Abstract
The thymidine analog bromodeoxyuridine (BrdU) is incorporated into newly synthesized DNA and has been shown to increase the susceptibility of incorporating cells to ionizing radiation. However, in the absence of secondary stressors, BrdU is thought to substitute relatively benignly for thymidine and is commonly used to “birth-date” proliferative cells. We report a novel antiproliferative effect of BrdU on cancer cells, which is independent of its role in radiosensitization. A single, brief in vitro exposure to BrdU induces a profound and sustained reduction in the proliferation rate of all cancer cells examined. Cells do not die but variably up-regulate some senescence-associated proteins as they accumulate in the G1 phase of the cell cycle. Bromodeoxyuridine also impairs the proliferative capacity of primary tumor-initiating human glioma cells and may therefore represent a means of targeting cancer stem cells. Finally, conservative in vivo BrdU regimens—in the absence of any other treatment—significantly suppress the progression of gliomas in the highly aggressive, syngeneic RG2 model. These results suggest that BrdU may have an important role as an adjunctive therapeutic for a wide variety of cancers based on new insights into its effect as a negative regulator of cell cycle progression.
Introduction
5-Bromo-2′-deoxyuridine (BrdU) is a thymidine analog that was introduced in the 1950s as a mutagen to target rapidly dividing cancer cells [1–3] but is now used ubiquitously to birth-date dividing cells. Although there are a myriad of reports dealing with the consequences of BrdU incorporation into DNA chains [4–7], the variations in dosage and exposure time in these studies make it difficult to compare individual results. Perhaps because of this, and because incorporating cells seem to maintain relatively normal function—at least in the short term [8]—BrdU is generally thought to substitute relatively benignly for thymidine. Recent work, however, suggests that BrdU may play a role in premature senescence induction in a wide variety of cells [9,10].
Despite its extensive history, there is no accepted consensus mechanism of action for BrdU. It has been suggested that BrdU alters the stability of DNA, thereby increasing the risk of sister-chromatid exchanges, mutations, and double-strand breaks (reviewed in Taupin [11]). However, most of these effects are found only when BrdU incorporation is combined with secondary stressors. Early toxicity studies showed that BrdU can induce chromosomal breakage and increase the sensitivity of treated cells to ionizing radiation [3,12,13], and this radiosensitizing effect has continued to be pursued as an adjunctive therapy in the treatment of a variety of cancers. Bromodeoxyuridine readily crosses the blood-brain barrier, and it has been combined with conventional chemotherapy and radiation treatment in several clinical trials [14–19]. Although the clinical benefits of including BrdU as a radiosensitizer have been disappointing—showing, at best, modest improvements for some outcome measurements—it is possible that other therapeutic effects of BrdU were not appreciated, either because of interference by the other treatment modalities in these studies or because finer analytical resolution is required to discern them.
Surprisingly, little attention has been focused on examining the influence that BrdU alone may exert on cellular function. In the present study, we show that a single brief exposure to BrdU leads to a progressive and sustained impairment of cell cycle progression in all examined cancer cells in vitro. Treated cells do not die but gradually accumulate in the G1 phase of the cell cycle while showing a variable up-regulation of some senescence-associated proteins. Clonal analysis of primary human glioma tumor cells with stem cell—like characteristics shows that a single pulse of BrdU can impair the proliferative capacity of the progeny of the putative tumor-initiating cells over many population doublings. Therefore, BrdU may represent a means of selectively targeting cancer stem cells. Most importantly, we show that a brief oral or systemic regimen of BrdU leads to significantly delayed tumor progression in a highly aggressive syngeneic rat model of glioma.
Our results suggest that BrdU possesses therapeutic potential as an anticancer agent that is independent of its role as a radiosensitizer and that BrdU should be reassessed as an adjunctive therapeutic modality based on a new understanding of its antiproliferative effect.
Materials and Methods
Cell Culture
Cell lines were obtained from American Type Culture Collection (http://www.atcc.org): H9, human cutaneous T-cell lymphoma (#HTB-176); MG-63, human osteosarcoma (#CRL-1427); Saos-2, human osteosarcoma (#HTB-85); TT, human thyroid tumor (#CRL-1803); BJ, human fibroblasts (#CRL-2522); and RG2, rat glioma (#CRL-2433). Primary human glioma cells were generated from a surgical resection. Experiments were performed in triplicate cultures maintained in a 37°C humidified chamber containing 5% CO2.
In Vitro Drug Treatment and Quantification of Proliferative Activity
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted: BrdU (#B9285), BrdU (#B23151 from Molecular Probes, Eugene, OR), 5-chloro-2′-deoxyuridine (CldU; #C6891), 5-iodo-2′-deoxyuridine (IdU; #I7125), 5-aza-2′-deoxycytidine (AZA; #A3656), 5-fluorouracil (5-FU; #F6627), thymidine (#T1895), and cytidine (#C4654). Exposure times ranged from 1 minute to 24 hours, after which the medium was aspirated and replaced with fresh medium without analogs. Control and treated cultures received the same number of medium changes.
Cultures were initially plated at 2000 cells/cm2 and were quantified with a Z2 Coulter Counter (Beckman Coulter, Fullerton, CA) at various intervals after the removal of BrdU (range = 1 minute to several weeks).
Neurosphere cultures of primary human glioma cells were established and maintained as described [20].
Statistical Analyses
All analyses were performed with GraphPad InStat and Prism 4 (San Diego, CA). Two-group comparisons of cell counts were performed with the Student's t test. Multiple-group comparisons were performed with either a one-way or two-way analysis of variance (ANOVA). Tumor progression data is expressed as Kaplan-Meier survival curves.
For the compound statistical model (Figure 2A; Table W1), the ratio was estimated using mean (exp group)/mean (control group) for each cancer line/time point combination. The SD was computed according to Cochran's equation 6.4 [21]. Under the null hypothesis that the ratio is 1, the test statistic is a z-statistic, which has the standard normal distribution if the null is true. An asterisk (*) indicates significance using the Bonferroni correction for multiple tests.
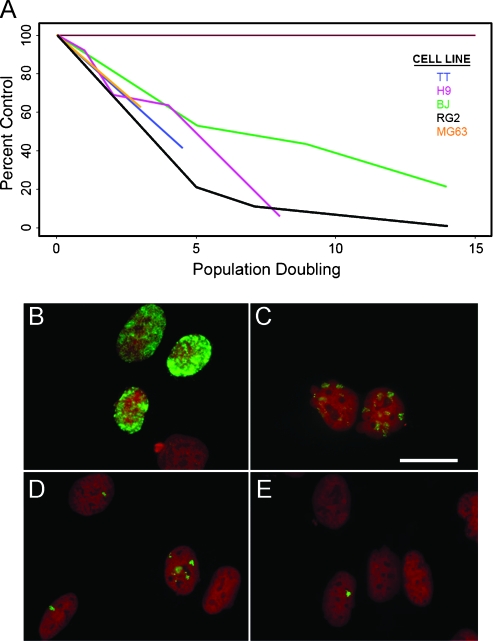
Figure 2.
Proliferation suppression is common among all cancer cells examined and is independent of BrdU retention. (A) Our standard treatment paradigm of 50 µM BrdU for 24 hours causes a reliable suppression of expansion in various cell lines. The top panel shows the graphical representation of expansion by BrdU-treated cells (shown as percent control) over a range of 0 to 14 population doublings after removal of BrdU (see statistical analysis of this model in Table W1). MG63 human osteosarcoma cells were exposed to a single, 18-hour pulse of 50 µM BrdU and assessed over time for BrdU retention. At 24 hours after exposure (B), greater than 95%of all cells show substantial BrdU immunoreactivity (green) within the nucleus. The amount and intensity of BrdU label progressively declines at 6 (C), 11 (D), and 13 (E) days after exposure. Cell nuclei are counterstained with propidium iodide (red). Scale bar in (C), 20 µm and applies to all panels.
Immunolabeling
Cells were processed for BrdU immunolabeling as previously described [22]. Briefly, cells were incubated for 2 hours in a 1:1 ratio of 2x SSC/formamide at 65°C. After a wash in 2x SSC, the cells were then incubated for 30 minutes at 37°C in 2N HCl. Finally, the cells were equilibrated at room temperature for 10 minutes in borate buffer, followed by standard indirect immunofluorescence detection of BrdU with a rat anti-BrdU antibody (#ab6326; Abcam, Cambridge, MA).
For cleaved caspase-3 and γH2A.X immunolabeling, cells were grown to ∼75% confluence on polyornithine-coated glass coverslips. The medium was removed, and the cells were fixed by incubating in 4% paraformaldehyde in PBS at room temperature for 15 minutes then washed with PBS for 5 minutes. Cells were prepared for immuno-cytochemistry by first blocking at room temperature for 1 hour in PBS plus 0.01% Triton X-100 (PBSt) containing 10% fetal bovine serum (FBS). Primary antibodies were then applied to the cells for 1 hour in PBSt plus 10% FBS with moderate agitation at 37°C. The antibodies used were either cleaved caspase-3 (at 1:400, #9661S; Cell Signaling Technology, Danvers, MA) or γH2A.X phospho-S139 (at 1:200, #ab2893; Abcam). Residual primary antibody was removed by three 5-minute washes with PBS, and secondary antibodies were applied at room temperature for 1 hour in PBSt plus 10% FBS. Residual secondary antibodies were removed by three 5-minute washes in PBS. The coverslips were placed onto glass slides and Vectashield mounting medium plus DAPI (H-1200; Vector Laboratories, Burlingame, CA) was applied immediately before coverslipping.
Flow Cytometry
Cell cycle analysis (propidium iodide). Cells were fixed overnight in 70% ethanol and then incubated for 1 hour at 4°C in PBS containing 50 µg/ml of propidium iodide (#P-4170; Sigma-Aldrich) and RNase A (#R6513; Sigma-Aldrich). Samples were processed with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed with FlowJo Flow Cytometry Analysis Software (Tree Star, Inc., Ashland, OR).
Annexin V. Cells were harvested at various time points after BrdU administration (50 µM) to assess Annexin V staining using the Vybrant Apoptosis Assay Kit #9 (V35113; Molecular Probes, Carlsbad, CA). Briefly, cell pellets were obtained by centrifugation and resuspended at 1 x 106 cells/ml in 1x annexin binding buffer (ABB). Annexin V (APC) and SYTOX green stain were added to the cell suspension and incubated with 5% CO2 for 15 minutes at 37°C. The cell suspension was diluted with 1x ABB, gentlymixed, and analyzed by flow cytometry (530/660 nm). Populations were separated based on high and low levels of red and green fluorescence.
JC-1. We performed the JC-1 assay to determine whether BrdU exposure causes any changes in mitochondrial membrane potential. Control and BrdU-treated cells were suspended in warm medium at 1 x 106 cells/ml. The positive control sample was treated with CCCP and incubated at 37°C for 5 minutes. All groups received 2 µM JC-1 and were incubated at 37°C, 5% CO2 for 30 minutes. Cells were washed once and resuspended in 500 µl of PBS. Samples were then analyzed on a flow cytometer with 488-nm excitation using the appropriate emission filter for Alexa Fluor 488 dye and R-phycoerythrin. Cells were gated to exclude debris, and standard compensation was performed using the CCCP-treated sample.
Western Blot Analysis and Densitometry
Proteins were isolated from adherent cell lines (RG2 and BJ) by incubating with RIPA extraction buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 1% sodium dodecyl sulfate) containing protease inhibitor cocktail (Calbiochem, San Diego, CA) on flasks over an ice bath for 5 minutes followed by cell scraping. Extracted protein was quantified against a bovine serum albumin standard using a DC protein assay reagent kit (Bio-Rad, Hercules, CA). Equal protein was loaded onto Bis-Tris 4% to 12% density gradient gels, and electrophoresis was performed using NuPAGE MES [12(N-morpholino) ethane sulfonic acid] reducing buffer system (Invitrogen, Carlsbad, CA) for 50 minutes at 200 V. Proteins were transferred onto nitrocellulose membranes for 1 to 1.5 hours at 30 V. Nonspecific binding was blocked with 5% bovine milk in Tris-buffered saline plus 0.05% Tween 20. Membranes were incubated in a blocking buffer at room temperature for 1 hour before being probed with the following primary antibodies: phosphospecific (Ser249,Thr252) antiretinoblastoma (at 1:500, PC640; Oncogene Research Products, San Diego, CA), p21 (at 1:500, #ab7960; Abcam), antihuman retinoblastoma protein (1:200, #554136; BD Pharmingen, San Jose, CA), and antiactin (at 1:2000, A-4700; Sigma). Membranes were incubated in primary antibody at 4°C overnight and then probed with an appropriate secondary antibody for 1 hour at room temperature. Finally, immunopositive proteins were detected by autoradiography using ECL reagents (GE Healthcare Life Sciences, UK), and densitometry was quantified with Image J software.
Subcutaneous Tumors and In Vivo BrdU Administration
A bolus of either untreated or pretreated RG2 glioma cells (1 x 106 cells in 250 µl of PBS) was injected subcutaneously between the scapulae of anesthetized adult male Fisher 344 rats as previously described [23]. Pretreated RG2 cells were treated with 50 µM BrdU for 24 hours before implantation. Tumors were measured every other day in two dimensions with digital calipers, and tumor volume was calculated [(π/6) x W2 x L (W = shortest dimension and L = longest dimension)]. The experimental end point was defined as a tumor volume ≥3000 mm3. At end point, euthanasia was performed by transcardial perfusion with 200 ml of 4% paraformaldehyde in PBS under deep sodium pentobarbital anesthesia (150 mg/kg, i.p.).
BrdU administration, i.p. Untreated RG2 cells were implanted into 10 animals as described previously. The BrdU regimen was initiated when palpable tumors had reached a volume of 200 mm3. Half of the animals received three i.p. injections of BrdU (300 mg/kg) per day for 2 days, whereas the other half served as controls and received an equal number and volume of sterile saline injections.
BrdU administration, oral. Again, untreated RG2 cells were implanted subcutaneously into 20 animals as described. Immediately after implantation, half of the animals were provided with drinking water containing BrdU (0.8 mg/ml), and half received normal drinking water. All animals were provided with freshly prepared water (either with or without BrdU) each day for 7 days, ad libitum. On the eighth day after implantation, all animals were placed on normal drinking water for the duration of the experiment.
A dose of 300 mg/kg corresponds to a clinical dose of 1800 mg/m2. The rats received three of these doses per day for 2 days, thus receiving a total of 10,800 mg/m2. The drinking water dose (based on the standard 20-ml/day consumption by adult rats) is 640 mg/m2 per day for 7 days, or a total of 4480 mg/m2. By way of comparison, previous clinical trials (e.g., Kinsella et al. [14]) included BrdU as a radiosensitizer as part of a multimodal therapy-treated patients with 350 mg/m2 for continuous 12-hour infusions every day for 14 days or 4900 mg/m2 total. Thus, the treatment range in our study is generally in accord with previous human clinical applications, because, although our injected BrdU was theoretically approximately twice what humans received, it is known that BrdU is active in plasma only for approximately 2 hours. Therefore, the continuous infusion used in the human trials likely resulted in more widespread BrdU incorporation than our injection paradigm.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling
Apoptotic cells from control and BrdU-treated cultures were visualized using a fluorimetric terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (DeadEnd Fluoremetric TUNEL System G3250; Promega, Madison, WI) according to the manufacturer's recommendations. This assay measures the fragmented DNA of apoptotic cells by incorporating fluorescein-labeled dUTP at the 3′ ends of DNA strands. As a positive control, some cells were processed for TUNEL after 30 minutes of incubation with DNase. Cells were counterstained with Vectashield + DAPI (H-1200; Vector Laboratories), and cell death was quantified by counting the number of TUNEL+ nuclei on each of three coverslips at five predetermined stage coordinates. The criterion for apoptotic cells was intentionally liberal to avoid undercounting (i.e., nuclei with even the faintest evidence of fluorescein label were considered positive).
Telomere Length
To determine whether the effects of BrdU are related to changes in telomere length, we performed a TeloTAGGG assay (catalog no. 2209136; Roche, Indianapolis, IN). Briefly, genomic DNA was isolated (#11814770001; Roche) and digested with Hinf1 and Rsa1 enzymes. After digestion, the DNA fragments were separated by gel electrophoresis and transferred to a nylon membrane for Southern blot analysis. The blotted DNA fragments were hybridized to a digoxigenin (DIG)-labeled probe specific for telomeric repeats and incubated with a DIG-specific antibody covalently coupled to alkaline phosphatase. Finally, the immobilized telomere probe was visualized by virtue of alkaline phosphatase-metabolizing CDP-Star, a highly sensitive chemiluminescence substrate.
Telomerase Activity
We used the TRAPeze ELISA kit (#S7750;Chemicon, Danvers,MA) assay to determine levels of telomerase activity in our control and BrdU-treated cells. Briefly, the sample cells' telomerase adds a number of telomeric repeats (GGTTAG) onto the 3′ end of the biotinylated telomerase substrate oligonucleotide (b-TS), and the extended products are then amplified by polymerase chain reaction. The extension/amplification was performed with biotinylated primer andDNP-labeled dCTP. Thus, the telomeric repeat amplification protocol (TRAP) products are tagged with biotin and DNP residues, and the labeled products can be immobilized onto streptavidin-coated microtiter plates through biotin-streptavidin interaction, and then detected by anti-DNP antibody conjugated to horseradish peroxidase (HRP). The amount of TRAP products was determined by means of the HRP activity using substrate TMB and subsequent color development.
Results
BrdU Administration Reduces Cancer Cell Population Expansion Over Time
RG2 rat glioma cells were treated once with 0, 1, 10, or 50 µM BrdU for 24 hours, and cumulative growth curves were obtained over 18 days (Figure 1A). Control and treated cells were quantified and replated at equal densities on days 5, 12, and 18 after treatment. At all time points, the BrdU-treated cells demonstrate a statistically significant dose-responsive decrease in cell numbers, which becomes more pronounced with increasing rounds of replication (data are expressed as population doublings over time, and statistical analysis was performed on total cell counts). To more finely analyze the temporal effect of BrdU on cell number, we repeated this experiment with nonadherent H9 human lymphoma cells, quantifying at 1, 2, or 4 days after exposure (Figure 1B). These results show that there is a delay of approximately 48 hours before statistically significant differences in cell number are detected. Most cell lines examined fail to display any sign of recovery. However, when we followed H9 cells that were treated for 24 hours with 50 µM BrdU and quantified periodically over 2 months, we found the initial dramatic reduction in the rate of proliferation was followed by a gradual recovery to near normal levels over the course of 61 population doublings (data not shown).
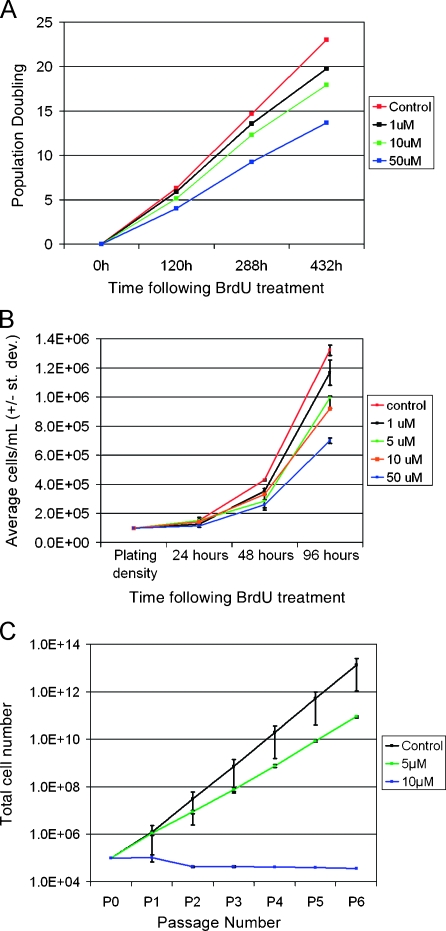
Figure 1.
BrdU induces a progressive, dose-responsive suppression of cancer cell line and cancer stem cell population expansion. (A) RG2 rat glioma cells treated for 24 hours with 1, 10, or 50 µM BrdU show a dose-responsive reduction in the rate of population doubling over 18 days after removal of BrdU. Cells from all groups were replated at equal density at 120 and 288 hours to prevent overgrowth of the culture vessels. At all time points, BrdU-treated cells lag significantly behind controls, regardless of dose (one-way ANOVA, Tukey-Kramer post hoc test of significance was performed on the basis of total cell counts at each time point; P < .001, n = 3 for all groups). (B) Finer temporal analysis reveals that significantly slowed expansion is apparent as early as 48 hours after BrdU. H9 human lymphoma cells treated for 24 hours with 1, 5, 10, or 50 µM BrdU are not significantly different from control at 24 hours after BrdU exposure, yet by 48 hours, all treated groups lag significantly behind control, and the degree of lag is dose-responsive (one-way ANOVA, Bonferroni post hoc test of significance; P <. 001, n = 3 for all groups). (C) Tumor-initiating cancer stem cells isolated from a primary human glioma were treated with a single pulse of 5 or 10 µM BrdU and grown in NS cultures. Neurospheres were passaged, quantified, and replated every 4 to 7 days. Both doses severely suppress cancer stem cell population expansion. Data are represented as mean ± SD.
We next examined the effect of BrdU administration on primary cancer cells with stemlike properties using the neurosphere (NS) assay [24,25]. Neurospheres are clonal structures consisting of progeny derived from a single founder cell capable of long-term self-renewal and multilineage differentiation [26]. Neural stem-like cells capable of forming NS are present in primary gliomas [27] and represent tumor-initiating cells in serial transplantation paradigms [20,28]. Neurospheres derived from primary human gliomas and treated with a single pulse of either 5 or 10 µM BrdU show a dose-dependent reduction in the rate of population doubling compared to untreated control cells (Figure 1C).
The inhibitory effect of BrdU on proliferation is common to all mammalian cells that we have tested. Figure 2A is a graphical representation of BrdU-induced reduction in expansion rate for a number of cell lines (Table W1 for statistical results). Expansion is expressed as percent of control, and all treated cells show a dramatic, sustained, and statistically significant reduction in the rate of expansion compared to matched, untreated controls. Because our paradigm includes only a single pulse of BrdU, and because treated cells are affected for numerous population doublings, we reasoned that impaired proliferation, while requiring initial BrdU incorporation into cellular DNA, is maintained even as the amount of retained BrdU decreases due to dilution with each round of cell division. We exposed MG63 human osteosarcoma cells to 50 µM BrdU for 18 hours and assessed BrdU immunolabeling over 2 weeks. At 24 hours after treatment, greater than 95% of cells are BrdU+, and the labeling is characteristically spread over the entire nucleus (Figure 2B). By day 6 (Figure 2C), most cells are still decorated, but the pattern is patchy and less intense. On day 11 (Figure 2D), only approximately half of all cells still express immunodetectable amounts of BrdU, and the patchy pattern is more pronounced. Finally, by day 13 (Figure 2E), only occasional cells are labeled, and these typically show only a single focal point within the nucleus. At later times, nuclear BrdU is not detected (data not shown). These data demonstrate that proliferation suppression does not depend on the continued presence of BrdU within the DNA.
Single-Pulse BrdU Does Not Result in Increased DNA Damage or Apoptosis
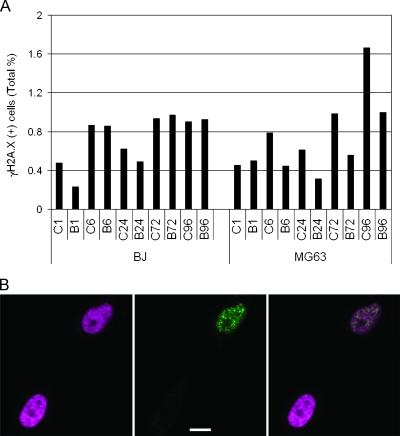
Reports associating BrdU with DNA damage generally involve the use of a secondary stressor such as irradiation, rendering it difficult to discern whether BrdU alone has an effect. It is plausible that BrdU exposure induces DNA damage that can cause stress in other cellular components, leading to amplification of apoptosis [29]. We labeled control and BrdU-treated cells with γH2A.X at various time points after BrdU administration to determine whether DNA damage, specifically double-strand breaks, is induced. We found with BJ and MG63 cells that there are no consistent differences in the number of γH2A.X-positive cells between treated and control groups during the first 4 days after exposure to BrdU. At no time did the percentage of positive cells in either control or treated groups exceed 2% of the total population, and in some instances, the percentage was higher in the controls (Figure 3). Next, we examined increased BrdU-mediated sensitivity to photolysis, as described by Michishita et al. [30]. Treated cells that were protected from ambient light for 5 days still show profound expansion suppression, demonstrating that DNA damage due to irradiation does not account for the observed effect (Figure W1).
Figure 3.
BrdU does not lead to increased γH2A.X immunoreactivity. (A) BrdU-treated (B) and matched control (C) BJ or MG63 cells immunostained for γH2A.X at 1, 6, 24, 72, and 96 hours after a single 24-hour pulse of BrdU, and positive cells are expressed as the percentage of the total cell population. Although there is a trend toward greater γH2A.X expression in both control and treated cells with increasing time in culture, there are no consistent differences between matched control and treated samples in either cell type. (B) Representative photomicrographs showing examples of γH2A.X(+) and γH2A.X(-) MG63 cells 96 hours after a single 24-hour pulse of 50 µM BrdU. The panel on the left shows DAPI staining of two nuclei (pseudocolored magenta). The middle panel shows γH2AX immunolabeling (green) of the same field of view. The right-hand panel is an overlay of two panels. Characteristic γH2AX(+) foci are present in the upper nucleus, indicating double-strand DNA breaks. Scale bar, 10 µm.
To assess BrdU-mediated apoptosis, we examined cells with the TUNEL assay that detects DNA fragmentation characteristic of apoptotic cells. As with the γH2A.X labeling, we found only negligible increases in TUNEL+-treated cells (Figure W2A) that cannot account for the profound reduction in expansion rate, because TUNEL+ cells never accounted for more than 0.5% of the total population. Because the TUNEL assay predominately detects late-stage apoptosis, we also assessed cells for mitochondrial membrane potential, cleaved caspase-3, and Annexin-V staining, which all detect early apoptotic events.
An early event in apoptosis is the collapse of the electrochemical gradient across the mitochondrial membrane, and the measurement of mitochondrial membrane potential is a method to detect apoptosis. We compared the mitochondrial membrane potential of treated and control MG63 cells at 1 and 7 days after a 24-hour pulse of 50-µM BrdU. These results (Figure W3) fail to reveal differences in mitochondrial membrane potential between control and BrdU-treated cells.
Activation of cleaved caspase-3 is another early event in cellular apoptosis. We examined BJ and MG63 cells for caspase-3 expression over 4 days after a 24-hour pulse of BrdU (Figure W2B). As with the TUNEL results, the percentage of cells expressing caspase in all groups was very low, never exceeding 1% of the total. Additionally, there were no consistent differences between treated cells and matched controls in either group. Thus, three methods for detecting apoptosis indicated that the level of cell death in BrdU-treated cultures is very low and not significantly different from the control level.
Finally, we examined Annexin V binding that reveals the loss of plasma membrane asymmetry that allows phosphatidylserine, normally located in the inner layer, to be exposed on the cell surface. Such loss of asymmetry is thought to be associated with cells that will eventually execute an apoptotic program. H9, Saos-2, and BJ cells were examined after a single 24-hour exposure to BrdU (50 µM). In addition, a dose-response study was carried out with MG63 cells. The results with Annexin V are highly variable and somewhat confusing (Figure W2, C and D). There is a dose-responsive increase in the percentage of cells labeled with Annexin V, with greater than 60% of all cells either dead or Annexin V(+) after exposure to 50 µM BrdU for 24 hours. The results with the other cell lines are highly variable. Treated H9 cells seem to show a slight increase in the Annexin V(+) population, whereas there is no increase in treated Saos-2 cells. BJ cells also fail to reveal differences in Annexin V levels between treated and control groups, but the base level of expression is approximately 50% even in the untreated controls. Because the Annexin V results are so dramatically different from the results obtained with other markers of apoptosis, and because there is large intra-cell line variability in both baseline Annexin V expression and degree of change after BrdU exposure, we believe that these results do not accurately reflect cell death in our culture paradigm. This is supported by reports in the literature demonstrating that Annexin V labeling can sometimes be reversible [31] and that Annexin V labeling can increase even without eventual cell death [32].
Antiproliferation Follows Even Low-Dose BrdU Administration
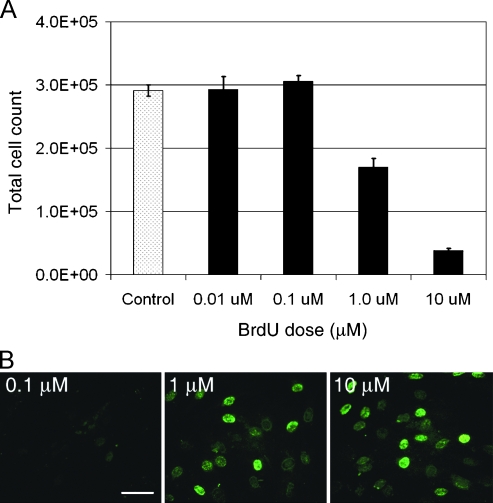
To determine the lowest effective dose of BrdU for slowing expansion, we treated RG2 cells with 0.01, 0.1, 1.0, or 10 µM BrdU for 18 hours and quantified after 8 days. A dose-response is again apparent, with 10 µM eliciting a stronger effect than 1.0 µM. However, doses lower than 1.0 µM fail to alter expansion (Figure 4A). Identical results were also obtained with BJ cells (data not shown). Anti-BrdU labeling reveals that only doses of 1 µM or higher result in immuno-detectable levels of BrdU within the cell nuclei. At 24 hours after treatment, BrdU is seen in more than 95% of BJ cells treated with 1.0 or 10 µM BrdU, whereas cells treated with 0.1 µM BrdU or lower fail to demonstrate any immunolabeling (Figure 4B). This finding demonstrates that BrdU must incorporate into cellular DNA at immunodetectable levels to elicit the antiproliferation effect.
Figure 4.
Transient, low-dose BrdU suppresses expansion rate. (A) RG2 cells were treated with a single 18-hour pulse of 0.01, 0.1, 1.0, or 10 µM BrdU and were quantified 8 days later. Cells receiving a 0.01 or 0.1 µM dose were not significantly different from control at 8 days, whereas expansion of both the 1.0 and 10 µM groups was significantly suppressed (one-way ANOVA with a Tukey-Kramer post hoc test of significance; P < .001. n = 3 for each group.). (B) BrdU doses that fail to suppress expansion also fail to immunolabel treated cells. Both the 1 and 10 µM groups demonstrate BrdU immunolabeling (green) of nearly all cells 24 hours after BrdU exposure, whereas the 0.1 µM group does not contain immunodetectable BrdU. Scale bar, 20 µm. Data are represented as mean ± SD.
BrdU Alters the Cell Cycle Profile
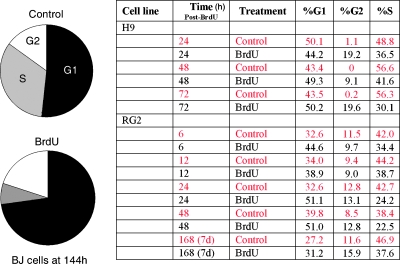
Because BrdU leads to a reduced cellular expansion over time that is not the result of increased cell death, we hypothesize that there must be an alteration in the cell cycle profile of treated cells. We treated asynchronous BJ fibroblasts with 50 µM BrdU for 24 hours and compared their cell cycle profile to control cells after 1 week. As expected, there is a statistically significant reduction in the proportion of BrdU treated cells in S-phase that is offset by an increase in the fraction of treated cells in G0/G1 (Figure 5). Finer analysis with similarly treated RG2 cells reveals that, as early as 6 hours after BrdU administration, there is a statistically significant reduction in the proportion of cells in S-phase in treated groups. The ratio of treated to control cells in S-phase varies, but at all times over 1 week after exposure, there are fewer BrdU-treated cells in S-phase. H9 cells treated for 24 hours with 50 µM BrdU also show a reduction in the proportion of cells in S-phase by 24 hours after BrdU exposure, and this reduction remains relatively stable over the next 2 days (data not shown). These findings demonstrate that BrdU exposure leads to a rapid alteration in cell cycle distribution that precedes a detectable delay in expansion rate as measured by total cell quantification.
Figure 5.
BrdU alters the cell cycle profile. Cell cycle kinetics of asynchronous control and treated cells were assessed using flow cytometry (propidium iodide staining) after a 24-hour pulse of 50 µM BrdU. At 144 hours after exposure, treated BJ cells (pie charts) show a substantial increase in the percentage of cells in G1/G2 with a corresponding reduction in S-phase. The table on the right shows the results of finer temporal analysis with H9 and RG2 cells. Even as early as 6 hours after BrdU exposure, there is a reduction in the percentage of cells in S-phase that persists over time.
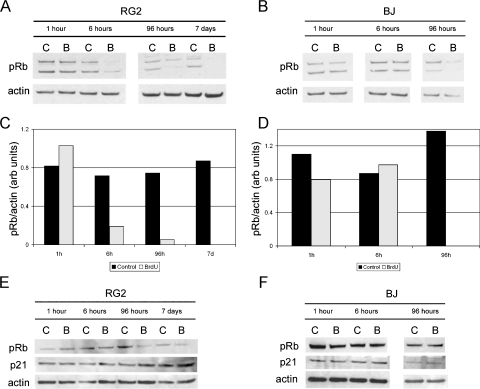
The increase in the population of BrdU-treated cells in G1 suggests the possibility that these cells are either exiting the cell cycle or are unable to traverse the restriction point. The control of cellular proliferation is tightly regulated by various intrinsic and extrinsic factors. However, the retinoblastoma protein (pRb) is recognized as a guardian of the restriction point and cell cycle progress [33]. To test the effect of BrdU on pRb phosphorylation, RG2 and BJ cells were administered BrdU (50 µM) at 0 hours, and cell lysates were collected at various postadministration time points (1, 6, and 96 hours and 7 days) for Western blot analysis. At 24 hours, the BrdU containing medium in the 96-hour and 7-day culture flasks was replaced with BrdU-free medium. The level of phosphorylated pRb in BrdU-treated RG2 cells is negligible at 6 hours after administration, and there is no detectable expression at 96 hours or at 7 days (Figure 6, A and C). Additionally, phosphorylated pRb expression is no longer detectable in BrdU-treated BJ cells by 96 hours after administration (Figure 6, B and D). The time at which the level of phosphorylated pRb declines corresponds with the G1 accumulation (Figure 5). The decrease in expression level seems to be phosphorylation-specific because the levels of total pRb are comparable between control and BrdU-treated cells, particularly in the BJ cells, at these time points (Figure 6, E and F).
Figure 6.
Phosphorylation of pRb is reduced in some cell types after BrdU exposure, whereas total pRb and p21 remain unchanged. (A and B) RG2 and BJ cells were exposed to a single 24-hour pulse of 50 µM BrdU and analyzed for phosphorylated pRb (Ser249, Thr252) by Western blot analysis at 1, 6, and 96 hours and 7 days after administration. (C and D) Densitometric analysis was performed to quantitate the changes in phosphorylated pRb protein levels in RG2 and BJ samples normalized to actin. There is a dramatic reduction in phospho-Rb beginning at 6 hours in the RG2 cells, and at 96 hours in the BJ cells. (E and F) Similarly treated RG2 and BJ cells were analyzed for total Rb, and p21 protein expression by Western blot analysis. Total Rb decreases slightly in RG2 cells at 1 week after administration but is not altered in BJ cells. In neither cell type is p21 expression altered because of BrdU exposure. (Amount of protein loaded/well: (A and B) RG2 1 hour, 6 hours: 17 µg; RG2 96 hours, 7 days: 20 µg; BJ 1 hour, 6 hours: 15 µg; BJ 96 hours: 17 µg; (C and D) RG2: 15 µg; BJ: 17 µg.)
Most of the cell lines we use have abnormal expression patterns for many of the primary markers related to G1 arrest (Table W2), making it difficult to assign the effect of BrdU to any of the prominent cell cycle and/or senescence pathways. However, the expression profile of a key cell cycle protein, p21, has been reported as normal in both the RG2 and BJ cell lines. Interestingly, the level of p21 does not change after BrdU treatment (Figure 6, E and F). This result is not necessarily surprising when one considers the fact that both H9 and Saos-2 cells do not express p21 yet are still sensitive to BrdU treatment.
The slowed expansion and altered cell cycle profile of BrdU-treated cells resembles a senescent-like phenotype, and there is evidence that halogenated pyrimidines can induce senescence in a variety of cell types [9,10,29,34]. However, the expression levels of known senescence-associated proteins are not consistently altered by BrdU exposure. For instance, senescence-associated β-galactosidase (SAβ-gal) activity [35] is up-regulated in RG2 cells 24 hours after exposure to 10 µM BrdU (Figure W4). In contrast, there is no detectable SAβ-gal activity in severely suppressed MG63 cells (data not shown). Similar ambiguous results were obtained in relation to telomerase activity and telomere maintenance. Telomere erosion during cellular replication has been shown to activate DNA damage signaling pathways that can inhibit subsequent cell cycle progression and induce senescence [36]. To examine BrdU-mediated perturbation of telomerase activity as a mechanism of slowed cell cycle progression, we performed TRAP analysis on control and BrdU-treated RG2 cells. Telomeric repeat amplification protocol analysis performed 24 and 48 hours after treatment reveals strongly reduced telomerase activity (Figure W5A). Again, however, this reduction is variable and cell line-specific because MG63 cells fail to demonstrate a reduction in telomerase activity, even 3 weeks after exposure (data not shown). Furthermore, the telomerasenegative BJ and Saos-2 cell lines also demonstrate BrdU-mediated proliferation suppression (Figure 2), suggesting that telomerase activity is not directly involved in BrdU-mediated antiproliferation but may itself be reduced in telomerase-positive cells subsequent to cell cycle alterations.
Under some circumstances, telomeres can be maintained by a telomerase-independent mechanism referred to as alternative lengthening of telomeres [37]. Because telomere length can be maintained in the absence of telomerase activity, and because BrdU induces slowed proliferation in cells regardless of telomerase activity, we looked for evidence of telomere shortening through terminal restriction fragment analysis in H9 cells but failed to detect differences in average telomere length between treated and control cells (Figure W5B).
The relationship between cellular metabolism and cell cycle control is not well understood. However, it has been reported that energy deprivation can prevent passage through the G1-S cell cycle checkpoint [38]. Whereas BrdU can incorporate into mitochondrial DNA [39], little is known about how it affects mitochondrial health and/or function. Mitochondrial membrane potential is a key indicator of cellular viability and is critical for ATP production. As we have already shown (Figure W2), BrdU does not lead to perturbed mitochondrial membrane potential either 1 day or 1 week after treatment. Identical results were obtained with both H9 and BJ cells (data not shown).
BrdU-Mediated Antiproliferation Is Not Blocked by the Addition of Cytosine or Thymidine
While BrdU normally pairs with adenosine during DNA replication, it is also known to frequently mispair with guanine [40–45]. Bromodeoxyuridine triphosphate (BrdUTP) is an inhibitor of ribonucleoside diphosphate reductase, which ultimately leads to a deficiency in the conversion of cytidine diphosphate to deoxycytidine diphosphate [40,42]. High BrdUTP concentrations, therefore, may prevent the formation of dCTP substrate for DNA synthesis. With a decrease in dCTP pools, BrdUTP becomes increasingly competitive for sites opposite template guanines, an effect that can be mitigated by the addition of excess deoxycytidine [45].
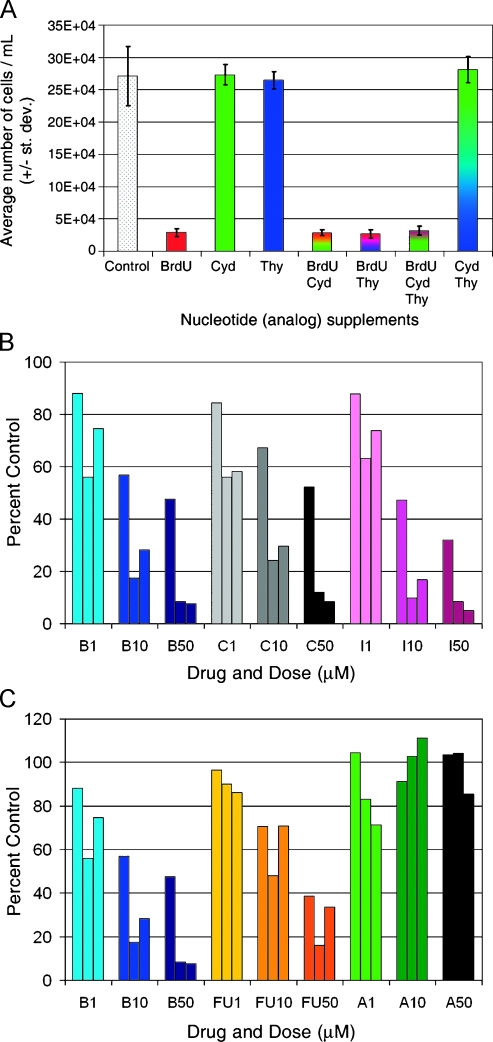
We followed the expansion rates of H9 cells treated with equimolar BrdU, thymidine, or cytidine, both alone and in combination (Figure 7A). The reduced cellular expansion produced by 50 µM BrdU is abrogated neither by the coadministration of equimolar thymidine or cytidine nor by a combination of thymidine and cytidine. Furthermore, neither thymidine nor cytidine, alone or in combination, significantly reduces proliferation rate compared to untreated controls cells. Finally, the antiproliferative effect of 50 µM BrdU is not diminished even when coadministered with up to 250 µM cytidine (data not shown). These results suggest that antiproliferation arises neither from BrdU outcompeting cytidine during DNA synthesis nor by simply altering the intracellular milieu by the addition of excess nucleotides.
Figure 7.
BrdU-mediated proliferation suppression is not antagonized by excess cytidine, is matched by similar halogenated pyrimidines, and surpasses current anticancer nucleoside analogs. (A) 50 µM BrdU, deoxycytidine (dCyd), and deoxythymidine (dThy) were applied to H9 human lymphoma cells in factorial combinations for 24 hours, and cellular expansion was assessed 1 week later. All of the groups receiving BrdU showed statistically indistinguishable reductions in expansion compared to control (P < .001). In contrast, groups receiving dCyd, dThy, or dCyd+dThy demonstrated expansion equivalent to control levels. One-way ANOVA with a Tukey-Kramer post hoc test of significance. n = 3 for each group. Error bars represent SD. (B) BrdU (B), CldU (C), and IdU (I) were compared for the ability to suppress expansion of H9 human lymphoma cells. Each analog was administered for 18 hours at 1, 10, or 50 µM and cells were quantified weekly for 3 weeks (weekly counts correspond to a left-to-right progression of color-coded triplicates of bars). At all doses and time points, the three halogenated pyrimidines produce remarkably similar, statistically significant reductions in cell number compared to untreated controls. (C) In the same experiment, the therapeutic anticancer nucleoside analogs 5-fluorouracil (FU) and 5-azacytidine (A) were also examined for their ability to suppress expansion of H9 cells in the same paradigm. Approximately 50 µM FU is slightly more effective than 50 µM BrdU at week 1, but by week 2, 50 µM BrdU is substantially better at suppressing expansion. At week 3, 50 µM BrdU suppression has not changed, whereas 50 µM FU suppression has started to recover toward control. At all other doses and time points BrdU is as effective as or more effective than FU at suppressing expansion, and BrdU suppression persists longer. 5-Azacytidine shows variable and transient suppression of expansion to near 80% of control levels. Counts were analyzed with a two-way ANOVA followed by a Tukey-Kramer post hoc test of significance. n = 3 for each group. Data are represented as mean ± SD.
Halogenated Pyrimidines Suppress Proliferation More Robustly Than Current Anticancer Nucleosides
To determine whether halogenated pyrimidines structurally similar to BrdU also perturb proliferation, we analyzed the effect of CldU and IdU on H9 cell expansion weekly for three consecutive weeks after an 18-hour exposure to 1, 10, or 50 µM of each thymidine analog. All three analogs produce a statistically significant dose-responsive reduction in proliferation that is remarkably similar in degree (Figure 7B). Furthermore, this antiproliferation is nonsynergistic, because combinatorial administration does not strengthen the effect (data not shown).
In the same experiment, we compared expansion among cells treated with either BrdU or one of two anticancer nucleosides, 5-FU or AZA. Each analog was administered to H9 cells at 0, 1, 10, or 50 µM for 18 hours, and cells were quantified weekly for three consecutive weeks (Figure 7C). At 1 week, AZA-treated cells are not significantly different from controls, whereas both BrdU- and 5-FU-treated cells are reduced by 50% to 60%. At 2 weeks, AZA-treated cells still match control levels, whereas the BrdU and 5-FU groups have dropped to approximately 10% of control. At week 3, the BrdU-treated cells are maintained near 10% of control level, whereas the 5-FU treated cells recover to approximately 35%. 5-Aza-2′-deoxycytidine showed substantial variability, with some treated cells demonstrating a statistically significant reduction to approximately 80% of control, but this reduction is not maintained over time (data not shown). These results suggest that the suppressive effect of single-pulse BrdU on cancer cell expansion is more effective than AZA and is more persistent than 5-FU.
BrdU Administration Slows Glioma Tumor Progression In Vivo
We chose a syngeneic, invasive, nonimmunogenic rat glioma model [46–48] to test whether the proliferation suppression of BrdU has a meaningful in vivo correlate. RG2 tumors are refractory to therapeutic modalities, rendering them nearly impossible to treat efficiently or cure. Their invasive pattern of growth and uniform lethality after an inoculum of relatively few cells make them a particularly attractive model to test new therapeutic modalities [49].
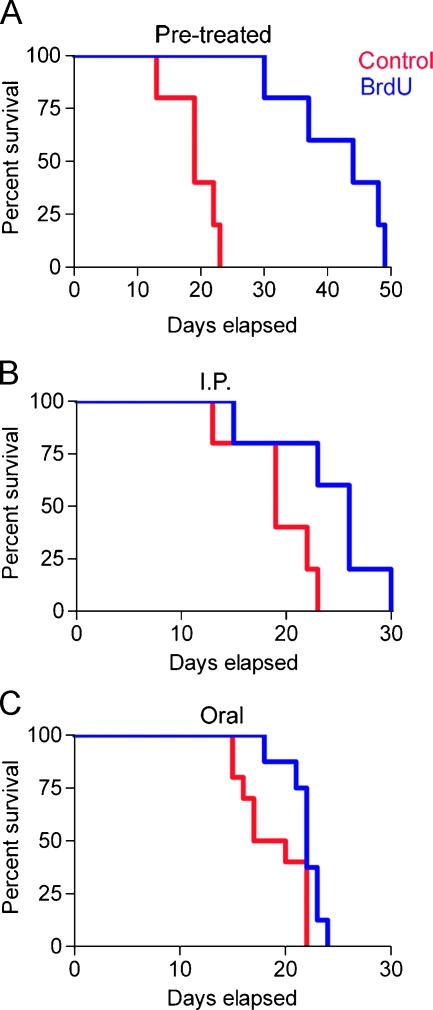
First, we injected a bolus of RG2 cells—either untreated or pretreated in vitro for 24 hours with 50 µM BrdU—subcutaneously into Fisher 344 rats and followed tumor progression. All animals eventually develop tumors, but the survival time (defined as 3000-mm3 tumor volume) is dramatically delayed in animals receiving pretreated cells (Figure 8A). This result clearly demonstrates that proliferation suppression induced by in vitro application of BrdU is maintained in the in vivo environment.
Figure 8.
BrdU administration slows tumor progression in a syngeneic in vivo glioma model. RG2 rat glioma cells, either untreated or pretreated with 50 µM BrdU, were injected subcutaneously in Fisher 344 rats. Tumor progression was monitored by taking measurements every other day and calculating tumor volume. Animals were euthanized when the tumor volume reached the critical end point (3000 mm3). (A) Animals that received the RG2 glioma cells that had been pretreated with 50 µM BrdU for 24 hours show a significant delay in tumor progression when compared to the control animals (P = .002). (B) Animals that received subcutaneous injections of untreated RG2 cells but were administered BrdU by i.p. injections (300 mg/kg x 1.5 days) also show significant delays in tumor progression (P = .02). Additionally, (C) animals that received subcutaneous injections of untreated RG2 cells and were administered BrdU in their drinking water (0.8 mg/ml for 7 days) show a significant delay in tumor progression (P = .04). Kaplan-Meier survival curves (X2 test with associated P value). n = 10 for each group.
In a second set of experiments, we tested whether tumor progression can be slowed by in vivo administration of BrdU. Animals were inoculated with naive RG2 cells and then treated with either i.p. or oral BrdU. After implantation, one group of animals received six i.p. injections of BrdU (300 mg/kg) over 2 days (Figure 8B), while a second group was placed on ad libitum drinking water containing 0.8 mg/ml BrdU for 1 week (Figure 8C). In both cases, there is a statistically significant increase in survival in the animals receiving BrdU, indicating that BrdU is effective at slowing the growth of cancer cells in vivo even when it is administered after tumor initiation. Even a modest increase in survival time after a conservative dosing regimen is highly encouraging when one considers the refractory nature of these tumors.
Discussion
Bromodeoxyuridine has a long history as a potential anticancer drug, and it is known that at high doses and in combination with secondary stressors, such as ionizing radiation, BrdU can have lethal consequences for incorporating cells. The present findings are surprising in that our BrdU regimen is exceedingly mild, even by current experimental pulse-chase birth-dating paradigms in which cells incorporate BrdU and continue to function in an apparently normal manner. Furthermore, the suppressed proliferation we describe occurs in the absence of secondary insults that would stress the cells ability to maintain homeostasis or undergo DNA repair.
A role for BrdU in senescence induction of mammalian cells has recently been described in the gerontology field [33], but this role is not yet widely appreciated by the larger scientific community and not previously been applied as an approach to slow tumor progression. Emerging in vivo studies underscore the importance of cellular senescence in altering the growth properties of tumors in humans and rodents [49,50], and we show here that cancer cells treated with single-pulse BrdU show some signs consistent with senescence. Both the altered cell cycle profile and the up-regulation of SAβ-gal are common manifestations of a senescent phenotype; however, SAβ-gal up-regulation varies widely by cell line, with some showing no enzyme activity even during severe suppression of proliferation rate, and there is evidence suggesting that SAβ-gal is not necessarily a selective marker of senescence [51]. It seems, then, that BrdU exposure does not lead to classically defined “senescence” but to a generalized slowing of proliferation and does not, in our experimental paradigm, lead to crisis or subsequent cell death. Furthermore, the battery of cell lines we have examined all show similar proliferation suppression after BrdU exposure yet differ widely in the status of quintessential senescence markers (Table W2). This variability makes it difficult to define a “universal” mechanism of action and suggests that these molecular players, while possibly involved in a cell type-dependent manner, are neither required nor causative for the suppressive effect of BrdU. Our cell cycle data indicate that BrdU-treated cells accumulate in G1 suggesting that these cells either may exit the cell cycle or are unable to traverse the restriction point. Although the control of cellular proliferation is tightly regulated by various intrinsic and extrinsic factors, pRb is generally regarded as the master regulator of the restriction point and is now implicated in cellular senescence. Retinoblastoma protein is believed to control a senescence-initiating pathway that may synergize with, but is distinct from, telomere loss [33,52]. Our results showing perturbed proliferation that corresponds with hypo-/unphosphorylated pRb yet seems to be independent of telomere maintenance supports this theory. The post-administration times at which the levels of phosphorylated pRb become undetectable in BrdU-treated RG2 and BJ cells almost perfectly correspond to the accumulation of these cells in G1. However, results showing similar proliferation suppression in pRb null Saos-2 human osteosarcoma cells (data not shown) again indicates that pRb is not required for BrdU's effect.
Proliferation was suppressed in all of the cells that we examined, and prior studies have also demonstrated the ubiquitous susceptibility of mammalian cells to BrdU [9]. There is even evidence that BrdU slows replication in thymidine-auxotrophic yeast (unpublished observation; [53]), suggesting that BrdU-mediated proliferation suppression in all eukaryotic cells may be affected through a common yet still undefined mechanism. Cancer stem cells seem particularly susceptible to the antiproliferative effect of BrdU. The NS-forming assay allows us to study the behavior of stem cell-like tumor-initiating cells, and a single pulse of BrdU reliably and dramatically slows the proliferation of clonally expanded progeny over numerous population doublings. This result strongly suggests that BrdU may be a potent therapeutic, targeting cancer stem cells and potentially slowing the regrowth of debulked primary tumors and/or the metastatic spread of secondary tumors. The wide penetrance of the antiproliferative effect, combined with the ability for rapid transport across the blood-brain barrier, makes BrdU an attractive candidate against all types of cancer. However, these same attributes also make it likely that indigenous stem cell pools will be adversely affected. Therefore, potential therapeutic BrdU dosing regimens will need to be carefully tested to avoid a permanent depletion of the stem cells and long-term progenitors needed for maintaining tissue homeostasis.
We consider delayed in vivo tumor progression in the extremely aggressive RG2 model to be the most important aspect of our study. Glioblastoma multiforme is the most common primary (i.e., nonmetastatic) brain tumor of humans. Despite advances in cytoreductive and cytotoxic therapies, the prognosis for this neoplasm remains dismal, with a median survival time of approximately 12 months [54–56]. This has fostered an intense interest in the search for alternative therapeutic modalities that may prove to be more effective or that may augment standard surgical, radiologic, or chemotherapeutic treatments for these neoplasms [57]. The RG2 glioma model has been posited as the equivalent of human glioblastoma multiforme. The fact that brief administration times used in our study result in statistically significant delays in the growth of naive tumor cells raises the possibility that BrdU alone may be capable of producing biologically significant therapeutic gains under optimized dosing schedules. In addition, the dramatically delayed progression of BrdU-pretreated cells (treated before implantation) suggests that BrdU may prove effective against secondary metastatic tumor formation. Future studies will be directed at examining the homing and invasiveness of BrdU incorporating cells and their progeny.
In reassessing BrdU as a potential anticancer drug, it is important to ask why the clinical trials of BrdU as a radiosensitizer were relatively ineffective at extending survival. Although these previous studies were concerned only with the extent to which BrdU augmented the standard chemical and radiation therapies, one would expect that the antiproliferative effect of BrdU should still have been evident. On the basis of our present findings, we can offer reasonable speculation as to why results from these earlier trials were so ambiguous. First, these clinical studies were performed solely based on in vitro evidence of BrdU radiosensitization [3,12,58]; thus, the study designs did not have the benefit of in vivo models that may have revealed dosing schedules and drug interaction effects that either optimize or attenuate BrdU incorporation. Second, in all of the clinical trials, BrdU was added to an existing, multimodal therapy that involved both chemotherapeutics and fractionated irradiation. Under these circumstances, it is not clear to what extent BrdU was systemically available during stages of active tumor cell division. Related to this is the question of dose and treatment length optimization; without the benefit of animal models, it simply was not possible to determine sufficient dosing schedules to maximize therapeutic outcome. Even our present results with in vivo BrdU administration do not necessarily represent an optimized treatment paradigm, as we were intentionally conservative in our approach and were able to elicit a therapeutic effect while staying well below side effect limitations. It remains for future studies to extend these results to define the most effective treatment regimen from a cost/benefit perspective.
Supplementary Material
Acknowledgments
The authors thank Barry E. Flanary for advice on telomeres and telomerase; Eleni Papadopulos-Eleopulos for insightful discussions of thymidine analogs; Rachael Watson and Padraic Levings for help with osteosarcoma cell culture; Linda J. Young for statistical advice; and Erin M. Dunbar for critical reading of the manuscript.
Footnotes
This work was supported by a National Institutes of Health/National Institute of Neurological Disorders and Stroke grant NS056019 (E.D.L.), by University of Florida Department of Anatomy & Cell Biology/University of Florida Shands Cancer Center startup funds (E.D.L.), and by the James S. McDonnell Foundation (W.J.S.). E.D.L. owns stock in RegenMed, Inc., which may or may not receive royalties from the University of Florida because of the work reported herein.
This article refers to supplementary materials, which are designated by Tables W1 and W2 and Figures W1–W5 and are available online at www.neoplasia.com.
References
- 1.Hakala MT. Mode of action of 5-bromodeoxyuridine on mammalian cells in culture. J Biol Chem. 1959;234:3072–3076. [PubMed] [Google Scholar]
- 2.Hakala MT. Effect of 5-bromodeoxyuridine incorporation on survival of cultured mammalian cells. Biochim Biophys Acta. 1962;61:815–823. doi: 10.1016/0926-6550(62)90064-6. [DOI] [PubMed] [Google Scholar]
- 3.Djordjevic B, Szybalski W. Genetics of human cell lines: III. Incorporation of 5-bromo- and 5-iododeoxyuridine into the deoxyribonucleic acid of human cells and its effect on radiation sensitivity. J Exp Med. 1960;112:509–531. doi: 10.1084/jem.112.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Littlefield JW, Gould EA. The toxic effect of 5-bromodeoxyuridine on cultured epithelial cells. J Biol Chem. 1959;235:1129–1133. [PubMed] [Google Scholar]
- 5.Stellwagen RH, Tomkins GM. Differential effect of 5-bromodeoxyuridine on the concentrations of specific enzymes in hepatoma cells in culture. Proc Natl Acad Sci USA. 1971;68:1147–1150. doi: 10.1073/pnas.68.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn DB, Smith JD. Effects of 5-halogenated uracils on the growth of Eschericia coli and their incorporation into deoxyribonucleic acids. Biochem J. 1957;67:494–506. doi: 10.1042/bj0670494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terzaghi BE, Streisinger G, Stahl FW. The mechanism of 5-bromouracil mutagenesis in the bacteriophage T4. Proc Natl Acad Sci USA. 1962;48:1519–1524. doi: 10.1073/pnas.48.9.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 9.Michishita E, Nakabayashi K, Suzuki T, Kaul SC, Ogino H, Fujii M, Mitsui Y, Ayusawa D. 5-Bromodeoxyuridine induces senescence-like phenomena in mammalian cells regardless of cell type or species. J Biochem. 1999;126:1052–1059. doi: 10.1093/oxfordjournals.jbchem.a022549. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Minigawa S, Michishita E, Ogino H, Fujii M, Mitsui Y, Ayusawa D. Induction of senescence-associated genes by 5-bromodeoxyuridine in HeLa cells. Exp Gerontol. 2001;36:465–474. doi: 10.1016/s0531-5565(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 11.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2006;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Hsu TC, Somers CE. Effect of 5-bromodeoxyuridine on mammalian chromosomes. Proc Natl Acad Sci USA. 1961;47:396–403. doi: 10.1073/pnas.47.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson RL, Szybalski W. Molecular radiobiology of human cell lines: V. Comparative radiosensitizing properties of 5-halodeoxycytidines and 5-halode-oxypyrimidines. Radiat Res. 1963;20:252–262. [PubMed] [Google Scholar]
- 14.Kinsella TJ, Russo A, Mitchell JB, Rowland J, Jenkins J, Schwade J, Myers CE, Collins JM, Speyer J, Kornblith P, et al. A phase I study of intermittent intravenous bromodeoxyuridine (BUdR) with conventional fractionated irradiation. Int J Radiat Oncol Biol Phys. 1984;10:69–76. doi: 10.1016/0360-3016(84)90414-0. [DOI] [PubMed] [Google Scholar]
- 15.Phillips TL, Levin WA, Ahn DK, Gutin PH, Wilson CB, Prados MD, Wara WM, Flam MS. Evaluation of bromodeoxyuridine in glioblastoma multiforme: a Northern California cancer center phase II study. Int J Radiat Oncol Biol Phys. 1991;21:709–714. doi: 10.1016/0360-3016(91)90690-6. [DOI] [PubMed] [Google Scholar]
- 16.Robertson JM, McGinn CJ, Walker S, Marx MV, Kessler ML, Ensminger WD, Lawrence TS. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Clin Invest. 1997;39:1087–1092. doi: 10.1016/s0360-3016(97)00550-6. [DOI] [PubMed] [Google Scholar]
- 17.Robertson JM, Ensminger WD, Walker S, Lawrence TS. A phase I trial of intravenous bromodeoxyuridine and radiation therapy for pancreatic cancer. Clin Invest. 1997;37:331–335. doi: 10.1016/s0360-3016(96)00527-5. [DOI] [PubMed] [Google Scholar]
- 18.Groves MD, Maor MH, Meyers C, Kyritsis AP, Jaeckle KA, Yung WK, Sawaya RE, Hess K, Bruner JM, Peterson P, et al. A phase II trial of high-dose bromodeoxyuridine with accelerated fractionation radiotherapy followed by procarbazine, lomustine, and vincristine for glioblastoma multiforme. Clin Invest. 1999;45:127–135. doi: 10.1016/s0360-3016(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 19.Prados MD, Seiferheld MS, Sandler HM, Buckner JC, Phillips T, Schultz C, Urtasun R, Davis R, Gutin P, Cascino TL, et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int J Radiat Oncol Biol Phys. 2004;58:1147–1152. doi: 10.1016/j.ijrobp.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 21.Cochran WG. Sampling Techniques. 3rd Ed. New York, NY: Wiley; 1977. [Google Scholar]
- 22.Laywell ED, Kearns SM, Zheng T, Chen KA, Deng J, Chen HX, Roper SN, Steindler DA. Neuron-to-astrocyte transition: phenotypic fluidity and the formation of hybrid asterons in differentiating neurosphere. J Comp Neurol. 2005;493:321–333. doi: 10.1002/cne.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariani CL, Kouri JG, Streit WJ. Rejection of RG-2 gliomas is mediated by microglia and T lymphocytes. J Neurooncol. 2006;79:243–253. doi: 10.1007/s11060-006-9137-x. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds BA, Rietze RL. Neural stem cells and neurospheres—re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 25.Marshall GP, II, Reynolds BA, Laywell ED. Using the neurosphere assay to quantify neural stem cells in vivo. Curr Pharm Biotechnol. 2007;8:141–145. doi: 10.2174/138920107780906559. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 27.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 28.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 29.Lin S-C, Chueh S-C, Hsiao C-J, Li T-K, Chen T-H, Liao C-H, Lyu P-C, Guh J-H. Prazosin displays anticancer activity against human prostate cancers: targeting DNA and cell cycle. Neoplasia. 2007;9:830–839. doi: 10.1593/neo.07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michishita E, Kurahashi T, Suzuki T, Fukuda M, Fujii M, Hirano H, Ayusawa D. Changes in nuclear matrix proteins during the senescence-like phenomenon induced by 5-chlorodeoxyuridine in HeLa cells. Exp Gerontol. 2002;37:885–890. doi: 10.1016/s0531-5565(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 31.Hammill AK, Uhr JW, Scheuermann RH. Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp Cell Res. 1999;251:16–21. doi: 10.1006/excr.1999.4581. [DOI] [PubMed] [Google Scholar]
- 32.Holder MJ, Barnes NM, Gregory CD, Gordon J. Lymphoma cells protected from apoptosis by dysregulated Bcl-2 continue to bind annexin V in response to B-cell receptor engagement: a cautionary tale. Leuk Res. 2006;30:77–80. doi: 10.1016/j.leukres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 34.Minagawa S, Nakabayashi K, Fujii M, Scherer SW, Ayusawa D. Early BrdU-responsive genes constitute a novel class of senescence-associated genes in human cells. Exp Cell Res. 2005;304:552–558. doi: 10.1016/j.yexcr.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lange T. Telomeres. Woodbury, NY: Cold Spring Harbor Press; 2006. Mammalian telomeres; pp. 341–387. [Google Scholar]
- 37.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 38.Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell. 2005;9:843–854. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Davis AF, Clayton DA. In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell Biol. 1996;135:883–893. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashman CR, Davidson RL. Bromodeoxyuridine mutagenesis in mammalian cells is related to deoxyribonucleotide pool imbalance. Mol Cell Biol. 1981;1:254–260. doi: 10.1128/mcb.1.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman ER, Davidson RL. Bromodeoxyuridine mutagenesis in mammalian cells: mutagenesis is independent of the amount of bromouracil in DNA. Proc Natl Acad Sci USA. 1978;75:4982–4986. doi: 10.1073/pnas.75.10.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meuth M, Green H. Induction of a deoxycytidineless state in cultured mammalian cells by bromodeoxyuridine. Cell. 1974;2:109–112. doi: 10.1016/0092-8674(74)90099-3. [DOI] [PubMed] [Google Scholar]
- 43.Moore EC, Hurlbert RB. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966;241:4802–4809. [PubMed] [Google Scholar]
- 44.Hopkins RL, Goodman MF. Deoxyribonucleotide pools, base pairing, and sequence configuration affecting bromodeoxyuridine- and 2-aminopuridine-induced mutagenesis. Proc Natl Acad Sci USA. 1980;77:1801–1805. doi: 10.1073/pnas.77.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson RL, Kaufman ER. Bromodeoxyuridine mutagenesis in mammalian cells is stimulated by thymidine and suppressed by deoxycytidine. Nature. 1978;276:722–733. doi: 10.1038/276722a0. [DOI] [PubMed] [Google Scholar]
- 46.Ko L, Koestner A, Wechsler W. Morphological characterization of nitrosourea-induced glioma cell lines and clones. Acta Neuropathol. 1980;51:23–31. doi: 10.1007/BF00688846. [DOI] [PubMed] [Google Scholar]
- 47.Aas AT, Brun A, Blennow C, Stromblad S, Salford LG. The RG2 rat glioma model. J Neurooncol. 1995;23:175–183. doi: 10.1007/BF01059948. [DOI] [PubMed] [Google Scholar]
- 48.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 49.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Hornsby PJ. Fibroblast stimulation of blood vessel development and cancer cell invasion in a subrenal capsule xenograft model: stress-induced premature senescence does not increase effect. Neoplasia. 2007;9:418–426. doi: 10.1593/neo.07205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Severino J, Allen RG, Balin S, Cristofalo VJ. Is β-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 52.Thomas DM, Yang H-S, Alexander K, Hinds PW. Role of the retinoblastoma protein in differentiation and senescence. Cancer Biol Ther. 2003;2:124–130. [PubMed] [Google Scholar]
- 53.Fujii M, Ito H, Hasegawa T, Suzuki T, Adachi N, Ayusawa D. 5-Bromo-2′-deoxyuridine efficiently suppresses division potential of the yeast Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2002;66:906–909. doi: 10.1271/bbb.66.906. [DOI] [PubMed] [Google Scholar]
- 54.Akman F, Cooper RA, Sen M, Tanriver Y, Kentli S. Validation of the Medical Research Council and a newly developed prognostic index in patients with malignant glioma: how useful are prognostic indices in routine clinical practice? J Neurooncol. 2002;59:39–47. doi: 10.1023/a:1016353614525. [DOI] [PubMed] [Google Scholar]
- 55.Basso U, Ermani M, Vastola F, Brandes AA. Non-cytotoxic therapies for malignant gliomas. J Neurooncol. 2002;58:57–69. doi: 10.1023/a:1015839111005. [DOI] [PubMed] [Google Scholar]
- 56.Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26:397–409. doi: 10.1053/ctrv.2000.0191. [DOI] [PubMed] [Google Scholar]
- 57.Mariani CL, Rajon D, Bova FJ, Streit WJ. Nonspecific immunotherapy with intratumoral lipopolysaccharide and zymosan A but not GM-CSF leads to an effective anti-tumor response in subcutaneous RG-2 gliomas. J Neurooncol. 2007;85:231–240. doi: 10.1007/s11060-007-9415-2. [DOI] [PubMed] [Google Scholar]
- 58.Phillips TL, Bodell WJ, Uhl B, Ross GY, Rasmussen J, Mitchell JB. Correlation of exposure time, concentration, and incorporation of IdURD in V-79 cells with radiation exposure. Int J Radiat Oncol Biol Phys. 1989;16:1251–1255. doi: 10.1016/0360-3016(89)90293-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.