Abstract
BAFF-R-dependent activation of the alternative NF-κB pathway plays an essential role in mature B cell survival. Mutations leading to overexpression of NIK and deletion of the TRAF3 gene are implicated in human multiple myeloma. We show that overexpression of NIK in mouse B lymphocytes amplifies alternative NF-κB activation and peripheral B cell numbers in a BAFF-R-dependent manner, whereas uncoupling NIK from TRAF3-mediated control causes maximal p100 processing and dramatic hyperplasia of BAFF-R-independent B cells. NIK controls alternative NF-κB signaling by increasing the protein levels of its negative regulator TRAF3 in a dose-dependent fashion. This mechanism keeps NIK protein levels below detection even when they cause B cell hyperplasia, so that contributions of NIK to B cell pathologies can easily be overlooked.
Keywords: IκB kinase, NF-κB, Hyperplasia, p100 processing, knockin
B cells are central players in adaptive immune responses and immunity, mainly through their ability to differentiate into antibody-producing plasma cells and long-lived memory B cells upon antigen-encounter. Their survival and function critically depend on extracellular cues, which upon recognition by cell-surface receptors induce defined gene expression programs via activation of specific signal transduction cascades and transcription factors. Deregulation of these signaling pathways can lead to autoimmunity and lymphomagenesis. The NF-κB signal transduction cascade has emerged as a critical player in all of these processes (1–3).
Mammalian cells contain five members of the NF-κB/Rel transcription factor family; namely, NF-κB1/p50, NF-κB2/p52, RelA, c-Rel, and RelB. The p50 and p52 proteins are produced through partial proteolytic degradation from their inactive precursors p105 and p100, respectively. Whereas the bulk of p105 is constitutively turned over to yield p50, p52 is predominantly generated in an inducible fashion. NF-κB factors participate as dimers in the regulation of immune responses and inflammation by controlling the expression of cytokines, chemokines, cell-adhesion molecules, and proteins involved in cellular proliferation and the combat of apoptosis. In most cells NF-κB activity, is kept in check through the presence of inhibitor of κB (IκB) molecules. The IκBs as well as p105 and p100 share ankyrin repeats, enabling them to sequester and inhibit DNA binding of NF-κB dimers. NF-κB proteins can approximately be subdivided into two pools: p50, Rel-A, or c-Rel bind mostly to IκB proteins, whereas RelB preferentially associates with p100 (2).
NF-κB activation can accordingly be subdivided into two main branches, which have been termed canonical and alternative pathway. Stimulation of receptors inducing canonical NF-κB results in rapid activation of the IκB Kinase (IKK) complex, which consists of the IKK1/α and IKK2/β catalytic subunits and the NEMO/IKKγ regulatory protein. Induction of this complex leads to phosphorylation and subsequent proteasomal degradation of IκB proteins, liberating the associated NF-κB dimers to activate transcription of their target genes. The generation of p52 from p100, also known as p100 processing, is the hallmark of the alternative pathway of NF-κB signaling. Its activation leads via NF-κB inducing kinase (NIK) and IKK1 to enhanced p52/RelB-mediated gene expression (2, 4, 5).
In mature resting B cells, both pathways of NF-κB activation are induced and their generation and survival depends on both, demonstrated by gene disruption in the mouse at the level of transcription factors and signaling proteins (6, 7).
It has been shown that the binding of B cell-activating factor of the TNF family (BAFF or BLyS) to BAFF-R (BR3), one of its three receptors, the others being transmembrane activator, calcium modulator, and cyclophilin ligand interactor (TACI), and B cell maturation antigen (BCMA) is required and sufficient to sustain the p100 processing needed for B cell survival (7). The main role of the alternative NF-κB branch in mature B cells appears to be the transmission of a survival signal elicited by BAFF, whose limited availability regulates the size of the peripheral B cell pool. Recent B cell-specific ablation studies of tumor necrosis factor receptor-associated factors (TRAFs) TRAF2 (8) and TRAF3 (9, 10) in mice have revealed striking phenotypic similarities to BAFF-transgenic mice, such as a pronounced B cell hyperplasia due to enhanced cell survival. On a molecular level, lack of TRAF2 or TRAF3 prominently induces robust p100 processing, independent of the presence of BAFF (8–10). The most straightforward explanation for this phenomenon is that the absence of TRAF2 or TRAF3 increases the protein concentration of NIK, as seen in TRAF3-deficient fibroblasts and transformed B cells (11–13), and suggested by the stabilization of NIK achieved through the removal of its TRAF3-binding domain (T3BD) (13). However, elevated amounts of NIK protein could not be detected in primary B cells lacking TRAF2 (8) or TRAF3 (9, 10).
The alternative pathway has been implicated in hematopoietic malignancies through chromosomal abnormalities leading to the production of truncated p100 proteins with diminished NF-κB-inhibitory ability (3, 4). Recently, two independent studies uncovered genetic aberrations affecting components of NF-κB activation, mostly assigned to the alternative branch, in human multiple myeloma cell lines (HMCL) and in 9% (14) or 17% (15) of patient cohorts with multiple myeloma (MM). These aberrations led to the absence of negative regulators of NF-κB, such as TRAF3, TRAF2, cellular inhibitor of apoptosis proteins 1 and 2 (c-IAP1/2), and cylindromatosis protein (CYLD) (14, 15) or to overexpression of NIK (14, 15). Collectively, these studies indicate that deregulation of the TRAF3-NIK axis might play an important role in lymphomagenesis.
We previously showed that selective constitutive activation of canonical NF-κB through expression of a constitutively active IKK2 (IKK2ca) in the B lineage led to hyperplasia of resting B cells and independence of BAFF:BAFF-R-interactions (16). In an effort to investigate the consequences of constitutive alternative NF-κB activity for B cells and to elucidate the importance of TRAF3/NIK interactions in vivo, we generated two conditional NIK knockin transgenes, encoding wild-type NIK or a NIK mutant lacking the T3BD (NIKΔT3) (13). Our results demonstrate that moderate overexpression of wild-type NIK leads to increased B cell numbers but not to BAFF-R independence, and suggest that this is due to negative feedback control of NIK involving NIK-mediated TRAF2/3 up-regulation. Uncoupling of the TRAF3-NIK interactions, however, leads to high levels of NIKΔT3 protein, causing massive B cell hyperplasia and freeing the B cells from their dependence of BAFF-R signals.
Results
Overexpression of Wild-Type NIK Modestly Induces Constitutive p100 Processing and B Cell Hyperplasia, Whereas Loss of TRAF3/NIK Interactions Strongly Induces It.
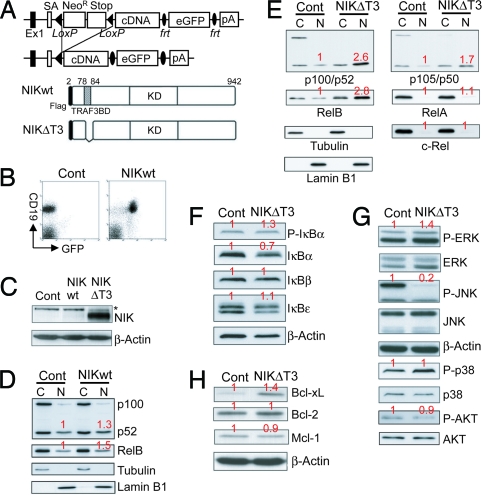
To generate a mouse model allowing the cell type-specific constitutive activation of the alternative branch of NF-κB activation we knocked a cDNA encoding wild-type NIK, preceded by a loxP-flanked neoR-Stop cassette and followed by Frt-flanked IRES-eGFP sequences into the ubiquitously expressed ROSA26 locus (Fig. 1A). Because TRAF3 was reported to induce proteosomal degradation of NIK through binding at a specific N-terminal motif (TRAF3-binding domain = T3BD) (13), we generated a second ROSA26-transgene that encodes a NIK mutant lacking this domain (NIKΔT3; Fig. 1A). B cell-specific expression of both transgenes mediated by CD19-cre was verified through flow cytometry (Fig. 1B). We failed to detect NIK protein by Western blot analysis in control and NIK-transgenic (NIKtg) B cells, but B cells from CD19-cre/R26StopFLNikΔT3 (B cellNIKΔT3) mice contained large amounts of the mutant NIKΔT3 protein (Fig. 1C). Correspondingly, expression of wild-type NIK from the ROSA26 locus leads to a minor increase in p100 processing and nuclear accumulation of RelB (Fig. 1D), whereas NIKΔT3 expression induces a near complete degradation of p100 to p52 and strongly enhanced nuclear levels of p52 and RelB (Fig. 1E), demonstrating that NIKΔT3 strongly activates the alternative pathway. Further analysis revealed that the presence of NIKΔT3 caused reduced amounts of cytoplasmic p105 and increased nuclear levels of p50 (Fig. 1E), in addition to increased IκBα phosphorylation, which corresponded to reduced IκBα protein (Fig. 1F) and elevated IκBα mRNA levels (Fig. 4C). These results indicate that NIKΔT3 induces canonical NF-κB activity, albeit to a lesser extent. However, NIKΔT3 does not significantly increase the nuclear levels of RelA or c-Rel (Fig. 1E) or cellular levels of the NF-κB target IκBε (Fig. 1F), which is strongly up-regulated in B cells with constitutive canonical NF-κB (16). In addition NIKΔT3-activity caused increased ERK and decreased AKT phosphorylation and potently inhibited JNK activation (Fig. 1G), in line with reports demonstrating that NF-κB activation can inhibit the JNK pathway (17). The expression of various anti-apoptotic Bcl-2 family proteins depends on NF-κB and BAFF:BAFF-R interactions, although the exact identity of these proteins remains controversial and might be cell-type- and context-dependent. We found that constitutive activation of p100 processing by NIKΔT3 led to elevated Bcl-xL, but not Bcl-2 and Mcl-1 levels (Fig. 1H).
Fig. 1.
NIKΔT3 expression strongly induces the alternative NF-κB pathway. (A) NIK or NIKΔT3 are expressed under control of the ROSA26 promoter after Cre-mediated deletion of the loxP-flanked STOP cassette. Flag-tagged wild-type NIK and NIKΔT3, which lacks the T3BD (amino acids 78–84) were used for this approach. KD, kinase domain. (B) B cell-specific expression of the IRES-eGFP-containing construct can be verified by FACS analysis. (C–H) Western blot analysis of extracts from control, NIKtg, and NIKΔT3tg B cells, as indicated above the individual blots. The levels of β-actin, tubulin, and lamin B1 are shown as loading controls for whole-cell, cytoplasmic (C), and nuclear (N) extracts, respectively. Numbers in red indicate average quantifications (normalized to the respective loading controls) relative to controls of four (D), five (E), three to six (F), four (G), or three to four (H) experiments.
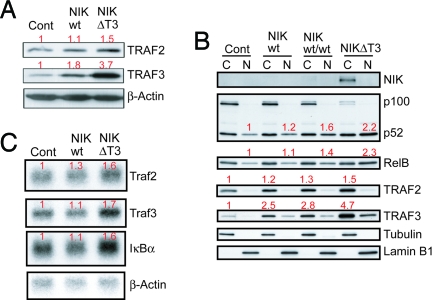
Fig. 4.
NIK protein level and activity are controlled by a negative feedback loop involving TRAF2/3. (A) Western blot analysis of TRAF2 and TRAF3 protein levels in purified splenic B cells of the indicated genotypes. (B) Western blot analysis of cytoplasmic (C) and nuclear (N) protein amounts of NIK, p100/p52, RelB, TRAF2, and TRAF3 in splenic B cells. (C) Northern blot analysis of Traf2, Traf3, and IκBα mRNA levels in CD43-depleted splenic B cells. Numbers in red indicate average quantifications (normalized to the respective loading controls) relative to controls of three (A), one (B), or two to three (C) experiments.
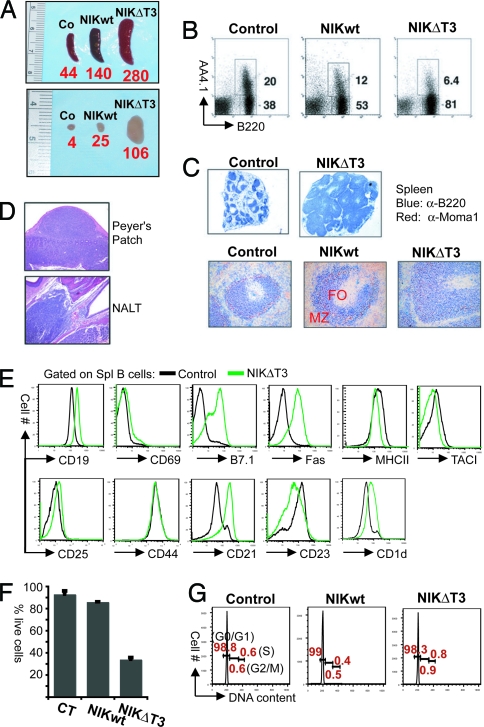
CD19-cre/R26StopFLNik (B cellNIK) mice had larger spleens and enlarged lymph nodes (Fig. 2A) because of a >3-fold increase in the number of mature peripheral B cells [Fig. 2B and supporting information (SI) Table S1]. Apart from a marked expansion of marginal zone (MZ) B cells (22.6 ± 1.7 × 106 in B cellNIK versus 4.9 ± 0.6 × 106 in control mice; n = 3–5; Fig. 2C and Fig. S1A) and elevated expression of CD23, NIKtg B cells did not appear activated or affected in their peripheral development (Fig. S1 A and B). The expression of NIKΔT3 in B cellNIKΔT3 mice led to massive splenomegaly and lymphadenopathy, with the spleens containing over six fold more mature AA4.1− B cells than those of control mice (Fig. 2A and Table S1). The cell-surface protein expression pattern of NIKΔT3tg B cells in spleen and lymph nodes was profoundly deregulated, resembling that of activated and MZ B cells (Fig. 2E and Fig. S2 A and B), so that a classification of B cells into subsets was not possible. Immunohistochemical analyses revealed an altered follicular structure (Fig. 2C), potentially due to the overabundance of B cells. This was also observed in Peyer's Patches and nasal-associated tissues, where lymphoid cells seemed to “spill over” from their normal confines into surrounding tissue (Fig. 2D). Constitutive NIK activity interfered with B1 cell development, because we detected a strong reduction in the B1 cell compartment in the peritoneal cavity (Fig. S2C). NIKΔT3tg B cells had strongly elevated levels of Fas, which rendered them susceptible to Fas-induced apoptosis (Fig. 2F), demonstrating that constitutive NIK-activity does not protect against this form of cell death.
Fig. 2.
B cell hyperplasia induced by NIK or NIKΔT3 expression. (A) Macroscopic appearance of spleen and lymph nodes. Bold red numbers below the spleens or lymph nodes indicate the average number of B cells (× 106) in three age-matched mice, respectively. (B) FACS analysis shows the proportions of transitional B220+AA4.1+ and mature B220+AA4.1− B cells in the spleens of control, B cellNIK, and B cellNIKΔT3 mice. Numbers next to individual gates refer to the percentages of transitional and mature splenic B cells of total lymphocytes. (C) Immunohistochemical analyses of spleen sections with anti-B220 (blue) and anti-MOMA1 (red) shows the ring of metallophilic macrophages (red) encircling the B cell (blue) follicles (FO). Blue staining outside the follicles represents MZ B cells. (Magnification: Upper, ×40; Lower, ×200.) (D) Hematoxylin/eosin staining of Peyer's patch and nasal-associated lymphoid tissue (NALT) sections from a B cellNIKΔT3 mouse. (E) FACS analysis of cell-surface marker expression on NIKΔT3tg and CD19-cre control B cells. Results are representative of at least three independent experiments. (F) Susceptibility of ex vivo isolated cells of the indicated genotypes to Fas-induced apoptosis. The percentages of life Fas-treated cells of life control antibody-treated cells are shown as mean and standard deviation of three independent experiments. (G) DNA-content analysis of ex vivo isolated control, NIKtg, and NIKΔT3tg B cells. The percentages of cells in different phases of the cell cycle (G0/G1, G2/M, and S) are indicated in red; one representative plot of three independent experiments is shown.
Neither the expression of NIK or NIKΔT3 induced proliferation of mature peripheral B cells, which were essentially all in a resting state (Fig. 2G). The increased amounts of Bcl-xL suggest that the B cell hyperplasia caused by NIKΔT3 expression could be due to enhanced survival. Indeed, NIKΔT3tg B cells isolated from spleen and lymph nodes survived much longer in culture than control B cells (Fig. S2D and SI Materials and Methods).
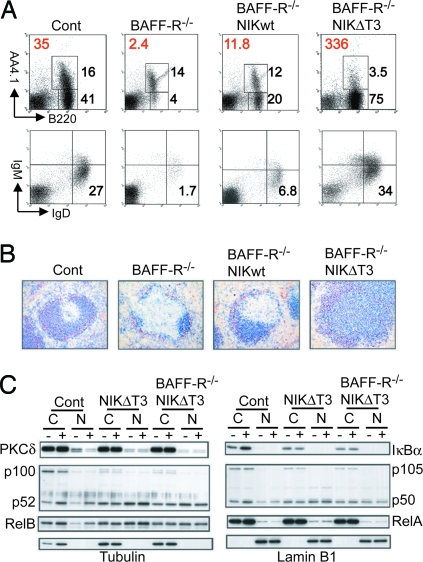
Expression of NIKΔT3, but Not of Wild-Type NIK, Can Liberate B Cells from Their Dependence of BAFF-R Signaling.
To evaluate, to what extent expression of wild-type and mutant NIK can substitute for BAFF:BAFF-R signals, we generated B cellNIK and B cellNIKΔT3 mice on a BAFF-R-deficient background (18). Examination by flow cytometry (Fig. 3A) and immunohistochemistry (Fig. 3B) demonstrated that enforced expression of wild-type NIK, compensated for the loss of BAFF-R only to a small extent. Expression of NIKΔT3 from the same locus, on the other hand, rendered the B cells completely BAFF-R-independent (Fig. 3 A and B). Biochemical analysis of in vitro cultured B cells revealed BAFF- and BAFF-R-independent p100 processing and nuclear p52 and RelB accumulation in NIKΔT3tg B cells, that exceeded the effects achieved by BAFF treatment of wild-type B cells (Fig. 3C). BAFF-induced increases in IκBα protein levels are not observed in NIKΔT3tg B cells, relating perhaps to the overall lower amounts of IκBα in these cells. The lack of BAFF-induced nuclear RelA accumulation further confirms that in our experimental settings BAFF:BAFF-R-interactions activate the canonical NF-κB pathway only weakly, if at all (16). In the absence of BAFF, protein kinase Cδ (PKCδ) translocates into the nucleus, an event that is most likely proapoptotic because ablation of PKCδ protects B cells from death due to loss of BAFF:BAFF-R-induced signals (19). The nuclear accumulation of PKCδ observed in the absence of BAFF or the BAFF-R was abolished in the presence of NIKΔT3 (Fig. 3C), placing this event downstream of NIK-mediated p100 processing.
Fig. 3.
Uncoupling of NIK from TRAF3-mediated negative regulation renders B cells independent of BAFF-R signaling. (A) FACS analysis of splenic B cell populations. Bold red numbers indicate the average number of mature B220+AA4.1− splenic B cells (× 106) (n = 2–4). The numbers next to individual gates refer to the percentages of transitional B220+AA4.1+ and mature splenic B cells (Upper) or of mature follicular IgMlowIgDhigh B cells (Lower) of total lymphocytes. (B) Immunohistochemical analysis of spleen sections. Anti-B220 (blue) stains follicular B cells inside the ring of MOMA1+ (red) metallophilic macrophages. (Magnification: ×200.) (C) Cytoplasmic (C) and nuclear (N) levels of PKCδ, p100/p52, RelB, IκBα, p105/p50, and RelA analyzed by Western blotting in extracts prepared from B cells cultured in medium with or without 500 ng of BAFF/ml for 24 h. Tubulin and lamin B1 levels demonstrate purity and equal loading of cytosolic and nuclear fractions, respectively. This experiment was repeated once with identical results.
Negative Feedback Involving NIK and TRAF2/3 Controls the Extent of Alternative NF-κB Activation.
Equivalent GFP expression from the IRES-eGFP sequences in NIKtg and NIKΔT3tg B cells (data not shown) indicated that removal of the sequences encoding the T3BD did not destabilize the NIK mRNA. Still, neither control nor NIKtg B cells contained detectable amounts of NIK, whereas NIKΔT3tg B cells contained high levels of the mutant protein (Fig. 1C). Examination of the protein levels of negative regulators of alternative NF-κB revealed that NIKtg B cells had elevated levels of TRAF3 and, to a lesser extent, of TRAF2, which were further increased in NIKΔT3tg B cells (Fig. 4A). This suggested a negative feedback-regulation, possibly through NIK-dependent transcriptional activation of TRAF2/3. Traf2 is a known target of NF-κB activity (20) and analysis of mRNA levels by Northern blotting revealed that NIKwt and NIKΔT3 expression induced an increase in Traf2 message (Fig. 4B), that correlated well with the increase in TRAF2 protein (Fig. 4A). The Traf3 gene has not been reported as a transcriptional target of NF-κB proteins. We therefore determined the Traf3 transcriptional initiation sites and promoter region and identified four NF-κB binding consensus sequences (κB sites) (Fig. S3A). The responsiveness of different Traf3-promoter-Luciferase constructs to alternative NF-κB activation was evaluated through transient expression of p52 and RelB (Fig. S3B). These analyses showed that the first two κB sites (κB1 and κB2) can transmit p52/RelB-mediated transcriptional activation of luciferase by the Traf3 promoter (Fig. S3B). Analysis of Traf3 mRNA levels by RT-PCR revealed a small but reproducible induction of Traf3 message by BAFF in control B cells, comparable to NIKΔT3tg B cells (Fig. S3C). However, the NIKΔT3-mediated increase in Traf3 message (Fig. 4C) can most likely not fully account for the strong increase in TRAF3 protein level observed in these cells (Fig. 4A). Further analysis, including B cells carrying two copies of the wild-type NIK knockin transgene, showed that the increase in TRAF3 and TRAF2 protein levels correlated in a dose-dependent fashion with NIK-activity as assessed by p100 processing and nuclear accumulation of p52 and RelB (Fig. 4B).
Discussion
Signaling induced by BAFF:BAFF-R-interactions is critical for the generation and survival of mature peripheral B cells. Excess availability of BAFF leads to B cell hyperplasia and to autoimmunity due to, at least in part, defective negative selection of autoreactive B cells (21, 22). Therefore, competition for limited resources of BAFF is, from the transitional stages of B cell development onwards, the main principle regulating the size of the mature B cell pool and peripheral B cell tolerance. Certain lymphomas can apparently overcome this limitation by producing their own BAFF (23). Of the intracellular signaling events induced by BAFF, the processing of p100 to p52 has been linked most convincingly to its prosurvival properties. To what extent in this context other events, such as activation of the AKT (24) and ERK (25) pathways, are downstream of, and therefore dependent on NIK and alternative NF-κB activation, remains to be elucidated. Constitutive activation of the alternative (shown here) and canonical pathway (16), as well as enforced expression of Bcl2 (16) are all sufficient to keep PKCδ in the cytoplasm, suggesting that nuclear translocation of PKCδ is a late event in the chain of events leading to the death of B cells due to lack of BAFF.
Removal of the T3BD dramatically increases NIKΔT3 protein stability (13), and v-ABL-transformed TRAF3-deficient B cells contain detectable NIK protein levels in contrast to control B cells (12), suggesting that BAFF:BAFF-R-induced relief of TRAF3-mediated repression of NIK protein levels leads to activation of alternative NF-κB and B cell survival. In TRAF2- or TRAF3-deficient primary B cells, however, no increase in NIK protein could be detected, despite strong induction of p100 processing (8–10).
To address the consequences of TRAF3-mediated control of NIK-activity for B cell physiology, we expressed wild-type NIK and NIKΔT3 in the B lineage under control of the endogenous ROSA26 locus. Overexpression of NIK induces B cell hyperplasia but did not lead to detectable NIK protein levels. Therefore, B cell-specific increases in NIK expression, undetectable by Western blot analysis, can induce p100 processing and markedly increase the number of mature peripheral B cells, especially MZ B cells. The NIK-mediated B cell hyperplasia is BAFF-R-dependent (Fig. 5 B and C), leading us to propose that elevated levels of NIK act as an amplifier of BAFF signals. It is likely, that higher expression levels of NIK than those mediated by the ROSA26 locus will allow the survival of more B cells in the absence of BAFF:BAFF-R-interactions, but these cells should still be hyper-responsive to BAFF. With respect to lymphomagenesis, this implies that translocations or amplifications of the NIK gene may render cells more sensitive to, but not fully independent of, BAFF signals.
Fig. 5.
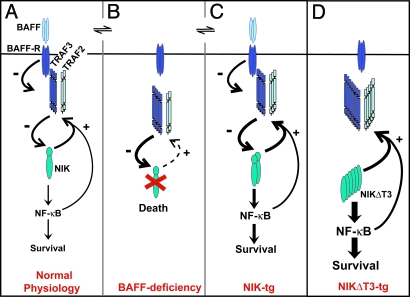
Scheme of BAFF:BAFF-R-induced alternative NF-κB activation via TRAF2, TRAF3, and NIK. During normal B cell physiology (A), BAFF:BAFF-R interactions induce the degradation of TRAF3, which binds to NIK at the T3BD and together with TRAF2 negatively regulates NIK protein levels. NIK activity stimulates B cell survival via the activation of predominantly alternative NF-κB and increases the TRAF2 and, more prominently, TRAF3 protein levels in a negative feedback interaction. (B) In the absence of BAFF:BAFF-R interactions, TRAF3 accumulates and together with TRAF2 induces the complete degradation of NIK, leading to shutdown of alternative NF-κB activity and cellular apoptosis. (C) In the presence of BAFF, increased expression of NIK leads to a steady state of enhanced NF-κB signaling and B cell survival and enhanced levels of TRAF2 and TRAF3, which keep NIK protein level below detection. Absence of BAFF (B) still results in TRAF2/3-mediated degradation of NIK and apoptosis. The activity of increased de novo generated NIK mostly likely slightly increases BAFF-independent survival. (D) NIKΔT3 induces BAFF-independent maximal p100 processing and B cell survival and very high TRAF2 and TRAF3 protein levels, which, however, cannot impact on NIKΔT3 protein levels.
Ablation of TRAF2 or TRAF3 in murine B cells induces hyperplasia, most likely due to elevated levels of NIK protein, even though this could not be confirmed by Western blot analysis. Similarly, deletion of the TRAF3 genomic locus in HMCLs does not induce high levels of NIK protein (14, 15). Our data show that increases in NIK protein levels undetectable by Western analysis can strongly increase mature B cell numbers, suggesting that increased NIK activity is the mechanism responsible for enhanced B cell survival due to absence of TRAF2 or TRAF3.
The disruption of NIK-TRAF3 interactions through deletion of the T3BD of NIK, expressed from the ROSA26 locus, leads to high steady-state protein levels of the mutant NIKΔT3 (Fig. 5D) and an increase in B cell numbers far exceeding the effects achieved by expression of wild-type NIK from the same locus or ablation of TRAF3 or TRAF2 or of both (10). This could be due to the combined effects of NIK overexpression and loss of negative regulation by TRAF2/3, because neither of these events alone produces detectable NIK protein levels in primary cells. B cell-specific NIKΔT3 expression profoundly disturbs normal B cell physiology, as evidenced by the disrupted lymphoid microarchitecture, abnormal cellular surface phenotype and altered intracellular signaling. These changes illustrate the dramatic consequences, be they direct or indirect, of deregulated NIK signaling in B cells.
The exact mechanisms of the BAFF:BAFF-R-TRAF2/3-NIK interplay remain to be elucidated and most likely involve additional players. We show that the steady-state amounts of TRAF2 and TRAF3 protein are regulated in a dose-dependent manner by NIK and that the T3BD of NIK is essential for the control of NIK protein levels in vivo. In resting mature B cells TRAF3 levels are kept low through BAFF:BAFF-R interactions (10). This could allow NIK to accumulate, albeit at still very low levels, and to activate NF-κB signaling, thereby ensuring B cell survival (Fig. 5A). Our results imply that the activity of the alternative NF-κB branch is kept under control through a negative feedback mechanism involving NIK-induced increase in the protein levels of the negative regulators TRAF2 and TRAF3 (Fig. 5A). While in the case of TRAF2 this appears to occur through a NIK-dependent transcriptional activation of the traf2 gene, the increase in TRAF3 protein levels likely involves transcriptional and posttranscriptional mechanisms. In the absence of BAFF:BAFF-R-mediated degradation signals TRAF3 accumulates (10), at least partially mediated through NIK-dependent mechanisms, and this in turn causes a depletion of NIK, followed by termination of alternative NF-κB signaling and apoptosis (Fig. 5B).
Loss of the negative regulator TRAF3 and overexpression of NIK belong to the more frequent genetic aberrations causing the deregulation of NF-κB activity recently described for human multiple myeloma (14, 15). The JJN3 HMCL harbors a translocation into the NIK locus producing a fusion protein lacking the T3BD (15). The translocated NIK allele is expressed at much lower level than the intact allele, yet abundant amounts of only the fusion protein can be detected in extracts from JJN3 cells, underscoring the importance of the T3BD for the proper regulation of NIK protein levels. NIK was identified in human B cells as a TRAF2-interacting protein and a NF-κB inducing kinase (26). Subsequent loss-of-function analyses in the mouse suggested that NIK's activity is limited to the induction of alternative NF-κB (4, 5). These results support the notion that BAFF:BAFF-R interactions mainly stimulate the alternative branch via IKK1 and NIK, which is further underlined by our finding that even strong overproduction of NIKΔT3 leads to only weak stimulation of events associated with canonical NF-κB activity. However, although most of the experimental evidence points to a minor role of BAFF in the induction of canonical NF-κB in murine B cells, there is evidence that BAFF treatment can induce significant canonical NF-κB activity also in the mouse (27, 28). Therefore, the exact mechanism of BAFF-induced canonical NF-κB and its importance in the context of human and murine B cell survival remain to be determined.
The recent finding that NIK protein accumulation in murine embryonic fibroblasts, achieved through ablation of TRAF3 or long-term stimulation of the LTβR, results in an amplification of canonical NF-κB activity (29) suggests that this activity could be mediated by NIK. In human B cell lines, treatment with BAFF, CD40L, or CD70 induced robust, NIK-dependent IκBα degradation and RelA nuclear accumulation (30). In NIKΔT3tg B cells we observed increased p50 levels as most prominent indicator of canonical NF-κB signals. We are now evaluating the dependence of NIKΔT3tg B cells on canonical NF-κB activation through ablation of NEMO.
Our results demonstrate that the alternative pathway of NF-κB activation is controlled through a negative feedback mechanism involving NIK-induced elevation of the protein levels of its negative regulators TRAF2/3. Overexpression of wild-type NIK leads to B cell hyperplasia caused by the amplification of BAFF-induced alternative NF-κB signals. The disruption of the interaction between TRAF3 and NIK, on the other hand, induces constitutive BAFF-independent activation of the alternative pathway and leads to a dramatic accumulation of mature B cells in secondary lymphoid organs to an extent that compromises their structural integrity. With respect to B cell malignancies our results imply that deregulated NIK expression could contribute to lymphomagenesis even if it remains undetectable at the protein level.
Methods
Genetically Modified Mice.
Mice expressing NIK from the ROSA26 locus in a tissue-specific manner were generated as described previously for IKK2ca (16). All mice were of a C57BL/6 genetic background and were bred and maintained in specific pathogen-free conditions; all mouse protocols were approved by the Harvard University Institutional Animal Care and Use Committee and by the Immune Disease Institute.
Flow Cytometry and Cell Purifications.
Single-cell suspensions were stained with the following monoclonal antibodies: anti-CD19 (ID3), anti-CD21 (7G6), anti-CD23 (B3B4), anti-CD1d (1B1), anti-Fas (Jo2), anti-CD80, anti-MHCII, anti-CD69, anti-CD25, and anti-CD44 from BD Pharmingen; and anti-AA4.1 from eBioscience. All samples were acquired on a FACSCalibur (BD PharMingen), and results were analyzed by using Flow-Jo and CellQuest software. B cells were purified by MACS (Miltenyi) to a purity of >85%.
Fas-Induced Apoptosis and Measurement of DNA Content.
B cells were purified ex vivo by using CD43-depletion by MACS (Miltenyi) and incubated overnight in the presence of Fas or control antibody. The total number of live cells was evaluated by cell counting. For DNA content, B cells were ethanol-fixed, RNaseA-treated, and stained with propidium iodide as described in ref. 16.
Immunohistochemistry.
For staining of B cells and macrophages, frozen 6-μm sections were thawed, air-dried, acetone-fixed, and stained for 1 h at room temperature in a humidified chamber with biotinylated rat anti-B220 and rat anti-MOMA-1 (Cedarlane) followed by HRP-conjugated goat anti-rat IgG and alkaline phosphatase-conjugated streptavidin.
Western and Northern Blot Analysis.
Western blot analysis was performed essentially as described in ref. 16. For Northern blot analysis, 5 μg of total RNA prepared from splenic B cells was electrophoresed on 1% agarose gel containing formaldehyde and transferred to Hybond N+ membrane (GE Healthcare). The coding region of cDNAs for Traf2, Traf3, IκBα, and β-actin were used as probes for hybridization.
Supplementary Material
Acknowledgments.
We thank P. Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA) for providing the plasmids pROSA26–1 and pROSA26-R; Y. M. Hsu and M. Scott (Biogen Idec, Cambridge, MA) for the gift of hBAFF; and D. Ghitza, C. Aristoff, and A. Monti for technical support. This work was supported by National Institutes of Health Grants AI057947, AI054636, and CA92625, and by grants from the Sandler Program for Asthma Research, the Mitsubishi Pharma Research Foundation, and the Leading Project for Regenerative Medicine. Y. Sasaki received a fellowship from the Uehara Memorial Foundation. M.S.-S. is a recipient of an Emmy Noether Grant from the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805186105/DCSupplemental.
References
- 1.Courtois G, Gilmore TD. Mutations in the NF-κB signaling pathway: Implications for human disease. Oncogene. 2006;25:6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 2.Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 3.Karin M, Cao Y, Greten FR, Li ZW. NF-κB in cancer: From innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 4.Beinke S, Ley SC. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejardin E. The alternative NF-κB pathway from biochemistry to biology: Pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Sen R. Control of B lymphocyte apoptosis by the transcription factor NF-κB. Immunity. 2006;25:871–883. doi: 10.1016/j.immuni.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-κB. Nat Rev Immunol. 2005;5:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 8.Grech AP, et al. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-κB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 11.He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. Specificity of TRAF3 in its negative regulation of the noncanonical NF-κB pathway. J Biol Chem. 2007;282:3688–3694. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- 12.He JQ, et al. Rescue of TRAF3-null mice by p100 NF-κB deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-κB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 14.Annunziata CM, et al. Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keats JJ, et al. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki Y, et al. Canonical NF-κB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Papa S, et al. The NF-κB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 19.Mecklenbrauker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A. Regulation of B-cell survival by BAFF-dependent PKCδ-mediated nuclear signalling. Nature. 2004;431:456–461. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- 20.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-κB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 21.Brink R. Regulation of B cell self-tolerance by BAFF. Semin Immunol. 2006;18:276–283. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: Fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol. 2004;4:347–354. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKCβ- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craxton A, Draves KE, Gruppi A, Clark EA. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202:1363–1374. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 27.Hatada EN, et al. NF-κB1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-κB2 p100 to p52 in quiescent mature B cells. J Immunol. 2003;171:761–768. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- 28.Shinners NP, et al. Bruton's tyrosine kinase mediates NF-κB activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J Immunol. 2007;179:3872–3880. doi: 10.4049/jimmunol.179.6.3872. [DOI] [PubMed] [Google Scholar]
- 29.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-κB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci USA. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-κB activation pathways by NF-κB-inducing kinase. Immunity. 2004;21:477–489. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.