factor, VEGF, interleukin (IL-)1α, IL-6, or tumor necrosis factor (TNF)-α [17]. Furthermore, AGE induce endothelial hyperpermeability and have been shown to be chemotactic for monocytes in vitro and in vivo [17]. Although the role of scavenger receptors in atherogenesis is controversial [19], induction of scavenger receptor class A and CD36 in macrophages due to AGE may represent another pro-atherogenic mechanism [17]. In summary, AGE formation due to hyperglycemia may promote development of atherosclerotic lesions by various well-known pro-atherogenic receptors [20].
Aminoguanidine is a hydrazine compound reacting with early intermediates of AGE formation and was the first inhibitor of AGE formation to be tested in clinical trials but had serious side effects and was therefore not marketed [21]. Instead of inhibiting AGE formation, other anti AGE drugs may promote breakdown of AGE-protein crosslinks [21]. One such component is LR-90, which recently was shown not only to inhibit AGE formation and crosslinking, but also to have atheroprotective properties as demonstrated in the aorta of diabetic animals [21]. One mechanism might be inhibition of pro-inflammatory mediators in monocytes as shown by reduction of CCL2 production as well as reduced adherence to human umbilical vein endothelial cells (HUVEC) of THP-1 cells, a monocytic cell line, after treatment with LR-90 [22]. Out of the drugs currently used to treat diabetes, metformin and pioglitazone have been shown to reduce AGE formation in vitro [21]. Interestingly, acetylsalicylic acid – a drug widely use in patients suffering from atherosclerotic disease – and pentoxifyllin – a drug used in patients with peripheral artery disease – also inhibit non-enzymatic glycation [23]. Finally, the β-HMG CoA reductase inhibitor cerivastatin has been shown to inhibit AGE formation [24].
An alternative way to inhibit AGE effects may be inhibiting its interaction with RAGE either by using blocking antibodies or soluble receptor for AGE (sRAGE). In Apoe−/− mice, which were diabetic after STZ injection, administration of sRAGE dose-dependently reduced development of atherosclerotic lesions independent of lipid, glucose or insulin levels [18]. In a subsequent study, sRAGE stabilized established atherosclerotic lesions in STZ-treated Apoe−/− mice as shown by reduced VSMC and macrophage content in plaque [25]. These findings were recently extended into a model for type 2 diabetes using Apoe−/− db/db (these are mice which lack the receptor for leptin, an adipokine discussed below), which after sRAGE treatment displayed smaller lesions size and reduced expression of inflammatory markers like VCAM-1, tissue factor or matrix metalloproteinase-9 (MMP-9) in the vasculature [26].
Protein kinase C (PKC)
Protein kinase C (PKC) phosphorylates proteins at serin and threonine residues. Of eleven known isoforms, eight are activated by diacylglycerol (DAG) [5,6]. Intracellular hyperglycemia induces PKC activity via increased levels of diacylglycerol (DAG) resulting mainly from DAG de novo synthesis [5,6]. Indirect PKC activation may be due to RAGE engagement or polyol pathway activation [5,6] or activation of 12/15-LO [7,8]. Various effects of PKC activation have been described which may promote atherogenesis.
Adhesion of human monocytes to HUVEC is promoted by oxLDL and can be blocked by pre-treating monocytes with PKC-inhibitors [20]. Many adhesion molecules are induced in endothelial cells by hyperglycemias, specifically PKCδ has been demonstrated to mediate thrombin-induced ICAM-1 expression in HUVEC [20]. Glucose itself also increases monocyte adhesion to the endothelium [27]. Glucose-induced expression of the scavenger receptor LOX-1 in macrophages can be inhibited by treatment with a PKCβ inhibitor [20]. Similarly, glucose-induced proliferation of rat vascular smooth muscle cells is mediated by PKCα through induction of transforming growth factor (TGF)-β, an effect that is inhibited by either antisense oligonucleotides or pharmacological inhibition [20].
Many different PKC inhibitors have been used in vitro [20]. Ruboxistaurin, a PKCβ- specific inhibitor, has been in clinical development for microvascular disease but results have not been definitive enough to recommend approval at this stage [6]. So far, no PKC inhibitor has been tested for its ability to prevent atherogenesis in diabetic animals or humans. However, several drugs currently used in diabetes therapy also have inhibitory effects on PKC. For example, peroxisome proliferator activated receptor (PPAR)-γ agonists have been demonstrated to inhibit hyperglycemia-induced PKC activity in endothelial or vascular smooth muscle cells [28,29]. It has to be noted, that recent clinical data indicate an increased number of cardiovascular events in patients treated with the PPARγ agonist rosiglitazone [30] emphasizing the complexity of interactions of this drug class which will further be discussed below.
Hexosamine pathway
The hexosamine pathway converts fructose-6-phosphate to glucosamine-6-phosphate through glutamine-fructose-6-phosphate amidotransferase (GFAT) activity, thereby providing substrates for synthesis of proteoglycans and glycoproteins [5]. Hyperglycemia-induced activity of GFAT has been associated with transcription of transforming growth facto-β1 (TGF-β1) and plasminogen activator inhibitor-1 (PAI-1) in bovine aortic endothelial cells (BAEC) [31]. This effect was inhibited by treatment with azaserine, a GFAT inhibitor [31]. Similarly, inhibition of GFAT by antisense oligonucleotides resulted in reversal of hyperglycemia-induced inhibition of eNOS activity in BAEC, a finding that was confirmed in vivo in aortas from rats with STZ-induced diabetes [32]. Glucosamine may activate protein kinase C and thereby induce pro-atherogenic mechanisms as discussed above [5]. The exact effects of the hexosamine pathway on macrovascular diabetic complications remain unclear, especially since it has recently been demonstrated that glucosamine – an intermediate producte of this pathway – improved the barrier function of BAEC in vitro and resulted in a decrease of THP-1 monocytic cell binding to these cells by about 50 % [33]. Most strikingly, intraperitoneal administration of glucosamine to Apoe−/− mice for twelve weeks resulted in a moderate reduction of atherosclerotic lesion size [33]. Thus, the hexosamine pathway may well be related to microvascular diabetic complications, but may not represent a strong link between diabetes and atherosclerosis.
12/15-lipoxygenase (12/15-LO) pathway
12- and 15-lipoxygenase are enzymes that insert oxygen at the 12 or 15 carbon position into arachidonic acid leading to formation of 12(S)- and 15(S)-hydroxyeicosatetraeonic acid [8]. 12/15-LO is expressed in endothelial cells, smooth muscle cells, monocytes and macrophages, its acitivity has been shown to be increased by hyperglycemia [8]. 12/15- LO-deficient mice crossbred with either ApoE−/− or LDLR−/− mice displayed decreased atherosclerotic lesion size [34,35].
Several effects of 12/15-LO activity are considered pro-atherogenic. 12/15-LO promotes oxidation of native to more atherogenic oxidized LDL [8]. Reduced IL-12 secretion upon stimulation with LPS suggests a role in the inflammatory response [8]. 12/15-LO seems to be involved in hyperglycemia- as well as mmLDL-mediated adhesion of monocytes to the endothelium and promotes vascular smooth muscle cell hypertrophy [8]. Also, 12(S)- HETE, one of the products of 12-LO, promotes monocyte-adhesion to endothelial cells, probably in part by inducing the fibronectin splice variant CS-1 and VCAM-1 on endothelial cells [8]. Interestingly, 12-LO deficient mice are resistant to STZ-induced diabetes suggesting not only a role for 12-LO in diabetes-induced atherosclerosis but also in pathogenesis of diabetes itself [36]. This notion is supported by the findings that the LO-inhibitor masoprocol can improve insulin sensitivity in a rat model of type 2 diabetes
In humans, 12/15-LO activity is likely to play a role as diabetics show higher urinary excretion of the 12-LO metabolite 12(S)-HETE than healthy controls [8]. Furthermore, indirect evidence for 15-LO activity has been found in early atherosclerotic lesions from humans supporting its pro-atherogenic role [37]. However, it has also to be noted that some metabolites of the 12/15-LO system seem to have anti-inflammatory effects, e.g. 13-hydroxyoctadecadieonic acid (13-HODE), which reduces platelet adhesion to endothelial cells and binds to PPARγ thereby reducing macrophage expression of matrix metalloproteinase-9 (MMP-9) and pro-inflammatory cytokines [38]. Even though, pharmacological inhibitors of LO have been tested in experimental settings in animals, so far no clinical trials have been undertaken. However, this may be of special interest as the 12/15-LO system is not only related to diabetes and atherosclerosis but also seems to be a link between hyperglycemia-related and insulin-resistance-associated pro-atherogenic features of diabetes, which will be discussed below.
Pro-atherogenic mechanisms in diabetes in addition to hyperglycemia
Reduction of hyperglycemia has been convincingly shown to reduce microvascular diabetic complications [39]. However, even if normoglycemia was achieved, diabetics still displayed a higher risk for developing atherosclerosis than non-diabetics [39]. This suggests that other mechanisms than hyperglycemia may be important for atherogenesis in diabetics, which may especially apply to type 2 diabetes. Type 2 diabetes is often preceded by or associated with the metabolic syndrome, a risk factor complex for cardiovascular disease consisting of atherogenic dyslipidemia, insulin resistance accompanied by glucose intolerance, abdominal obesity, a proinflammatory and prothrombotic state and hypertension [40,41].
Diabetes-associated hyperlipidemia
Type 2 diabetes is associated with hyperlipidemia (elevated triglycerides, decreased high density lipoprotein (HDL) and increased low density lipoprotein (LDL) levels [40]. Furthermore, in addition to oxidation of lipoproteins hyperglycemia may lead to glycation leading to formation of pro-atherogenic glycoxidation products [42] in analogy to formation of advanced glycation end products (AGE) as already discussed. Even if LDL levels are normal, the composition of LDL particles is still altered and therefore more atherogenic as compared to healthy individuals [43]. Therapy with fibrates and statins has been demonstrated effective to reduce cardiovascular events, probably not only by normalizing lipid profile but also by pleiotropic anti-inflammatory effects [44].
Mechanisms related to insulin resistance and hyperinsulinemia
There are many proposed mechanisms leading to insulin resistance and subsequent hyperinsulinemia – both key features of type 2 diabetes (Figure 2). One prominent idea is that impaired mitochondrial activity results in dysregulation of intracellular fatty acid metabolism [45]. Increased intracellular fatty acids may activate PKCβ and δ via DAG and thereby lead to serine phosphorylation of the insulin receptor substrate (IRS)-1, resulting in reduced glucose transport [45]. These defects seem to be closely related to increased body fat. Indeed, visceral abdominal fat has important endocrine functions, leading to increased serum levels of TNF-α, interleukin-6, plasminogen activator inhibitor (PAI-)1, and reduced expression of adiponectin [46]. These changes in adipokines – these are cytokines produced by adipose tissue – could also lead to reduced insulin signaling and activation of cytokines, transcription factors such as NFκB and activated mitogen kinases such as JNK, both also leading to insulin resistance. We have created a model of genetic insulin resistance on the ApoE−/− background by targeted insulin receptor (IR) and IRS-1 deletion and have found evidence for increased atherosclerosis (Galkina et al., submitted).
Figure 2. Pro-atherogenic mechanisms of diabetes associated with insulin resistance.
Multiple mechanisms associated with insulin resistance may promote diabetic atherosclerosis in type 2 diabetes: 1. Secretion of adipokines from adipose tissue (adiponectin, apelin, or leptin), 2. fatty acid binding protein (FABP) aP2 secretion from adipocytes, macrophages (Mϕ) or foam cells, 3. C peptide as a decomposition product of proinsulin, and 4. diabetic hyperlipidemia. Lipoprotein lipase (LPL) is inversely associated with insulin resistance, non-enzymatically glycated hemoglobin (HbA1c), and atherosclerosis. IRS-1 = insulin receptor substrate-1, SR-A = scavenger receptor class A
In humans, insulin resistance has been associated with low expression of IRS-1 [47]. In addition, patients displayed reduced serum levels of adiponectin and reduced gene expression of PPARγ, fatty acid binding protein (FABP) aP2 and lipoprotein lipase (LPL) in adipose tissue. Even at younger AGE and having a normal body mass index, individuals with low IRS-1 expression displayed not only an increased waist/hip ratio and an unfavorable lipid profile, but also significant intimal thickening of their carotid arteries, suggesting higher susceptibility to atherogenesis [47]. Insulin resistance resulting in higher daily insulin doses has been shown to be a risk factor for atherogenesis in type 2 but not in type 1 diabetics [48], however, it is unlikely that insulin itself leads to increased atherogenesis. It is likely that follow-up studies of the inflammatory pathways in visceral fat could lead to new therapeutic targets for reducing atherosclerosis since the innate immune system is activated in visceral fat and components of the innate immune system (e.g. IL-12 or toll-like receptor-4 (TLR4)) have been clearly implicated in atherogenesis.
Adiponectin and other adipokines
Adipokines are cytokines produced by adipose tissue; they may be related to the metabolic syndrome and have pro- as well as antiatherogenic effects. Adiponectin serum levels have been demonstrated to be inversely correlated with insulin resistance and inflammatory state [46]. It reduces TNF-α-mediated adhesion of THP-1 monocytes to and induction of ICAM-1 in human coronary artery endothelial cells (HCAEC) [49]. In Apoe−/− mice, treatment with a recombinant adenovirus expressing human adiponectin resulted in increased adiponectin serum levels and decrease of atherosclerotic lesion size by about 30 % [50]. mRNA for VCAM-1 and scavenger receptor class A were significantly reduced in lesions from these mice [50]. Interestingly, PPARγ agonists are known to promote adipocyte differentiation and also to increase adiponectin transcription and serum levels [51]. However, clinical data suggest that PPARγ agonists may rather promote cardiovascular adverse events than be atheroprotective [30]. Telmisartan – an angiotensin receptor blocker administered in hypertensive patients – has been demonstrated to increase adiponectin transcription in human adipocytes [52].
The role of other adipokines like leptin or apelin is less clear. The role of leptin in atherogenesis is currently controversial as leptin (ob/ob) or leptin receptor (db/db) deficiency in Apoe−/− mice has resulted in attenuation of plaque formation [53] or increased atherogenesis [54]. Inactivating of the apelin receptor (APJ) in Apoe−/− mice resulted in reduced lesion size and oxidative stress, suggesting a role for apelin in atherogenesis [55].
Fatty acid-binding protein aP2
Fatty acid-binding protein aP2 (aP2) is member of the FABP family of cytoplasmatic proteins that bind hydrophobic ligands like saturated or unsaturated fatty acids. aP2 is expressed in adipocytes and macrophages and has been demonstrated in foam cells in human atherosclerotic plaque [56]. aP2−/− mice are obese but protected against insulin resistance [57]. aP2 deficiency also reduces atherosclerotic lesions in Apoe−/− mice [58]. Recently, pharmacological inhibition of aP2 has been demonstrated to reduce atherosclerosis in Apoe−/− mice, probably due to inhibition of foam cell formation, production of inflammatory chemokines and cytokines [59]. Atorvastatin, a β-HMG CoA reductase inhibitor, has been demonstrated to suppress aP2 in vitro [60].
C peptide
Another potential therapeutic target related to hyperinsulinemia is C peptide, a decomposition product of proinsulin, which may be more abundant when high amounts of insulin are needed in the body. C peptide has been shown to be expressed in atherosclerotic lesions from diabetic patients [61]. In vitro, C-peptide displayed chemotactic activity towards monocytes via a G-protein coupled receptor and phosphatidylinositol-3 kinase- (PI3K)-dependent mechanism [61] and induced proliferation of vascular smooth muscle cells [62]. Currently, there are no reports published on using C peptide as therapeutic target to prevent diabetic macrovascular complications. Thus, additional studies in this area will be required to clarify any role of C peptide.
Thromboxane receptor A2
The thromboxane A2 receptor (TP) may represent another therapeutic target in diabetic cardiovascular disease. Apoe−/− mice made diabetic by STZ injection showed decreased inflammation and atherosclerotic lesion size when treated with a TP inhibitor independently of platelet-derived thromboxane A2 production [63]. This finding may be explained by restoration of endothelial function as demonstrated by increase of endothelial NO synthase (eNOS) and reduced vascular cell adhesion molecule-1 (VCAM-1) expression [63]. S18886 (terubtroban) has been demonstrated to improve endothelial function in patients with coronary artery disease [64] and is currently compared with other antithombotic agents in a clinical trial (Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischaemic strOke or tRansient ischemic attack PERFORM). In addition, a recent study showed a role of S18886 to reduce oxidative stress and expression of 12/15-LO [65].
Lipoprotein lipase
Lipoprotein lipase is an enzyme hydrolyzing triglycerides in chylomicrones or very low density lipoproteins (VLDL) [66]. LPL mass and activity in post heparin plasma not only correlate inversely with triglycerides and non-enzymatically glycated haemoglobin (HbA1c) in type 2 diabetics, but also with insulin resistance [67] and future coronary artery disease even after adjustment for traditional risk factors like blood pressure, LDL serum levels, diabetes or body mass index [68]. LPL seems to be an excellent marker integrating type 2 diabetes and atherogenesis, but there are currently no studies testing for LPL inhibition in order to prevent cardiovascular disease.
Genes associated with diabetes and atherosclerosis
Gene expression studies of foam cell formation from macrophages using Affymetrix chips have revealed a role for high insulin and glucose levels in this process [69]. Furthermore, several genes have recently been identified in genome-wide association studies to be related to diabetes and atherosclerosis, thereby unveiling potential new therapeutic targets. The function of many of these genes is currently not completely understood. A common polymorphism in the PPARγ gene (PPARG) has been early reported to be related to reduced risk for the development of type 2 diabetes [70]. Similarly, a single nucleotide polymorphisms (SNP) in the gene for calpain-10 (CAPN10), a nonlysosomal cysteine protease of unknown function, has been demonstrated to be related to insulin resistance and subclinical atherosclerosis as defined by carotid intimal thickening [71]. Another polymorphism has been described in the ENPP1 (encoding ectonucleotide pyrophosphatase 1) gene. ENPP1 is an inhibitor for an inhibitor of insulin secretion associated with insulin resistance, development of diabetes before the age of 65 and myocardial infarction before the age of 50 [72]. Recently, a TNF-induced protein 3 (TNFAIP3, a negative regulator of NFκB) polymorphism was identified to modulate the risk for coronary artery disease in type 2 diabetics [73]. All of these common variants have been replicated in multiple populations, supporting their validity.
Independently of genome wide association studies, the haptoglobin gene (HP) has been shown to play a role in diabetic atherosclerosis [74]. Depending on the genotype, susceptibility for vascular complications in diabetes was found to be increased (higher in Hp2-2 than in Hp1-1 or Hp-2-1). Proposed mechanisms of this finding include differences in the antioxidant and CD163-mediated scavenging functions of Hp2-2. Even though, prospective data are lacking, this hypothesis is supported by the fact that individuals with the Hp2-2 genotype benefited from treatment with antioxidative vitamin E as seen in a retrospective analysis of the HOPE study, whereas in patients with the Hp1-1 vitamin E did not have any effect [75].
Conclusions
The incidence of diabetes and diabetes-related atherosclerosis has dramatically increased over the past years [3]. Even though prevention of diabetes by a reasonable life style should be the first step in fighting this epidemic, it is unlikely to be sufficient [4]. Considering the overlap of pathogenetic mechanisms of diabetes and atherosclerosis, it seems promising to identify therapeutic targets shared by both diseases [2,3].
Many of the pro-atherogenic mechanisms of diabetes may be related to hyperglycemia and thereby promote macrovascular complications as discussed above. Aspects of the metabolic syndrome seem to represent promising therapeutic targets. This is supported by the finding that achieving normoglycemia alone is not sufficient to reduce the cardiovascular risk to that of non-diabetic individuals [39]. Interestingly, some of the drugs that have been used to treat cardiovascular or diabetic patients for a long time actually targeted some of these mechanisms even before they were identified (e.g. PPARγ agonists). However, there is still room for improvement, not only because long term prognosis of diabetic patients is still dissatisfactory, but also as there are still many mechanisms which have not been approached yet (e.g. LPL). Furthermore, the link to inflammatory pathways triggered by visceral fat has to be studied in more detail.
Genome-wide association screens have identified polymorphisms in genes associated with diabetes as well as atherosclerosis. Even though the exact function of many of these genes is still unknown, this broad approach may help to identify subgroups benefiting from specific therapeutic interventions (e.g. antioxidants) or even reveal new therapeutic targets for both diseases. The fact that rosiglitazone – a PPARγ agonist, which according to our current pathophysological understanding of diabetic atherosclerosis should be protective – has recently been associated with a potential for higher cardiovascular mortality in type 2 diabetics underlines the complex relation between atherosclerosis and diabetes [30]. Thus, careful studies will be necessary to confirm our concepts of diabetic macrovascular disease. It is known that aggressive therapy of cardiovascular risk factors other than diabetes, i.e. hyperlipidemia or hypertension, can improve the long-term prognosis of diabetic patients with cardiovascular disease [39]. Identifying and approaching new therapeutic targets may help to further improve their prognosis.
Table 1.
Diabetes-related pro-atherogenic mechanisms and therapeutic approaches
| Mechanism | Strategic approach to target | Common drugs targeting this mechanism | Experimental therapies/trials | Refs |
|---|---|---|---|---|
| Polyol pathway | Inhibit the rate limiting enzyme aldose reductase (AR) | • Epalrestat (marketed in Japan to treat polyneuropathy) | Overexpression of human AR increases atherosclerosis in type 1 diabetic mice (LDLR−/−, STZ injection) | [11,12] |
| AGE formation | Inhibit AGE formation and protein cross-linking, breakdown crosslinks, block RAGE ligation | • Metformin • Pioglitazone • Acetylsalicylic acid • Pentoxifyllin • Cerivastatin |
RAGE antibodies and sRAGE reduce atherosclerosis in type 2 diabetic mice (Apoe−/−, db/db) | [25,26] |
| Protein kinase C | Inhibit certain PKC isoforms | • Ciglitazone, troglitazone (not marketed) | Plethora of pharmacological inhibitors used in vitro and in vivo | Reviewed in [20] |
| 12-/15- lipoxygenase | Inhibit 12-/15-lipoxygenase | - | 12/15-LO deficiency reduces athersclerosis in Apoe−/− and LDLR−/− mice | Reviewed in [8,38] |
| Diabetic hyperlipidemia | Reduce LDL, increase HDL serum levels, ameliorate composition of LDL particles | • Statins • Fibrates |
- | Reviewd in [44] |
| Adiponectin | Increase of adiponectin serum levels | • Pioglitazone • Telmisartan |
Adenoviral overexpression of adiponectin reduced atherosclerosis in Apoe−/− mice | [50] |
| Apelin | Blocking of the apelin receptor (APJ) | - | APJ knock out reduces athersclerosis in Apoe−/− mice | [55] |
| Leptin | (Unclear as currently contradictory results in mouse experiments) | - | Contradictory results in leptin (ob/ob; Apoe−/−) or leptin receptor (db/db; Apoe−/−) deficient atherosclerotic mice | [53,54] |
| Fatty acid binding protein aP2 | Inhibit pharmacologically to reduce foam cells formation, cytokine and chemokine production | • Atorvastatin | Treatment of type 2 diabetic mice with BMS309403 reduced atherosclerotic lesions (ob/ob, Apoe−/−) | [59] |
| Lipoprotein lipase | (Marker for insulin sensitivity predicting risk of diabetes and atherosclerosis, role as therapeutic target unclear) | - | - | [67,68] |
| C peptide | Reduce formation by increasing insulin resistance | • PPARγ agonists | - | [61] |
| Thromboxane receptor A2 | Block the thromboxane A2 receptor, reduce levels of other eicosanoids | • Terutroban/S18886 (currently efficiency to prevent cardio- and cerebrovascular events is evaluated in the PERFORM study, which is supposed to be completed in 2010) | Treatment of type I diabetic mice (Apoe−/−, STZ injection) with S18886 reduced atherosclerotic lesions independent of platelet-derived thromboxane A2 production | [63] |
| Haptoglobin | Depending on genotype reduced antioxidative and scavenging capacity | • Antioxidants like vitamin E | Retrospective analysis of the HOPE study, no prospective study undertaken yet | [75] |
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (grant GL599/1-1 to C.A.G.), by NIH grant HL58108 (to K.L.), and by NIH grant HL55798 (to K.L. and J.L.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Adeghate E, et al. An Update on the Etiology and Epidemiology of Diabetes Mellitus. Ann NY Acad Sci. 2006;1084(1):1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of Medical Care in Diabetes - 2007. Diabetes Care. 2007;30 Suppl 1:S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 6.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55(6):498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep. 2007;7(3):242–248. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(9):1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 9.Spycher SE, et al. Aldose reductase induction: a novel response to oxidative stress of smooth muscle cells. FASEB J. 1997;11(2):181–188. doi: 10.1096/fasebj.11.2.9039961. [DOI] [PubMed] [Google Scholar]
- 10.Bohren KM, et al. The aldo-keto reductase superfamily. cDNAs and deduced amino acid sequences of human aldehyde and aldose reductases. J Biol Chem. 1989;264(16):9547–9551. [PubMed] [Google Scholar]
- 11.Vikramadithyan RK, et al. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115(9):2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L, et al. Addition of dietary fat to cholesterol in the diets of LDL receptor knockout mice: effects on plasma insulin, lipoproteins, and atherosclerosis. J Lipid Res. 2006;47(10):2215–2222. doi: 10.1194/jlr.M600146-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Hotta N, et al. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care. 2006;29(7):1538–1544. doi: 10.2337/dc05-2370. [DOI] [PubMed] [Google Scholar]
- 14.Oka M, Kato N. Aldose reductase inhibitors. J Enzyme Inhib. 2001;16(6):465–473. doi: 10.1080/14756360127568. [DOI] [PubMed] [Google Scholar]
- 15.Demaine AG. Polymorphisms of the aldose reductase gene and susceptibility to diabetic microvascular complications. Curr Med Chem. 2003;10(15):1389–1398. doi: 10.2174/0929867033457359. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava SK, et al. Hyperglycemia-induced activation of human erythrocyte aldose reductase and alterations in kinetic properties. Biochim Biophys Acta. 1986;870(2):302–311. doi: 10.1016/0167-4838(86)90234-7. [DOI] [PubMed] [Google Scholar]
- 17.Goldin A, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt AM, et al. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84(5):489–497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- 19.Moore KJ, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115(8):2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25(3):487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 21.Rahbar S, Figarola JL. Novel inhibitors of advanced glycation end-products. Arch Biochem Biophys. 2003;419(1):63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Figarola JL, et al. Anti-inflammatory effects of the advanced glycation end product inhibitor LR-90 in human monocytes. Diabetes. 2007;56(3):647–655. doi: 10.2337/db06-0936. [DOI] [PubMed] [Google Scholar]
- 23.Rahbar S, et al. Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin Chim Acta. 2000;301(1–2):65–77. doi: 10.1016/s0009-8981(00)00327-2. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto T, et al. Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. FASEB J. 2002;16(14):1928–1930. doi: 10.1096/fj.02-0030fje. [DOI] [PubMed] [Google Scholar]
- 25.Bucciarelli LG, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106(22):2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 26.Wendt T, et al. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185(1):70–77. doi: 10.1016/j.atherosclerosis.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Manduteanu I, et al. High glucose induces enhanced monocyte adhesion to valvular endothelial cells via a mechanism involving ICAM-1, VCAM-1 and CD18. Endothelium. 1999;6(4):315–324. doi: 10.3109/10623329909078498. [DOI] [PubMed] [Google Scholar]
- 28.Verrier E, et al. PPARgamma agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: role of diacylglycerol kinase. Circ Res. 2004;94(11):1515–1522. doi: 10.1161/01.RES.0000130527.92537.06. [DOI] [PubMed] [Google Scholar]
- 29.Yasunari K, et al. Mechanisms of action of troglitazone in the prevention of high glucose-induced migration and proliferation of cultured coronary smooth muscle cells. Circ Res. 1997;81(6):953–962. doi: 10.1161/01.res.81.6.953. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 31.Du XL, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97(22):12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du XL, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108(9):1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan W, et al. Distinct effects of glucose and glucosamine on vascular endothelial and smooth muscle cells: evidence for a protective role for glucosamine in atherosclerosis. Cardiovasc Diabetol. 2005;4:16. doi: 10.1186/1475-2840-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cyrus T, et al. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation. 2001;103(18):2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 35.George J, et al. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104(14):1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 36.Bleich D, et al. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103(10):1431–1436. doi: 10.1172/JCI5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhn H, et al. In vivo action of 15-lipoxygenase in early stages of human atherogenesis. J Clin Invest. 1997;99(5):888–893. doi: 10.1172/JCI119253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77(2):67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Sobel BE. Optimizing cardiovascular outcomes in diabetes mellitus. Am J Med. 2007;120 9 Suppl 2:S3–S11. doi: 10.1016/j.amjmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 41.Kahn R, et al. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005;48(9):1684–1699. doi: 10.1007/s00125-005-1876-2. [DOI] [PubMed] [Google Scholar]
- 42.Lopes-Virella MF, et al. The immunogenicity of modified lipoproteins. Ann N Y Acad Sci. 2005;1043:367–378. doi: 10.1196/annals.1333.043. [DOI] [PubMed] [Google Scholar]
- 43.Association AD. Dyslipidemia Management in Adults With Diabetes. Diabetes Care. 2004;27(90001):68S–71S. doi: 10.2337/diacare.27.2007.s68. [DOI] [PubMed] [Google Scholar]
- 44.Libby P, Plutzky J. Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma agonists. Am J Cardiol. 2007;99(4A):27B–40B. doi: 10.1016/j.amjcard.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Petersen KF, Shulman GI. New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14 Suppl 1:34S–40S. doi: 10.1038/oby.2006.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kershaw EE, Flier JS. Adipose Tissue as an Endocrine Organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 47.Jansson PA, et al. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. FASEB J. 2003;17(11):1434–1440. doi: 10.1096/fj.02-1132com. [DOI] [PubMed] [Google Scholar]
- 48.Wollesen F, et al. Insulin resistance and atherosclerosis in diabetes mellitus. Metabolism. 2002;51(8):941–948. doi: 10.1053/meta.2002.32721. [DOI] [PubMed] [Google Scholar]
- 49.Ouchi N, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto Y, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106(22):2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 51.Miyazaki Y, et al. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89(9):4312–4319. doi: 10.1210/jc.2004-0190. [DOI] [PubMed] [Google Scholar]
- 52.Moriuchi A, et al. Induction of human adiponectin gene transcription by telmisartan, angiotensin receptor blocker, independently on PPAR-gamma activation. Biochem Biophys Res Commun. 2007;356(4):1024–1030. doi: 10.1016/j.bbrc.2007.03.084. [DOI] [PubMed] [Google Scholar]
- 53.Wu KK, et al. Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis. 2005;181(2):251–259. doi: 10.1016/j.atherosclerosis.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 54.Chiba T, et al. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto T, et al. Requirement of Apelin-Apelin Receptor System for Oxidative Stress-Linked Atherosclerosis. Am J Pathol. 2007 doi: 10.2353/ajpath.2007.070471. ajpath.2007.070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu Y, et al. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165(2):259–269. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 57.Hotamisligil GS, et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 58.Makowski L, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7(6):699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furuhashi M, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Llaverias G, et al. Atorvastatin reduces CD68, FABP4, and HBP expression in oxLDL-treated human macrophages. Biochem Biophys Res Commun. 2004;318(1):265–274. doi: 10.1016/j.bbrc.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 61.Marx N, et al. C-peptide colocalizes with macrophages in early arteriosclerotic lesions of diabetic subjects and induces monocyte chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2004;24(3):540–545. doi: 10.1161/01.ATV.0000116027.81513.68. [DOI] [PubMed] [Google Scholar]
- 62.Walcher D, et al. C-Peptide induces vascular smooth muscle cell proliferation: involvement of SRC-kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase 1/2. Circ Res. 2006;99(11):1181–1187. doi: 10.1161/01.RES.0000251231.16993.88. [DOI] [PubMed] [Google Scholar]
- 63.Zuccollo A, et al. The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation. 2005;112(19):3001–3008. doi: 10.1161/CIRCULATIONAHA.105.581892. [DOI] [PubMed] [Google Scholar]
- 64.Belhassen L, et al. Improved endothelial function by the thromboxane A2 receptor antagonist S 18886 in patients with coronary artery disease treated with aspirin. J Am Coll Cardiol. 2003;41(17):1198–1204. doi: 10.1016/s0735-1097(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 65.Xu S, et al. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes. 2006;55(1):110–119. [PubMed] [Google Scholar]
- 66.Kobayashi J, et al. Serum lipoprotein lipase mass: clinical significance of its measurement. Clin Chim Acta. 2007;378(1–2):7–12. doi: 10.1016/j.cca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Hanyu O, et al. Lipoprotein lipase (LPL) mass in preheparin serum reflects insulin sensitivity. Atherosclerosis. 2004;174(2):385–390. doi: 10.1016/j.atherosclerosis.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi J, et al. Lipoprotein lipase mass and activity in post-heparin plasma from subjects with intra-abdominal visceral fat accumulation. Clin Endocrinol (Oxf) 1998;48(4):515–520. doi: 10.1046/j.1365-2265.1998.00485.x. [DOI] [PubMed] [Google Scholar]
- 69.Shashkin PN, et al. Insulin and glucose play a role in foam cell formation and function. Cardiovasc Diabetol. 2006;5:13. doi: 10.1186/1475-2840-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altshuler D, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26(1):76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 71.Goodarzi MO, et al. Association of the diabetes gene calpain-10 with subclinical atherosclerosis: the Mexican-American Coronary Artery Disease Study. Diabetes. 2005;54(4):1228–1232. doi: 10.2337/diabetes.54.4.1228. [DOI] [PubMed] [Google Scholar]
- 72.Bacci S, et al. The K121Q polymorphism of the ENPP1/PC-1 gene is associated with insulin resistance/atherogenic phenotypes, including earlier onset of type 2 diabetes and myocardial infarction. Diabetes. 2005;54(10):3021–3025. doi: 10.2337/diabetes.54.10.3021. [DOI] [PubMed] [Google Scholar]
- 73.Boonyasrisawat W, et al. Tag polymorphisms at the A20 (TNFAIP3) locus are associated with lower gene expression and increased risk of coronary artery disease in type 2 diabetes. Diabetes. 2007;56(2):499–505. doi: 10.2337/db06-0946. [DOI] [PubMed] [Google Scholar]
- 74.Asleh R, Levy AP. In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag. 2005;1(1):19–28. doi: 10.2147/vhrm.1.1.19.58930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levy AP, et al. The Effect of Vitamin E Supplementation on Cardiovascular Risk in Diabetic Individuals With Different Haptoglobin Phenotypes. Diabetes Care. 2004;27(11):2767. doi: 10.2337/diacare.27.11.2767. [DOI] [PubMed] [Google Scholar]