Abstract
The discovery of ghrelin O-acyltransferase (GOAT) opens the way to the design of drugs that block the attachment of an octanoyl group to the appetite-stimulating peptide hormone ghrelin, potentially preventing obesity. Here, we develop a biochemical assay that uses membranes from insect cells infected with baculovirus encoding mouse GOAT. The GOAT-containing membranes transferred the [3H]octanoyl group from [3H]octanoyl CoA to recombinant proghrelin in vitro. Transfer depended on the serine at residue 3 of proghrelin, which is the known site of acylation. GOAT also transferred [3H]octanoyl to a pentapeptide containing only the N-terminal five amino acids of proghrelin. GOAT activity could be inhibited by an octanoylated ghrelin pentapeptide, and its potency was enhanced 45-fold when the octanoylated serine-3 was replaced by octanoylated diaminopropionic acid. The data suggest that GOAT is subjected to end-product inhibition and this inhibition is better achieved with substrates having the octanoyl group attached through an amide linkage rather than the corresponding ester. These insights may facilitate the future design of useful inhibitors of GOAT.
Keywords: appetite control, in vitro, inhibitors, obesity, octanoylation of proghrelin
Ghrelin O-acyltransferase (GOAT) is the membrane-bound enzyme that attaches eight-carbon octanoate to a serine residue in ghrelin, a peptide hormone (1, 2). Ghrelin comprises the N-terminal 28 amino acids of proghrelin, a precursor of 94 amino acids that is cleaved proteolytically to release ghrelin. The octanoylated serine is the third amino acid from the N terminus of ghrelin, and its presence is essential for the physiologic actions of ghrelin in stimulating appetite and other neuroendocrine functions (3).
Most ghrelin is produced in a minor population of endocrine cells in the gastric mucosa, originally called X/A-like cells and now called ghrelin cells. After secretion into plasma, octanoylated ghrelin travels to the pituitary where it binds to a G protein-coupled receptor that triggers the release of growth hormone (3). The plasma concentration of ghrelin rises immediately before meals (4), and the octanoylated peptide enhances food intake when administered to rats (5) and humans (6–9). Inactivation of the gene encoding ghrelin or its receptor produces a modest resistance to obesity in mice that are presented with a high-fat diet (10, 11). These observations led to the hypothesis that interference with ghrelin action might protect against obesity in humans (8, 12).
One way to inhibit ghrelin action would be to inhibit GOAT. The enzyme is an attractive target because ghrelin is the only protein known to be octanoylated. Hence, GOAT inhibition is likely to alter only one protein. GOAT was first identified by transfection of candidate cDNAs into cultured endocrine cells that process proghrelin to ghrelin but fail to attach octanoyl (1). With these transfection assays, the enzyme attached octanoyl to the appropriate serine-3 in ghrelin, and the attachment reaction was shown to require the two amino acids in GOAT that are conserved in other membrane-bound O-acyltransferases (1, 2). GOAT is a highly hydrophobic protein with eight postulated membrane-spanning helices. It is presumed to be located in the endoplasmic reticulum where proghrelin is initially inserted (1).
Thus far, the action of GOAT has been studied only in intact cells (1, 2). In the current studies, we have established a biochemical assay for GOAT activity by using membranes from insect cells that express mouse GOAT as a result of infection with a recombinant baculovirus. Using purified recombinant proghrelin as a substrate, we characterized the enzymatic properties of GOAT, and we showed that the enzyme is potently inhibited by octanoylated peptides that comprise the first five amino acids of ghrelin. These five amino acids appear to constitute the substrate recognition site. These findings open the way to the design of small-molecule GOAT inhibitors.
Results
Establishment of in Vitro Assay for GOAT.
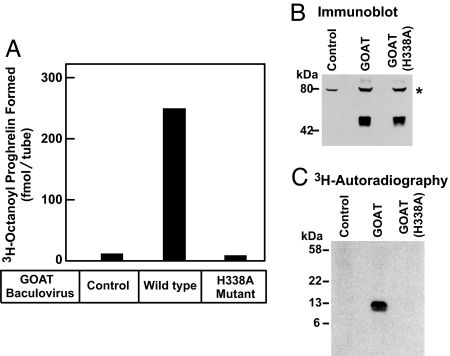
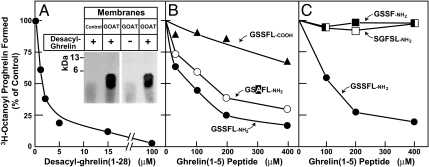
To establish a biochemical assay for GOAT activity, we infected Sf9 insect cells with a recombinant baculovirus encoding mouse GOAT with a His10 tag at the N terminus. The cells were disrupted with a Dounce homogenizer, and a crude membrane fraction was isolated by ultracentrifugation. Recombinant mouse proghrelin with eight histidines at the C terminus was prepared in Escherichia coli as described in Experimental Procedures. The GOAT-containing membranes were incubated with proghrelin-His8 in the presence of [3H]octanoyl CoA, and the octanoylated proghrelin formed in the reaction was isolated by adherence to nickel-coated beads. As shown in Fig. 1 A and B, membranes from uninfected Sf9 cells failed to transfer significant amounts of [3H]octanoyl to proghrelin. Abundant transfer was seen with membranes from cells expressing wild-type GOAT but not comparable amounts of the H338A mutant version. This mutation substitutes alanine for histidine-338, an essential catalytic residue that is required for GOAT-mediated octanoylation of proghrelin in transfected INS-1 cells (1). To confirm the attachment of [3H]octanoyl to proghrelin, we precipitated the proteins at the end of the reaction in Fig. 1A and subjected them to SDS/PAGE followed by autoradiography. The 3H-radioactivity was incorporated into a band with an apparent molecular mass of 12 kDa corresponding to proghrelin-His8 (Fig. 1C).
Fig. 1.
Establishment of in vitro octanoylation assay. (A) GOAT activity in membranes from Sf9 insect cells. On day 0, Sf9 cells were set up for experiments and infected on day 1 with baculovirus encoding wild-type GOAT or the H338A-mutant GOAT, both containing a N-terminal His10 tag. After infection for 48 h, the 100,000 × g membrane fraction from the uninfected cells (control) and the infected cells were prepared as described in Experimental Procedures. Each 50-μl reaction mixture contained 50 μg of membrane protein from the indicated cells, 5 μg of proghrelin-His8, 50 μg of myristyl ether CoA, and 1 μM [3H]octanoyl CoA (11 dpm/fmol). After incubation for 5 min at 37°C, the amount of [3H]octanoyl transferred to proghrelin-His8 was quantified by nickel chromatography and scintillation counting as described in Experimental Procedures. Each value represents the average of triplicate assays. (B) Immunoblot analysis of GOAT expressed in Sf9 cells. Aliquots of the membranes used in A (75 μg of protein) were subjected to immunoblot analysis with 1 μg/ml monoclonal anti-His antibody. The asterisk denotes an irrelevant cross-reacting band present in the membranes of Sf9 cells. Film was exposed for 10 s. (C) Autoradiography of [3H]octanoyl proghrelin-His8 formed in the in vitro assay. The reaction products from replicate assays in A were precipitated with 80% acetone, loaded onto 16% Tricine SDS/PAGE, transferred to a PVDF membrane, and subjected to autoradiography for 5 days as described in SI Experimental Procedures.
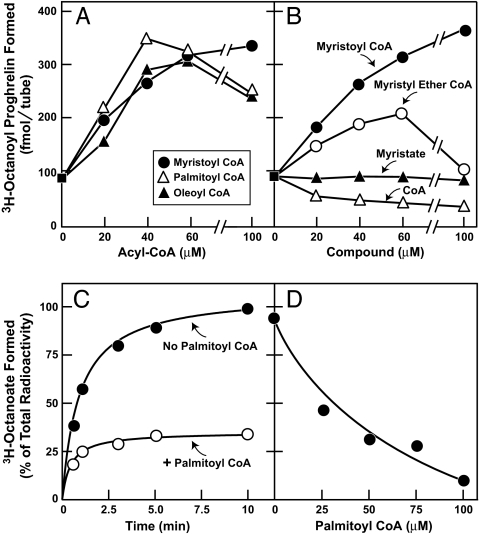
In preliminary experiments, we noted that GOAT activity was enhanced when we included long-chain fatty acyl CoA adducts in the assay. The reaction was stimulated equally by myristoyl CoA (14 carbons), palmitoyl CoA (16 carbons), or oleoyl CoA (18 carbons) (Fig. 2A). The reaction was also stimulated by myristyl ether CoA, but not by free myristate. The addition of free CoA partially inhibited the reaction (Fig. 2B), possibly because it competes for the octanoyl CoA-binding site on GOAT.
Fig. 2.
Action of long-chain acyl-CoAs in stimulating GOAT activity (A and B) by inhibiting deacylation of [3H]octanoyl CoA (C and D). Membranes from Sf9 cells infected with baculovirus encoding His10-GOAT were prepared as in Fig. 1A. Each 50-μl reaction mixture contained 50 μg of membrane protein, 5 μg of proghrelin-His8, 1 μM [3H]octanoyl CoA (11 dpm/fmol), and the indicated compound. (A and B) After incubation for 5 min at 37°C, the amount of [3H]octanoyl transferred to proghrelin-His8 was quantified by nickel chromatography and scintillation counting. Each value represents the average of duplicate assays. (C and D) After incubation at 37°C for the indicated time (C) or 5 min (D), the amount of [3H]octanoate formed in the reaction was quantified by TLC analysis as described in SI Experimental Procedures.
The explanation for the apparent stimulation of GOAT activity by fatty acyl CoAs became apparent when we measured the amount of [3H]octanoyl CoA that was cleaved to release [3H]octanoate during the incubation (Fig. 2 C and D). This cleavage is caused most likely by deacylases that are present in the GOAT-containing crude membrane preparation. In experiments not shown, we observed virtually no deacylation of [3H]octanoyl CoA when membranes were omitted from the reaction. To measure this deacylation, we incubated [3H]octanoyl CoA with the GOAT-containing membranes in the standard reaction and used TLC to separate [3H]octanoate from residual [3H]octanoyl CoA. In the absence of palmitoyl CoA, >90% of [3H]octanoyl CoA was converted to [3H]octanoate within 5 min. In the presence of 50 μM palmitoyl CoA, <30% of [3H]octanoyl CoA was cleaved (Fig. 2C). At 5 min, the amount of [3H]octanoate formed in the reaction declined as a function of the concentration of palmitoyl CoA (Fig. 2D). Therefore, all further assays were conducted in the presence of 50 μM palmitoyl CoA.
Under the conditions of this assay, the formation of [3H]octanoyl proghrelin was linear with time for 5 min (Fig. 3A). The reaction was not fully linear with the amount of GOAT-containing Sf9 membranes (Fig. 3B), possibly caused by the presence of deacylases and other competing reactions. The reaction showed saturation kinetics with respect to the concentration of the substrates [3H]octanoyl CoA (Fig. 3C) and proghrelin (Fig. 3D). The apparent Km values were 0.6 and 6 μM, respectively.
Fig. 3.
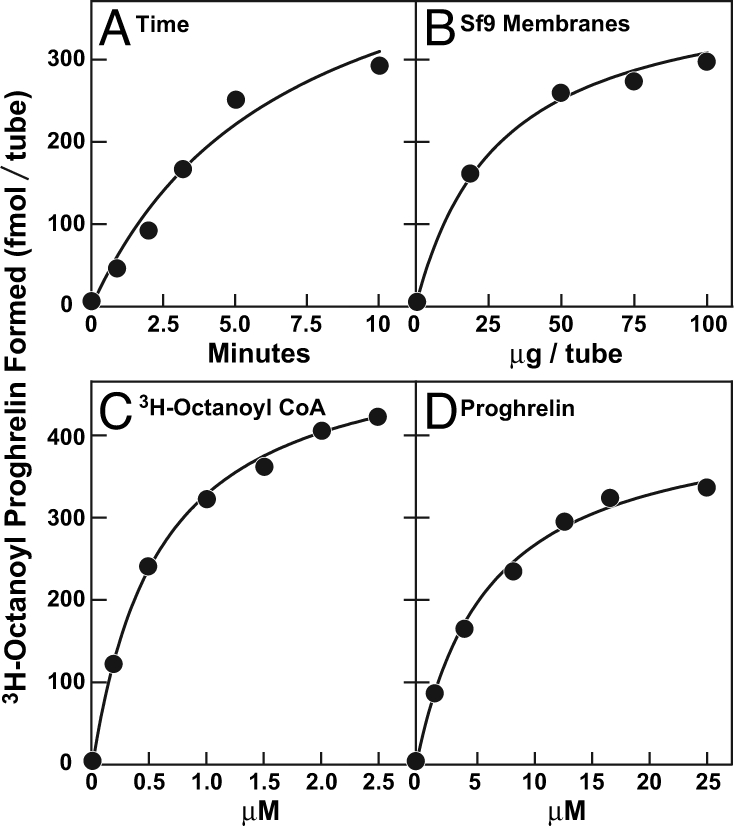
Characterization of GOAT activity in vitro. Membranes from Sf9 cells infected with baculovirus encoding His10-GOAT were prepared as in Fig. 1A. Unless otherwise indicated, each 50-μl reaction mixture contained 50 μg of membrane protein, 5 μg (424,000 fmol) of proghrelin-His8, 50 μM palmitoyl CoA, and 1 μM [3H]octanoyl CoA (50,000 fmol, 11 dpm/fmol). After incubation for 5 min at 37°C, the amount of [3H]octanoyl attached to proghrelin-His8 was quantified by nickel chromatography. (A) Time course. (B) Concentration curve of membrane protein. (C) Concentration curve of [3H]octanoyl CoA. (D) Concentration curve of proghrelin-His8. Each value represents the average of duplicate assays.
When assays were carried out at various pH values between 6.0 and 8.0, the highest rate of the reaction was at pH 7.0 in buffers containing either 50 mM Tris-Cl or Hepes-NaOH. The following additions to the assay had no effect on GOAT activity: 1 mM MgCl2, 1 mM CaCl2, 150 mM NaCl, 150 mM KCl, 400 mM sucrose, 1 mM sodium EDTA, 1 mM DTT, and 1 mM N-ethylmaleimide (data not shown).
GOAT Recognizes N-Terminal Sequence of Ghrelin.
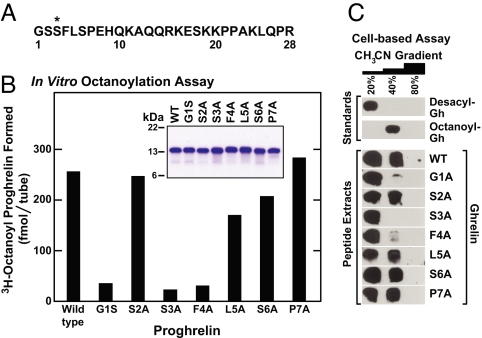
To test the sequence requirements for substrate recognition by GOAT, we prepared recombinant proghrelin substrates with a variety of substitution mutations in the region of the octanoylated serine (Fig. 4A). As expected, replacement of serine-3 with alanine nearly eliminated [3H]octanoyl transfer, whereas replacement of the adjacent serine-2 had no such effect (Fig. 4B). Replacement of the N-terminal glycine with serine markedly reduced the transfer reaction, as did the phenylalanine-to-alanine substitution at residue 4. These results implicate glycine-1, serine-3, and phenylalanine-4 as components of the recognition sequence for GOAT. A much smaller effect was seen when alanines were substituted for leucine-5 or serine-6, and no inhibitory effect was seen when proline-7 was replaced (Fig. 4B).
Fig. 4.
Identification of amino acids in proghrelin required for octanoylation. (A) Amino acid sequence of the ghrelin portion of mouse proghrelin. The asterisk denotes the octanoylated serine-3 residue. (B) Octanoylation of mutant proghrelins in vitro. Membranes from Sf9 cells infected with baculovirus encoding mouse His10-GOAT were prepared as described in Fig. 1A. Each 50-μl reaction mixture contained 50 μg of membrane protein, 50 μM palmitoyl CoA, 1 μM [3H]octanoyl CoA (11 dpm/fmol), and 5 μg of wild-type proghrelin-His8 or the indicated mutant version. Each value represents the average of duplicate assays. (B Inset) SDS/PAGE of purified wild-type and single amino acid mutants of proghrelin-His8. Each recombinant protein (5 μg) was subjected to 16% Tricine SDS/PAGE and visualized by Coomassie blue staining. (C) Octanoylation of mutant ghrelins in INS-1 cells. On day 0, 100-mm dishes of INS-1 cells were set up for experiments as described in Experimental Procedures. On day 2, the cells were cotransfected with 5 μg of pCMV-preproghrelin or its indicated mutant version and 0.1 μg of pCMV-GOAT. On day 4, each dish received a direct addition of 50 μM octanoate-albumin (final concentration). On day 5, three dishes of cells from each transfection were harvested and pooled, after which peptides were extracted from the cells, fractionated on reverse-phase chromatography, and subjected to immunoblot analysis with 1:1,000 dilution of rabbit anti-ghrelin antibody as described in Experimental Procedures. Film was exposed for 1 min.
The sequence requirements of the in vitro octanoylation reaction were essentially identical to those observed when GOAT was studied in a cell-based assay. To make these measurements, we used INS-1 cells, a line of rat insulinoma cells that were shown to process preproghrelin to acylated ghrelin when cotransfected with cDNAs encoding GOAT and preproghrelin (1). Octanoyl-ghrelin was separated from desacyl-ghrelin by elution from a reverse-phase column with 40% acetonitrile. As shown in Fig. 4C, wild-type ghrelin was octanoylated, but the reaction was blocked when glycine-1, serine-3, and phenylalanine-4 were each replaced with alanine. Replacement of serine-2, leucine-5, serine-6, and proline-7 had no effect.
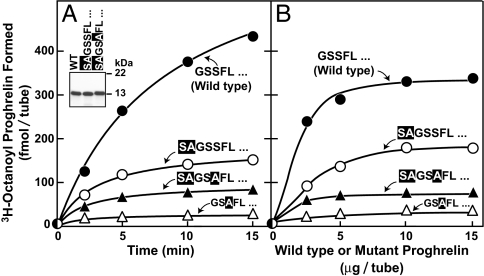
We next sought to determine whether an N-terminal extension of proghrelin would affect its octanoylation in the in vitro assay (Fig. 5). Addition of two extra amino acids (SA) at the N terminus of proghrelin-His8 markedly reduced its susceptibility to octanoylation, and the reaction was reduced further when the extended sequence bore a substitution of alanine for the serine corresponding to serine-3 in wild-type ghrelin (SAGSAFL…) (Fig. 5A). The residual acylation of the SAGSAFL… mutant was actually higher than that observed when serine-3 was replaced with alanine in the absence of the N-terminal extension (GSAFL… ) (Fig. 5A), suggesting the possibility that serine-4 in the mutant (corresponding to serine-2 in wild-type ghrelin) becomes the site of this residual octanoylation. All of the above decreases in GOAT-activity appeared to reduce the Vmax of the reaction rather than the Km (Fig. 5B).
Fig. 5.
Free N-terminal glycine residue of proghrelin is required for optimal GOAT activity. Membranes from Sf9 cells infected with baculovirus encoding His-10-GOAT were prepared as in Fig. 1A. Each 50-μl reaction mixture contained 50 μg of membrane protein, 5 μg of the indicated wild-type or mutant proghrelin-His8, 50 μM palmitoyl CoA, and 1 μM [3H]octanoyl CoA (11 dpm/fmol). After incubation at 37°C for the indicated time (A) or for 5 min (B), the amount of [3H]octanoyl attached to proghrelin-His8 was quantified by nickel chromatography. Each value represents the average of duplicate assays. Mutant residues in proghrelin are shaded. (A Inset) SDS/PAGE of purified recombinant wild-type or mutant versions of proghrelin-His-8. Each recombinant protein (5 μg) was subjected to 16% Tricine SDS/PAGE and visualized by Coomassie blue staining.
In addition to octanoylating proghrelin, GOAT also attached [3H]octanoyl to synthetic desacyl-ghrelin (1–28) in vitro as determined by SDS/PAGE and autoradiography (Fig. 6A Inset). When increasing amounts of desacyl-ghrelin (1–28) were added to the assay in the presence of proghrelin-His-8, 50% inhibition of octanoylation was achieved at a desacyl-ghrelin (1–28) concentration of 2 μM (Fig. 6A).
Fig. 6.
Ability of desacyl-ghrelin peptides to compete for octanoylation of proghrelin. Membranes from Sf9 cells infected with baculovirus encoding mouse His-10-GOAT were prepared as described in Fig. 1A. Each 50-μl reaction mixture contained 50 μg of membrane protein, 5 μg of proghrelin-His8, 50 μM palmitoyl CoA, 1 μM [3H]octanoyl CoA (11 dpm/fmol), and the indicated concentration of peptide. Each value represents the average of duplicate assays. The “100% of control values,” which represent the amount of [3H]octanoyl proghrelin formed in the absence of peptide, were 278 (A), 247 (B), and 244 (C) fmol per tube. (A Inset) Autoradiography of [3H]octanoyl ghrelin (1–28) formed in the in vitro assay. The reaction contained 50 μg of membrane protein from either uninfected Sf9 cells (control) or cells infected with baculovirus encoding GOAT as indicated, 50 μM palmitoyl CoA, 1 μM [3H]octanoyl CoA (11 dpm/fmol), and 20 μM desacyl-ghrelin (1–28) as indicated. After incubation for 5 min at 37°C, the samples were processed for autoradiography as in Fig. 1C, except that the film was exposed for 7 days.
Inhibition of GOAT by Ghrelin Pentapeptides.
A peptide corresponding to the first five amino acids of ghrelin (GSSFL-COOH) was a relatively weak inhibitor of the reaction (Fig. 6B). The potency was increased markedly when the C terminus was amidated (GSSFL-NH2) with 50% inhibition achieved at 80 μM. This amidated peptide retained most of its inhibitory activity even when serine-3 was changed to alanine (GSAFL-NH2) (Fig. 6B). In contrast, neither the amidated tetrapeptide GSSF-NH2 nor a scrambled amidated pentapeptide (SGFSL-NH2) showed any inhibitory activity (Fig. 6C).
We next sought to determine whether GOAT would transfer [3H]octanoyl to the ghrelin pentapeptide. For this purpose, we incubated the GOAT-containing membranes with [3H]octanoyl CoA and a saturating concentration of GSSFL-NH2. At the end of the incubation, we added unlabeled authentic octanoyl-GSSFL-NH2 peptide as a carrier, purified the radiolabeled peptide by reverse phase chromatography, and then subjected it to thin layer chromatography [supporting information (SI) Fig. S1]. The spot containing the octanoylated peptide was subjected to scintillation counting. In two independent experiments (Experiments A and B), GOAT transferred 171 and 209 fmol/tube of [3H]octanoyl to GSSFL-NH2 (Table 1 and Fig. S1). There was no transfer when the membranes were omitted from the reaction or when the peptide contained an alanine substituted for serine-3 (GSAFL-NH2). In Experiment A, we measured in parallel tubes the transfer of [3H]octanoyl to a saturating concentration of proghrelin by the standard nickel chromatography assay and found that 310 fmol per tube was transferred. We conclude that the amidated ghrelin pentapeptide is nearly as good a substrate as proghrelin in the GOAT assay.
Table 1.
Transfer of [3H]octanoyl from [3H]octanoyl CoA to GSSFL-NH2, a pentapeptide corresponding to the first five amino acids of ghrelin
| Additions to assay |
[3H]Octanoyl transferred, fmol per tube |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tube | Membranes | Wild-type GSSFL-NH2 | Mutant GSAFL-NH2 | Wild-type proghrelin | Experiment A | Experiment B | |||
| 1 | − | + | − | 18 | 14 | ||||
| 2 | + | − | − | 28 | 20 | ||||
| 3 | + | + | − | 171 | 209 | ||||
| 4 | + | − | + | 23 | 28 | ||||
| 5 | + | − | 8 | ||||||
| 6 | + | + | 310 | ||||||
Each 50-μl reaction mixture contained 50 μM palmitoyl CoA, 1 μM [3H]octanoyl CoA (11 dpm/fmol), and (where indicated) 50 μg membrane protein, 500 μM of the indicated peptide, or 8 μM proghrelin-His8. After incubation at 37°C for 10 min, each reaction mixture was subjected to either TLC analysis (tubes 1–4) or nickel chromatography assay (tubes 5 and 6) as described in SI Experimental Procedures. The complete TLC profile for Exp. A (tubes 1–4) is shown in Fig. S1.
Potent Inhibition of GOAT by Octanoylated [Dap3]-Ghrelin.
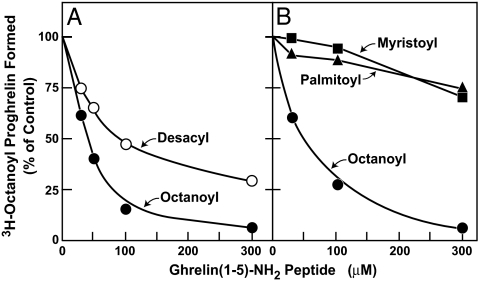
Fig. 7A shows an experiment designed to determine whether octanoylation of ghrelin (1–5)-NH2 would increase its potency as a GOAT inhibitor. When serine-3 in GSSFL-NH2 was octanoylated, its ability to inhibit GOAT increased relative to that of the desacyl-ghrelin (1–5)-NH2 peptide (50% inhibition at 45 μM vs. 80 μM, respectively). Substitution of 14-carbon myristoyl or 16-carbon palmitoyl for the octanoyl group eliminated the inhibitory activity of ghrelin (1–5)-NH2 (Fig. 7B).
Fig. 7.
Inhibition of GOAT activity by octanoyl-ghrelin (1–5)-NH2 peptide. Membranes from Sf9 cells infected with baculovirus encoding mouse His10-GOAT were prepared as described in Fig. 1A. Each 50-μl reaction mixture contained 50 μg of membrane protein, 5 μg of proghrelin-His8, 50 μM palmitoyl CoA, 1 μM [3H]octanoyl CoA (11 dpm/fmol), and the indicated concentration of peptide in a final concentration of 3% (vol/vol) DMSO. Each value represents the average of duplicate assays. The 100% of control values, which represent the amount of [3H]octanoyl proghrelin formed in the absence of peptide, were 205 fmol per tube (A) and 214 fmol per tube (B).
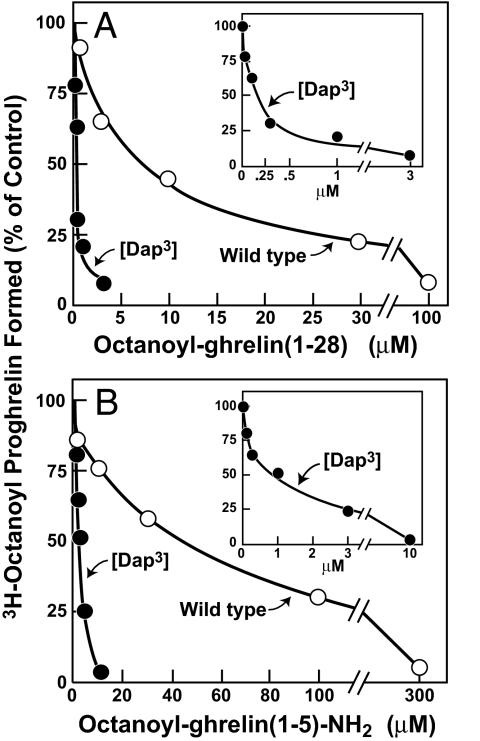
Fig. 8 shows that inhibition of GOAT is markedly enhanced when the octanoyl group is attached to ghrelin through an amide linkage rather than its less stable ester counterpart. We obtained a synthetic ghrelin (1–28) in which serine-3 was replaced by (S)-2,3-diaminopropionic acid (Dap) and where the distal amine of this residue was octanoylated. Fig. 8A shows that octanoylated [Dap3]-ghrelin (1–28) was 35-fold more potent than octanoylated wild-type ghrelin (1–28) in blocking GOAT activity in vitro (50% inhibition at 0.2 μM vs. 7 μM, respectively). A 45-fold increased potency was also observed when the octanoylated GSSFL (1–5)-NH2 peptide contained Dap in place of serine at residue 3 (50% inhibition at 1 μM vs. 45 μM, respectively).
Fig. 8.
Inhibition of GOAT activity by [Dap3]octanoyl-ghrelin (1–28) (A) and [Dap3]octanoyl-ghrelin (1–5)-NH2 (B). Assays were carried out as described in the legend to Fig. 7 except for the presence of different ghrelin peptides as indicated. [Dap3] denotes the substitution of a (S)-2,3-diaminopropionic acid residue for serine-3 in ghrelin (1–28) and ghrelin (1–5)-NH2. The insets show an expanded scale for the concentration curve of [Dap3]octanoyl-ghrelin (1–28) (A) and [Dap3]octanoyl-ghrelin (1–5)-NH2 (B).
Discussion
In the current studies, we have developed an in vitro biochemical assay for GOAT activity by using membranes from insect cells expressing mouse GOAT and purified recombinant proghrelin as the acyl acceptor. With this assay, we found that GOAT recognizes several amino acids in proghrelin that surround the site of octanoylation at serine-3. The crucial residues are glycine-1, serine-3, and phenylalanine-4. This recognition specificity is consistent with the fact that these three amino acids are absolutely conserved in all vertebrate ghrelins (3). A pentapeptide containing only the N-terminal five amino acids of proghrelin (GSSFL) inhibited the octanoylation of proghrelin in vitro only when its C terminus was amidated. The amidated pentapeptide was itself a substrate for GOAT (Table 1 and Fig. S1). The mutant GSAFL-NH2 pentapeptide also inhibited GOAT even though it is not octanoylated by the enzyme. Thus, the unacylated pentapeptides appeared to inhibit primarily by competing for the peptide binding site and not necessarily by serving as substrates for octanoylation.
Octanoylation of proghrelin was inhibited more efficiently when the amidated pentapeptide contained an octanoyl in ester linkage to serine-3, thereby mimicking the product of the reaction. Supporting the concept of end-product inhibition, we found that peptides containing longer acyl chains, i.e., myristoyl (14 carbons) and palmitoyl (16 carbons), were not potent inhibitors of the enzyme. Of particular importance was the finding that inhibitory activity was significantly increased when full-length ghrelin (1–28) or the amidated ghrelin pentapeptide contained an octanoylated diaminopropionic acid (Dap) in place of the octanoylated serine at residue 3. The increased inhibitory potency of the octanoylated ghrelin peptides with an amide linkage at residue 3, instead of an ester linkage, may help guide the design of potent small molecule inhibitors of GOAT.
The kinetics of the in vitro assay were complex, owing in large part to the use of crude membranes as a source of GOAT. For example, it was necessary to include long-chain acyl CoAs in the assay to prevent deacylation of the [3H]octanoyl CoA substrate. The deacylation of [3H]octanoyl CoA to [3H]octanoate was carried out by contaminating enzymes in the crude insect cell membranes. Precise measurements of kinetic parameters await the solubilization and purification of the enzyme.
An important question raised by our studies relates to the geometry of the acylation reaction with respect to the cytosolic and luminal faces of the membrane. In the cell, proghrelin must be octanoylated in the ER lumen after it is inserted cotranslationally by means of a classic signal sequence at its N terminus. It is likely that octanoylation occurs after the signal sequence is cleaved by signal peptide peptidase. This conclusion is supported by the finding that the addition of even two amino acids (SA) on the N-terminal end of proghrelin markedly reduced its ability to accept an octanoyl from GOAT in vitro. Thus, the active site of GOAT must face the luminal side of the ER membrane. In order for GOAT-containing membrane vesicles to acylate proghrelin in vitro, at least a fraction of the GOAT proteins must be oriented with their active sites facing the extraluminal side of the membrane. We attempted to increase this fraction by sonicating the vesicles, but we did not observe an increase in GOAT activity (data not shown). Moreover, all attempts to solubilize active GOAT from these Sf9 insect cell membranes have failed so far.
Another question relates to the fate of the reaction product, namely, octanoylated proghrelin. In experiments not shown, we found that all of the [3H]octanoyl proghrelin product remained bound to the insect membranes. We do not know whether it was bound specifically to GOAT or whether it was associated nonspecifically with the membranes by virtue of the hydrophobic octanoyl group. The observation that octanoylated ghrelin (1–28) and octanoylated ghrelin pentapeptide inhibit GOAT activity (Figs. 7 and 8) is consistent with the idea that the [3H]octanoyl proghrelin could remain bound to the enzyme, and this may account for the observation that the acylation reaction is linear with time only for ≈5 min. After this time, a locally sufficient concentration of octanoylated proghrelin may have accumulated to inhibit the reaction. If such end-product inhibition occurs in cells, then cells must have some acceptor molecule that removes octanoylated proghrelin from GOAT and allows the reaction to proceed catalytically.
Experimental Procedures
Materials.
We obtained fatty acyl CoAs and myristyl ether CoA (tetradecanyl CoA) from Avanti Polar Lipids; [3H-2,2′,3,3′]octanoyl CoA (60 Ci/mmol) and [3H-2,2′,3,3′]octanoate (60 Ci/mmol) from American Radiolabeled Chemicals; monoclonal anti-His antibody from GE Healthcare; and BSA (essentially fatty-acid free), solvents, and all other chemicals from Sigma unless otherwise specified. All acyl and desacyl versions of ghrelin (1–28) and ghrelin (1–5)-NH2, including [Dap3]octanoyl-ghrelin (1–28) and [Dap3]octanoyl-ghrelin (1–5), were obtained from Peptide International except for GSSFL-NH2, GSSFL-COOH, GSAFL-NH2, GSSF-NH2, and SGFSL-NH2, which were synthesized at the Protein Chemistry Technology Center at the University of Texas Southwestern Medical Center. Stock solutions for all peptides (1 or 10 mM) were made up in water except for palmitoyl-ghrelin (1–5)-NH2, myristoyl-ghrelin (1–5)-NH2, octanoyl-ghrelin (1–5)-NH2, and [Dap3]octanoyl-ghrelin (1–5)-NH2, which were made up in 100% DMSO. Fatty acids were bound to albumin at a final concentration of 5 mM fatty acid and 10% (wt/vol) BSA in 0.15 M NaCl at pH 7.4 as described in ref. 13. Expression vectors for mouse GOAT (pCMV-GOAT), preproghrelin (pCMV-preproghrelin), and various mutant versions were prepared as described in ref. 1.
Purification of Recombinant Mouse Proghrelin.
Mouse proghrelin was encoded as a fusion protein containing GST at the N terminus followed by a Tobacco Etch Virus (TEV) protease cleavage site (ENLYFQG). A His8 epitope tag was included at the C terminus as described in ref. 1. GST-proghrelin-His8 was expressed in E. coli, bound to glutathione-agarose beads, eluted with glutathione, and cleaved by recombinant TEV protease to release proghrelin-His8. The TEV protease cleaves in such a way that the N-terminal sequence of recombinant proghrelin-His8 is identical to that of authentic proghrelin. Proghrelin-His8 was purified by nickel-affinity chromatography (QIAGEN) and dialyzed with a 3,500 molecular weight cut-off (MWCO) dialysis membrane (Pierce) against 10 mM Tris-Cl (pH 8.5), 50 mM NaCl, 10% (vol/vol) glycerol, and 0.01% (wt/vol) CHAPS. The protein was concentrated with a 5,000 MWCO concentrator (Millipore) to a final concentration of 1 or 2 mg/ml and stored at −80°C. Mutant versions of proghrelin-His-8 were generated by site-directed mutagenesis (Stratagene), proteolyzed, and purified as described in ref. 2. The N-terminal sequences of all recombinant proteins were confirmed by Edman degradation.
Cell Culture, Transfection, and Baculoviral Infection.
For baculoviral infection of insect cells, mouse GOAT cDNA was inserted into pFastBac HT-A (His10 tag) (Invitrogen), and baculovirus was generated as described in ref. 14. Sf9 insect cells were cultured, set up on day 0 at a density of 5 × 105 cells per ml, and infected on day 1 with baculovirus encoding His-10-GOAT at a density of 1 × 106 cells per ml as described in ref. 14. Cells were harvested 48 h after infection and washed once with PBS.
Monolayers of rat INS-1 cells were cultured at 37°C in an atmosphere of 8.8% CO2 as described in ref. 1. On day 0, 1.5 × 106 INS-1 cells were plated onto each 100-mm dish in medium A (RPMI medium 1640 supplemented with 10 mM Hepes, 50 μM β-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin) containing 10% (vol/vol) FCS. On day 2, cells were transfected with plasmids by using FuGENE HD Transfection Reagent (Roche) according to the manufacturer's instructions. The total amount of transfected DNA in each experiment was constant and adjusted to 6 μg per 100-mm dish by the addition of pcDNA 3.1 vector. On day 3, the medium was switched to fresh medium containing 10% FCS. On day 5, cells were harvested for experiments.
In Vitro Octanoylation Assay.
Each pellet of Sf9 cells (obtained from 1 liter of cell culture) was disrupted with a Dounce homogenizer (40 strokes) on ice in 50 ml of buffer containing 50 mM Tris-Cl (pH 7.0), 150 mM NaCl, 1 mM sodium EDTA, 1 mM DTT, 100 μM bis (4-nitrophenyl) phosphate, 2.5 μg/ml aprotinin, 20 μg/ml phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A. After an initial centrifugation at 3,000 × g for 5 min at 4°C, the supernatant was further centrifuged at 100,000 × g for 30 min at 4°C to obtain a membrane fraction, which was stored at −80°C until the time of assay. Just before assay, the membranes were suspended in 50 mM Hepes-NaOH (pH 7.0) and then passed through a 22-gauge needle 10 times. After centrifugation at 1,000 × g for 1 min to remove aggregated materials, the supernatant (hereafter referred as membranes) was used for assays.
Unless otherwise stated, the standard assay mixture contained 50 mM Hepes-NaOH (pH 7.0), 50 μM palmitoyl CoA or myristyl ether CoA, 50 μg membrane protein, 5 μg recombinant wild-type or mutant versions of proghrelin-His-8 and 1 μM [3H]octanoyl CoA (11 dpm/fmol) in a final volume of 50 μl. After incubation at 37°C for 5 min, each reaction was stopped by the addition of 1 ml of buffer A [50 mM Tris-Cl at pH 7.5, 150 mM NaCl, and 0.1% (wt/vol) SDS], after which each sample was loaded onto a 0.2-ml nickel-affinity column (QIAGEN). The columns were washed at room temperature with 1 ml of buffer A followed by 3 ml of buffer A containing 25 mM imidazole. The His-tagged proghrelin was then eluted with 1 ml of buffer A containing 250 mM imidazole. Radioactivity present in the 250 mM imidazole eluate was quantified by liquid scintillation counting as described in ref. 1. Blank values were determined in parallel 50-μl reactions containing all components except proghrelin-His-8. These values ranged from 6 to 10 fmol per tube and were subtracted from all data points shown in the figures.
Other Experimental Procedures.
Procedures for TLC, 3H-autoradiography, reverse-phase chromatography, and immunoblot analysis are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments.
We thank our colleagues Arun Radhakrishnan, Hyock Kwon, and Patrick Harran for helpful suggestions; Patrick Harran for critical review of the manuscript; and Lisa Beatty for invaluable assistance with tissue culture. This work was supported by National Institutes of Health Grant HL20948 and a grant from the Perot Family Foundation.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. XM_001476434 and EU721729).
This article contains supporting information online at www.pnas.org/cgi/content/full/0805353105/DCSupplemental.
References
- 1.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endoc Metab. 2008;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamegai J, et al. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 6.Wren AM, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endoc Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 7.Small CJ, Bloom SR. Gut hormones and the control of appetite. Trends Endocrin Metabolism. 2004;15:259–263. doi: 10.1016/j.tem.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE. Ghrelin and short- and long-term regulation of appetite and body weight. Physio Behavior. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 10.Wortley KE, et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zigman JM, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zigman JM, Elmquist JK. In search of an effective obesity treatment: A shot in the dark or a shot in the arm? Proc Natl Acad Sci USA. 2006;103:12961–12962. doi: 10.1073/pnas.0605959103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate SREBP isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem. 2001;276:4365–4372. doi: 10.1074/jbc.M007273200. [DOI] [PubMed] [Google Scholar]
- 14.Radhakrishnan A, Sun L-P, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: Mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.