Abstract
The astonishingly efficient location and excision of damaged DNA bases by DNA repair glycosylases is an especially intriguing problem in biology. One example is the enzyme uracil DNA glycosylase (UNG), which captures and excises rare extrahelical uracil bases that have emerged from the DNA base stack by spontaneous base pair breathing motions. Here, we explore the efficiency and mechanism by which UNG executes intramolecular transfer and excision of two uracil sites embedded on the same or opposite DNA strands at increasing site spacings. The efficiency of intramolecular site transfer decreased from 41 to 0% as the base pair spacing between uracil sites on the same DNA strand increased from 20 to 800 bp. The mechanism of transfer is dominated by DNA hopping between landing sites of ≈10 bp size, over which rapid 1D scanning likely occurs. Consistent with DNA hopping, site transfer at 20- and 56-bp spacings was unaffected by whether the uracils were placed on the same or opposite strands. Thus, UNG uses hopping and 3D diffusion through bulk solution as the principal pathways for efficient patrolling of long genomic DNA sequences for damage. Short-range sliding over the range of a helical turn allows for redundant inspection of very local DNA sequences and trapping of spontaneously emerging extrahelical uracils.
Keywords: facilitated diffusion, search mechanism
The large amount of background DNA sequences present in all genomes presents a formidable challenge for enzymes that must locate and react with rare specific sites in DNA. A demanding example is that of DNA-repair glycosylases that use an extrahelical recognition mechanism to identify the specific features of a damaged DNA base while it is camouflaged in a vast amount of undamaged genomic DNA (1, 2). With almost unfathomable efficiency these enzymes excise a wide variety of oxidized, deaminated, or alkylated bases, thereby preventing adverse consequences to the coding content of the genome (3). Recent structural and NMR dynamic studies have established that the enzyme uracil DNA glycosylase (UNG) detects uracil in duplex DNA by trapping the base as it partially emerges from the base stack because of thermal breathing motions of the DNA (4), a mechanism that places stringent kinetic constraints on recognition (1).
How can such efficient site location events occur in the presence of nontarget DNA sequences? The mechanism may be appreciated by recognizing that these enzymes possess weak micromolar binding affinities for DNA lacking a specific damaged base lesion (5–8). Although nonspecific DNA binding sites behave as competitive inhibitors if located on another DNA molecule from that containing the site of damage, these sites can serve as a molecular conduit for facilitated location of the damage site when present on the same DNA chain. This phenomenon is known as “facilitated diffusion,” and for monomeric proteins with a single DNA binding site, may arise by two general pathways. The first pathway involves protein sliding along the DNA backbone, which serves to reduce the 3D-search process to a single dimension along the linear contour of the DNA. Because 1D diffusion is a thermally driven process, each step that the protein makes along the DNA contour has an equal probability of being in the direction of the damage, or alternatively, in the opposite direction (5, 9, 10). Thus, a hallmark of 1D sliding is that the probability of a protein reaching a specific site after leaving a defined position on the DNA contour decreases in proportion to the square of the number of base pair steps (n) between the initial and damaged sites (11). This property of 1D sliding makes it an extremely slow and highly redundant process for searching long stretches of DNA for damage, but ideal for scanning local sequences. The second facilitated pathway involves intramolecular hopping along the DNA chain (5). This mechanism serves to reduce the volume of the search process, but in contrast with the 1D pathway, the probability of the protein encountering the damaged site after departing a defined position on the DNA decreases with the inverse of the distance between the initial and damaged sites (1/r) (11). Hopping is more efficient than sliding over longer distances because it does not involve repetitive and fruitless searching of the same local DNA sequence.
The experiments in this article explore the nature of facilitated diffusion for the DNA repair enzyme uracil DNA glycosylase from Escherichia coli (UNG). This extensively studied enzyme excises uracil from U/A or U/G base pairs in DNA that arise from either misincorporation of dUTP during DNA replication, or alternatively, spontaneous or enzymatic cytosine deamination (12). As detailed above, sliding or hopping mechanisms for intramolecular site transfer can in principle be distinguished by their distinctive n2 or 1/r dependences, respectively. Consequently, by embedding two uracil sites within a single DNA chain at increasing site spacings, the site location mechanism of UNG may be deciphered. Our findings resolve previous conflicting reports concerning the relative contributions of sliding, hopping, and 3D bulk diffusion pathways for site location by UNG (13, 14), and call attention to the absolute requirement for short-range scanning along the DNA backbone for enzymes that trap damaged bases in their open state.
Results
Measuring Intramolecular Uracil Excision.
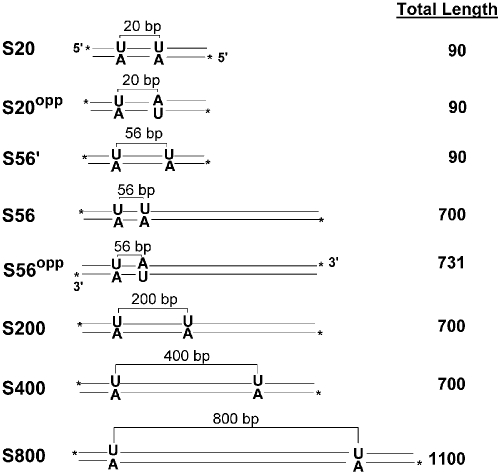
These studies required synthesis of large DNA substrates with two uracils spaced at defined distances from each other (Fig. 1; see also supporting information (SI) Methods and Figs. S1–S4) (15). Three substrates of 90 bp total length were synthesized in which the two uracils were located on either the same or opposite DNA strands with a 20-bp spacing (Fig. 1, S20 and S20opp), or on the same strand with a 56-bp spacing (S56′). The remaining substrates with longer total lengths of 700–1,100 bp had uracils incorporated on a single DNA strand with spacings of 56 (S56), 200 (S200), 400 (S400), and 800 (S800) bp. In addition, a 731-bp DNA was constructed in which the uracils were spaced by 56 bp on opposite strands (S56opp). The substrates were engineered such that the sequences 15 bp on either side of the uracil were identical to negate excision rate differences because of divergent sequences at the two sites.
Fig. 1.
Substrate constructs used in this study. Substrates with two uracils located on the same or opposite strands of the DNA duplex are shown along with the uracil site spacings. 32P end-labeling positions are shown with asterisks, and the total length of each substrate in base pairs is given on the right.
The intramolecular excision efficiencies were measured by using the approach originally used by Modrich and colleagues (16, 17) for restriction enzyme EcoRI, and later by Stanford et al. (9, 10) for EcoRV. In brief, a substrate containing two reactive sites is incubated with a small amount of enzyme such that the probability of two enzyme molecules reacting with a single substrate is extremely small. Under initial rate conditions, an enzyme molecule binds to the substrate at a random position and then diffuses to and excises one of the two uracils (kcl, Fig. 2). Once the uracil is excised from either of the sites in a primary cleavage event, the instantaneous position of the enzyme is then “marked” with respect to the other site. At that moment, one of two competing events takes place: (i) escape of the enzyme from the DNA domain such that it encounters a virgin substrate molecule rather than the singly excised substrate it just departed (kesc, Fig. 2), or (ii) intramolecular transfer and excision of the second uracil site in the same molecule in a secondary excision event (kintra, Fig. 2). The intramolecular transfer and excision efficiency (fintra, Eq. 1) depends on the product of the transfer probability [T = kintra/(kintra + kesc)], which decreases with site spacing, and the efficiency of uracil excision once the site is located [E = kex/(kex + koff)], where kex is the excision rate and koff is the off-rate from the uracil site.
Although Eq. 1 shows that fintra depends on two kinetically determined probabilities, the most useful experimental method to obtain fintra is to measure the stoichiometries of the single- and double-excision products as a function of time, which directly reflect the probabilities in Eq. 1 (Fig. 3A). Experimentally, the reactions are first quenched at various times with a potent and specific inhibitor of UNG (18, 19), and the uracil excision products are then converted to unique dsDNA fragments AB, BC, A, and C by using abasic site endonuclease and restriction endonuclease nicking reactions (SI Methods). The central aspect of this method is that the single-excision intermediates (fragments AB and BC) are consumed when UNG transfers successfully to the second site and excises the uracil. Accordingly, after postreaction processing, intramolecular transfer events are revealed by a stoichiometric excess of the products A and C, which are produced by both single- and double-excision events, compared with the singly excised intermediates AB and BC (Fig. 3A). The intramolecular transfer efficiency (fintra), which is described kinetically in Eq. 1, may therefore be equivalently expressed in terms of DNA fragment concentrations (Eq. 2). In the numerator of Eq. 2 is the number of single-enzyme substrate encounters resulting in excision at both uracil sites in a single encounter ([A] + [C] − [BC] − [AB]), and this is divided by the total excision products ([A] + [C] + [AB] + [BC]) (10, 16, 17).
 |
We note that fintra must be obtained by extrapolation to time 0 of the reaction, because as time progresses, the ratio in Eq. 2 reduces to [A] + [C]/[A] + [C] = 1. Thus, fintra is obtained from a plot of the apparent fintra values against time and extrapolating to the ordinate axis by using a second-order polynomial nonlinear best fit to the data (see below).
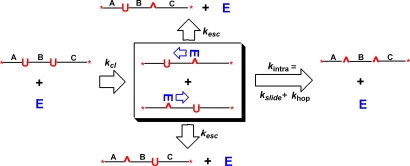
Fig. 2.
Intramolecular transfer between uracil sites by UNG. After cleaving the glycosidic bond to either uracil in a primary excision event, UNG will either escape to bulk solution (kesc) or translocate to the second uracil (kintra) by using a sliding (kslide) or hopping (khop) pathway. The efficiency of intramolecular transfer as compared with escape is revealed by the concentration of the double-excision product on the right relative to the two single-excision products at the top and bottom of the figure.
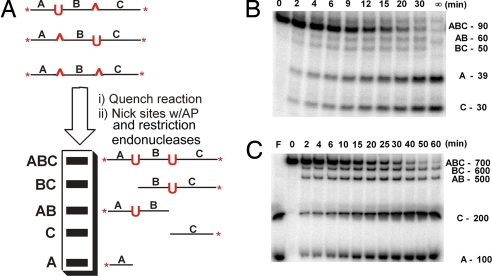
Fig. 3.
Assay and measurement of intramolecular excision events. (A) After quenching UNG with a potent and specific oligonucleotide inhibitor, the single- and double-excision products are digested to discrete dsDNA fragments by using abasic endonuclease and a nicking restriction enzyme. (B) Time course for excision of uracils from S20. (C) Time course for excision of uracils from S400. Reactions were at 37°C with 10 mM NaCl.
Intramolecular Transfer Between Sites Positioned on the Same DNA Strand.
The time course for reaction of UNG on substrates S20 through S800 was carried out with an enzyme-to-substrate ratio of 1:4,000 with a low-salt buffer (10 mM NaCl). Because a minimal binding site for UNG encompasses approximately one turn of the DNA helix (20), S20 has nearly overlapping binding sites. Visual inspection of the product band intensities for reaction of S20 (Fig. 3B) reveals that, at the earliest time points in the reaction, the concentrations of fragments A and C exceed those of BC and AB, indicating that intramolecular transfer is occurring. In significant contrast, the relative concentrations of fragments A, C, BC, and AB in the initial rate regime are nearly the same for S400, indicating that very little intramolecular site transfer occurs at this spacing (Fig. 3C). Similar experiments were performed by using substrates S56, S200, S400, and S800, and the apparent fintra values as a function of time were plotted with the corresponding second-order polynomial fits used to obtain the limiting values for fintra at zero time (Fig. 4A). The limiting values for fintra decreased from a maximum of 0.30 at the 20-bp spacing to zero at the longest spacing of 800 bp (Fig. 4B, Table 1). A recent study with UNG using a single short site spacing of 20 bp reported a small transfer efficiency of 0.4 at zero [NaCl], which is similar to the more extensive measurements reported here under different conditions (21). In addition, the strong distance dependence of the intramolecular transfer efficiency of UNG provides an explanation for previous descriptions of UNG as behaving both processively and distributively depending on the site spacing (13, 14). We also found that fintra at a 56-bp spacing decreased to 0.0 ± 0.05 (Table 1), when the salt concentration was increased to a level approximating the intracellular concentration (150 mM NaCl). The decrease in intramolecular site transfer as the ionic strength is increased is consistent with the expectation that lowering the binding affinity for nonspecific DNA should increase the escape rate of UNG from the DNA domain, but also reflects decreases in the uracil excision efficiency once the site is encountered (the steady-state rate of single-site excision is 30-fold slower in the presence of 150 mM NaCl).
Fig. 4.
Determination of intramolecular transfer probabilities (fintra). (A) Plot of fintra against time for each substrate. The curves are best fits to a second-order polynomial expression and the extrapolated value at zero time provides the true fintra. (B) fintra divided by the site excision efficiency (E) plotted as a function of uracil site spacing in nanometers. Spacings were calculated by using the wormlike chain model for the DNA polymer (23, 24). The solid curve is the best fit to Eq. 4 for hopping, and the 90% confidence intervals are plotted as dashed lines. The uracil spacings in base pairs are shown at the top of the plot for reference. The orange data point is for S56 with a NaCl concentration of 150 mM.
Table 1.
Intramolecular transfer efficiencies
| DNA | fintra* | fintra/E | Spacing, nm† |
|---|---|---|---|
| S20 | 0.30 ± 0.06 | 0.41 ± 0.08 | 6.7 ± 0.1 |
| S20opp | 0.31 ± 0.12 | 0.42 ± 0.16 | 6.7 ± 0.1 |
| S56′ | 0.19 ± 0.03 | 0.26 ± 0.04 | 18 ± 0.1 |
| S56 | 0.22 ± 0.02 (0 ± 0.03)‡ | 0.30 ± 0.03 (0 ± 0.04)‡ | 18 ± 0.1 |
| S56opp | 0.24 ± 0.02 | 0.33 ± 0.03 | 18 ± 0.1 |
| S200 | 0.09 ± 0.01 | 0.12 ± 0.01 | 56 ± 1 |
| S400 | 0.05 ± 0.02 | 0.07 ± 0.03 | 96 ± 2 |
| S800 | 0.0 ± 0.01 | 0.0 ± 0.01 | 151 ± 6 |
*Measured intramolecular excision efficiencies (fintra) were divided by the site excision efficiency E = 0.73. Errors are standard errors of the mean (n = 2–4).
†Uracil site spacings were calculated by using the wormlike chain model for the DNA polymer (35). An average value for the persistence length (Pl) of 53 nm was used based on single-molecule force-extension measurements at 10 mM sodium ion concentration at 25° C (23, 24). The two experimental values Pl = 47 nm and 58 nm provide lower and upper bounds for the uncertainty in the site spacings.
‡The value in parentheses was measured by using 150 mM NaCl (red data point, Fig. 4B).
Intramolecular Transfer Between Sites Positioned on Opposite DNA Strands.
If intramolecular transfer is observed for a substrate where the two uracil sites are embedded on opposite strands of the DNA duplex, then transfer must involve at least one dissociation event that allows the enzyme to reorient with respect to the DNA (9). In other words, substrates with sites on opposite strands are deficient in the single-strand sliding pathway for transfer, and any intramolecular transfer that is observed for such substrates must occur by at least one dissociation/reorientation event. To test this aspect of site transfer, we constructed substrates S20opp and S56opp with short site spacings for comparison with the analogous substrates S20 and S56. Short site spacings were chosen because sliding would be expected to make the largest fractional contribution to the observed intramolecular transfer efficiency with such substrates. As reported in Table 1, fintra was indistinguishable for substrates S20 and S20opp as well as S56 and S56opp. Thus, these data provide no evidence for a significant single-strand sliding component to intramolecular transfer even at short site spacings of 20 and 56 bp.
Determination of the Uracil Excision Efficiency.
As shown in Eq. 1, the overall intramolecular transfer efficiency depends not only on the probability of transfer but also the uracil excision efficiency once the site is reached (E). Determination of the excision efficiency requires measurement of the partitioning of UNG between uracil excision (kex) and dissociation from the site (koff). We obtained the partitioning ratio kex/koff from a pulse–chase experiment in which excess UNG was rapidly mixed with a 3′-32P-labeled 283-bp duplex containing a single uracil approximately in the center of the sequence. Our approach is conceptually similar to the classic steady-state kinetic partitioning methods of Rose (22), but is performed under conditions of excess enzyme and limiting substrate (Fig. 5A and SI Methods). When 1 μM UNG was mixed with 20 nM substrate for 2 ms and the reaction was quenched with 0.5 M HCl, 8 nM excision product was formed (Pq*) and 12 nM bound substrate was left unreacted (ES*, Fig. 5A, see also Fig. S4). In contrast, when the acid quench was replaced with 60 μM abasic DNA (KD ≈ 30 nM) to serve as a trap for UNG after it dissociated from the ES* complex, the 12 nM ES* present at 2 ms was converted to 3 nM free substrate (ST*), and 9 nM was excised to form product (PT*). Because PT*/ST* = kex/koff = 9/3, then the excision efficiency E = kex/(kex + koff) may be directly calculated as 0.73.
Fig. 5.
Determination of the uracil excision efficiency (E). (A) Schematic of pulse–chase kinetic partitioning experiment to determine the ratio of glycosidic bond cleavage (kex) to substrate dissociation (koff). Excess enzyme (1 μM) is rapidly mixed with 32P-labeled DNA substrate (20 nM) and after 2 ms of aging time the reaction is either quenched with 0.5 N HCl or chased with 60 μM unlabeled DNA trap. The ratio of bound substrate that dissociates (ST*) to that which reacts to form product (PT*) in the chase period gives the ratio kex/koff. (B) Product (squares) and substrate (triangles) concentrations during the chase time. The amount of product that is formed during the chase period must be corrected for the amount that was present after the 2-ms aging period (Pq*, dashed line); this correction is obtained from the acid-quenched samples (SI Methods). Because the trap is not 100% efficient, the released substrate can slowly rebind and react to form more product, thus the true ratio of PT*/ST* = kex/koff is obtained by extrapolation to zero time.
Discussion
Mechanism of Intramolecular Site Transfer.
The site-spacing-dependent transfer efficiencies reported in Table 1 provide unambiguous evidence that UNG uses facilitated diffusion to locate and excise the second uracil site. Encoded in the distance dependence of fintra = fslide + fhop are the contributions of the sliding (fslide) and hopping (fhop) pathways. To test explicitly whether the site-spacing dependence of fintra could be accommodated solely by a sliding mechanism we used Eq. 3 (10, 11). In Eq. 3, the fintra values
are first divided by the efficiency of excising the site once it is encountered (E = 0.73), so that the data depend now only on the site transfer efficiencies (T) rather than the product T × E (see Eq. 1) (10). The efficiency of intramolecular transfer by sliding depends on the probability of sliding as opposed to escape at each base pair step (n) along the way Ps = kslide/(kslide + kesc). Because Ps is always a fraction less than unity raised to the n2 power, then fslide decreases sharply as site spacing increases. As shown in Fig. S2, we were unable to adequately fit the data in Table 1 to Eq. 3. This is not entirely surprising because the escape rate of UNG from nonspecific DNA can be estimated to be approximately kesc = 200 s−1 at 37°C and 10 mM NaCl based on the measured KD = 1 μM and UNG's diffusion controlled on-rate of 2 × 108 M−1 s−1 (SI Methods). This very short residence time (τbind = 1/kesc = 5 ms) provides a small window for sliding to occur. In addition, it is difficult to reconcile the identical site transfer efficiencies for the substrates with the uracils on the same and opposite strands by a strand sliding mechanism, because excision on the opposite strand requires at least one dissociation event to reorient UNG with respect to the substrate polarity (Table 1). Thus, these combined data strongly indicate that dissociation and reorientation of UNG relative to the DNA strands is a frequent event even at a short site spacing of 20 bp, and consequently, fslide has a small contribution to intramolecular transfer when the spacing is ≥20 bp.
The probability of hopping to the second site and excising the uracil (fhop) will depend on the radius of the site (a), and the inverse of the mean distance between the first and second uracil sites (1/〈r〉) (Eq. 4) (11). In Eq. 4, the observed fintra values
are normalized to the site excision efficiency as done above for sliding, and 〈r〉 may be calculated by using the wormlike chain model for the DNA polymer (23, 24). The a/r dependence of the site hopping probability is anticipated because hopping is a reduced-volume 3D search and, thus, is proportional to the radius of the target site (a) and inversely proportional to the distance that must be traveled to reach that site. Although this analysis assumes a spherical target site, in general, the corrections for shape are small and would have little effect on the distance dependence of fintra (11). As shown by the nonlinear regression fit to the data in Fig. 4B, the site spacing dependence of fintra/E may be fit by using Eq. 4 with a target size a = 3.7 ± 0.4 nm (R2 = 0.85). The site size of approximately one helical turn of B DNA indicates that on landing within ≈10 bp of a uracil site, UNG locates and excises the uracil with an efficiency of 0.73. Over this short range, UNG likely translocates via sliding as required by the kinetic restraints detailed below.
Short-Range Sliding Is Vital for Extrahelical Uracil Trapping.
Although sliding occurs over a distance of only 10 bp, short-range sliding plays an indispensable function in uracil recognition by UNG. Recent crystallographic and NMR dynamic findings have established that the earliest event in uracil recognition is trapping of thymine and uracil bases after base pair breathing motions have ejected them from the DNA base stack into a transient exo binding site on UNG (4, 25, 26). For this mechanism to be physically plausible several parameters must be kinetically matched: the binding lifetime of UNG at a nonspecific landing site, the dynamic motions of the base pair that lead to exposure of the uracil, and the sliding rate (Fig. 6). The binding lifetime of UNG for undamaged DNA is estimated to be τbind = 5 ms (SI Methods), and therefore, UNG will scan ≈10 bp of DNA during this period, sampling each base pair for an average of 5 ms/10 bp = 0.5 ms/bp. The opening rate of T/A and U/A base pairs at 37°C may be estimated to be ≈8 ms−1 based on the enthalpies of T/A base pair opening determined by NMR imino proton exchange measurements over the range 0 to 20°C (a U/A base pair opens with a similar rate as T/A) (26–28). Therefore, if one U/A base pair is present within 10 bp of the landing site, four opening events will be sampled (i.e., 0.5 ms/bp × 8 opening events ms−1). Without short-range scanning of the duplex, trapping of a spontaneously emerging thymine or uracil base would be an improbable event, and therefore, a prohibitively inefficient mechanism. Such a mechanism likely applies to a previously described UNG mutant that excises thymine (29).
Fig. 6.
Short-range sliding by UNG allows trapping of extrahelical uracil bases. On landing within 10 bp of a uracil site, UNG scans the duplex with an average residence time of 0.5 ms per base pair. U/A base pairs spontaneously open with an estimated rate constant of 8 ms−1 at 37°C (see text and SI Methods). Thus, on average, UNG can sample four opening events during its binding lifetime. Binding from bulk solution to an extrahelical uracil is not a kinetically competent pathway for recognition (26).
A reasonable question is whether the in vitro mechanism proposed here with the bacterial UNG is sufficient to account for rapid repair of genomic DNA by human UNG. It may be calculated from the number of UNG molecules (3 × 1012 per liter) and base pairs in these in vitro reactions (≈1019 per liter), and the assumption that each base pair is inspected by the enzyme, that each UNG inspects on average three million base pairs before repair. Under these conditions, complete excision of the uracils occurs in <60 min. In human cells, similar calculations indicate that each UNG would only have to patrol ≈15,000 bp based on its steady-state abundance (≈200,000 per nucleus), and the number of base pairs in the genome (3 × 109) (3). Therefore, extrapolating from the in vitro studies, genomic uracils would be expected to be removed in a time frame of seconds.
Intramolecular Transfer Mechanisms of Enzymes.
In recent years several studies have revealed key insights into the nature of intramolecular DNA transfer by enzymes, which can be compared with the current findings with UNG. The dimeric restriction enzyme EcoRV was found to use both hopping and scanning to locate its 6-bp target sequence employing methods analogous to those used here (9, 10). In this case, the size of the landing site was estimated as 50 bp, which is five times larger than the 10-bp site size for UNG. In addition, 40% of the EcoRV molecules were found to dissociate before encountering a site ≈200 bp away (T = 37°C, 25 mM NaCl) (10), whereas 50% of UNG molecules dissociate when the separation is 30 bp (Fig. 4B). The larger landing site size for EcoRV likely reflects the increased probability of finding a 6-bp palindromic recognition sequence, as opposed to locating a single extrahelical base in the case of UNG. In addition, the greater DNA interaction surface of the EcoRV dimer would lead to a longer residence time on nontarget DNA as compared with UNG and a greater time window for sliding (30, 31). A single molecule study of the enzyme 8-oxoguanine DNA glycosylase (hOGG1) indicated that this enzyme slides over ≈400 bp of DNA before dissociation (25°C, 0.1 M NaCl) (32). However, hopping events were not observed because these experiments were performed by using flow conditions to stretch the DNA, and the time resolution of the single-molecule methodology was not sufficient to detect rapid association–dissociation events. The results of these three studies differ only in the relative amounts of sliding and hopping that occur, and suggest that hopping and short-range sliding over ≈10–100 bp will predominate in the intramolecular site location mechanisms of many, if not all enzymes.
Materials and Methods
Enzymes.
The purifications of E. coli UNG and human apurinic/apyrimidinic endonuclease (APE1) were described in refs. 33 and 34. All enzymes, with the exception of Exonuclease V (USB), shrimp alkaline phosphatase (Roche), and PfuUltraHigh-Fidelity DNA Polymerase (Stratagene) were purchased from New England Biolabs.
Oligonucleotides and DNA Substrate Synthesis.
In general, all oligonucleotides were purchased from Integrated DNA Technologies. A detailed protocol for the synthesis of substrates containing two uracil sites is included in the SI Methods.
Intramolecular Transfer Measurements and Analysis.
A detailed protocol is included in the SI Methods. In brief, end-labeled DNA (20 nM) in reaction buffer [0 mM Na-Hepes (pH 7.5), 0.002% Brij 35, 10 mM NaCl, and 1 mM DTT] was warmed to 37°C before initiation of the reaction with E. coli UNG (final concentration, 5 pM). Samples were removed at appropriate time intervals and UNG was specifically quenched by adding 5 μl of a potent inhibitory oligonucleotide (50 nM) and then chilling the quenched sample on ice. Discrete double-stranded DNA fragments were generated by specifically nicking each strand of the product with human abasic endonuclease (Ape1) and N. BbvCIB restriction enzyme. The nicking reaction was terminated with EDTA (38 mM) and the DNA fragments were resolved by electrophoresis by using a native 4% polyacrylamide gel in 1× TBE buffer. The gels were exposed to a phosphorimaging plate (Fujifilm), scanned by using a Fujifilm BAS-2500 phosphorpmager, and the DNA bands were quantified. The apparent fintra values were calculated as described in SI Methods, plotted as a function of time, and fitted to a second-order polynomial equation.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant GM56834 (to J.T.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801612105/DCSupplemental.
References
- 1.Stivers JT. Extrahelical damaged base recognition by DNA glycosylase enzymes. Chemistry. 2008;14:786–793. doi: 10.1002/chem.200701501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slupphaug G, et al. A nucleotide-flipping mechanism from the structure of human uracil-DNA glycosylase bound to DNA [see comments] Nature. 1996;384:87–92. doi: 10.1038/384087a0. [DOI] [PubMed] [Google Scholar]
- 3.Stivers JT, Jiang YL. A mechanistic perspective on the chemistry of DNA repair glycosylases. Chem Rev. 2003;103:2729–2759. doi: 10.1021/cr010219b. [DOI] [PubMed] [Google Scholar]
- 4.Parker JB, et al. Enzymatic capture of an extrahelical thymine in the search for uracil in DNA. Nature. 2007;449:433–438. doi: 10.1038/nature06131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halford SE, Marko JF. How do site-specific DNA-binding proteins find their targets? Nucleic Acids Res. 2004;32:3040–3052. doi: 10.1093/nar/gkh624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg OG, Winter RB, von Hippel PH. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981;20:6929–6948. doi: 10.1021/bi00527a028. [DOI] [PubMed] [Google Scholar]
- 7.von Hippel PH, Berg OG. Facilitated target location in biological systems. J Biol Chem. 1989;264:675–678. [PubMed] [Google Scholar]
- 8.Daniels DS, et al. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat Struct Mol Biol. 2004;11:714–720. doi: 10.1038/nsmb791. [DOI] [PubMed] [Google Scholar]
- 9.Gowers DM, Wilson GG, Halford SE. Measurement of the contributions of 1D and 3D pathways to the translocation of a protein along DNA. Proc Natl Acad Sci USA. 2005;102:15883–15888. doi: 10.1073/pnas.0505378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanford NP, Szczelkun MD, Marko JF, Halford SE. One- and three-dimensional pathways for proteins to reach specific DNA sites. EMBO J. 2000;19:6546–6557. doi: 10.1093/emboj/19.23.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg HC. Random Walks in Biology. Princeton, NJ: Princeton Univ Press; 1993. [Google Scholar]
- 12.Kavli B, Otterlei M, Slupphaug G, Krokan HE. Uracil in DNA-general mutagen, but normal intermediate in acquired immunity. DNA Repair (Amst) 2007;6:509–516. doi: 10.1016/j.dnarep.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Bennett SE, Sanderson RJ, Mosbaugh DW. Processivity of escherichia coli and rat liver mitochondrial uracil-DNA glycosylase is affected by NaCl concentration. Biochemistry. 1995;34:6109–6119. doi: 10.1021/bi00018a014. [DOI] [PubMed] [Google Scholar]
- 14.Purmal AA, et al. Uracil DNA N-glycosylase distributively interacts with duplex polynucleotides containing repeating units of either TGGCCAAGCU or TGGCCAAGCTTGGCCAAGCU. J Biol Chem. 1994;269:22046–22053. [PubMed] [Google Scholar]
- 15.Wang H, Hays JB. Simple and rapid preparation of gapped plasmid DNA for incorporation of oligomers containing specific DNA lesions. Mol Biotechnol. 2001;19:133–140. doi: 10.1385/MB:19:2:133. [DOI] [PubMed] [Google Scholar]
- 16.Terry BJ, Jack WE, Modrich P. Facilitated diffusion during catalysis by EcoRI endonuclease. nonspecific interactions in EcoRI catalysis. J Biol Chem. 1985;260:13130–13137. [PubMed] [Google Scholar]
- 17.Terry BJ, Jack WE, Modrich P. Mechanism of specific site location and DNA cleavage by EcoR I endonuclease. Gene Amplif Anal. 1987;5:103–118. [PubMed] [Google Scholar]
- 18.Krosky DJ, Song F, Stivers JT. The origins of high-affinity enzyme binding to an extrahelical DNA base. Biochemistry. 2005;44:5949–5959. doi: 10.1021/bi050084u. [DOI] [PubMed] [Google Scholar]
- 19.Krosky DJ, Schwarz FP, Stivers JT. Linear free energy correlations for enzymatic base flipping: How do damaged base pairs facilitate specific recognition? Biochemistry. 2004;43:4188–4195. doi: 10.1021/bi036303y. [DOI] [PubMed] [Google Scholar]
- 20.Parikh SS, et al. Uracil-DNA glycosylase-DNA substrate and product structures: Conformational strain promotes catalytic efficiency by coupled stereoelectronic effects. Proc Natl Acad Sci USA. 2000;97:5083–5088. doi: 10.1073/pnas.97.10.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidorenko VS, Mechetin GV, Nevinsky GA, Zharkov DO. Correlated cleavage of single- and double-stranded substrates by uracil-DNA glycosylase. FEBS Lett. 2008;582:410–414. doi: 10.1016/j.febslet.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Rose IA. Substrate trapping expt. Methods Enzymol. 1995;249:315–340. doi: 10.1016/0076-6879(95)49040-x. [DOI] [PubMed] [Google Scholar]
- 23.Baumann CG, Smith SB, Bloomfield VA, Bustamante C. Ionic effects on the elasticity of single DNA molecules. Proc Natl Acad Sci USA. 1997;94:6185–6190. doi: 10.1073/pnas.94.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang MD, Yin H, Landick R, Gelles J, Block SM. Stretching DNA with optical tweezers. Biophys J. 1997;72:1335–1346. doi: 10.1016/S0006-3495(97)78780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao C, Jiang YL, Krosky DJ, Stivers JT. The catalytic power of uracil DNA glycosylase in the opening of thymine base pairs. J Am Chem Soc. 2006;128:13034–13035. doi: 10.1021/ja062978n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao C, Jiang YL, Stivers JT, Song F. Dynamic opening of DNA during the enzymatic search for a damaged base. Nat Struct Mol Biol. 2004;11:1230–1236. doi: 10.1038/nsmb864. [DOI] [PubMed] [Google Scholar]
- 27.Leijon M, Leroy JL. Internal motions of nucleic acid structures and the determination of base-pair lifetimes. Biochimie. 1997;79:775–779. doi: 10.1016/s0300-9084(97)86936-2. [DOI] [PubMed] [Google Scholar]
- 28.Moe JG, Russu IM. Kinetics and energetics of base-pair opening in 5′-d(CGCGAATTCGCG)-3′ and a substituted dodecamer containing G. T mismatches. Biochemistry. 1992;31:8421–8428. doi: 10.1021/bi00151a005. [DOI] [PubMed] [Google Scholar]
- 29.Kavli B, et al. Excision of cytosine and thymine from DNA by mutants of human uracil-DNA glycosylase. EMBO J. 1996;15:3442–3447. [PMC free article] [PubMed] [Google Scholar]
- 30.Erskine SG, Halford SE. Reactions of the EcoRV restriction endonuclease with fluorescent oligodeoxynucleotides: Identical equilibrium constants for binding to specific and non-specific DNA. J Mol Biol. 1998;275:759–772. doi: 10.1006/jmbi.1997.1517. [DOI] [PubMed] [Google Scholar]
- 31.Winkler FK, et al. The crystal structure of EcoRV endonuclease and of its complexes with cognate and non-cognate DNA fragments. EMBO J. 1993;12:1781–1795. doi: 10.2210/pdb4rve/pdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blainey PC, van Oijen AM, Banerjee A, Verdine GL, Xie XS. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc Natl Acad Sci USA. 2006;103:5752–5757. doi: 10.1073/pnas.0509723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao G, et al. Crystal structure of Escherichia coli uracil DNA glycosylase and its complexes with uracil and glycerol: Structure and glycosylase mechanism revisited. Proteins. 1999;35:13–24. [PubMed] [Google Scholar]
- 34.Gorman MA, et al. The crystal structure of the human DNA repair endonuclease HAP1 suggests the recognition of extra-helical deoxyribose at DNA abasic sites. EMBO J. 1997;16:6548–6558. doi: 10.1093/emboj/16.21.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazur AK. Evaluation of elastic properties of atomistic DNA models. Biophys J. 2006;91:4507–4518. doi: 10.1529/biophysj.106.091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.