Abstract
Neural variability in responding to identical repeated stimuli has been related to trial-by-trial fluctuations in ongoing activity, yet the neural and perceptual consequences of these fluctuations remain poorly understood. Using functional neuroimaging, we recorded brain activity in subjects who reported perceptual decisions on an ambiguous figure, Rubin's vase-faces picture, which was briefly presented at variable intervals of ≥20 s. Prestimulus activity in the fusiform face area, a cortical region preferentially responding to faces, was higher when subjects subsequently perceived faces instead of the vase. This finding suggests that endogenous variations in prestimulus neuronal activity biased subsequent perceptual inference. Furnishing evidence that evoked sensory responses, we then went on to show that the pre- and poststimulus activity interact in a nonlinear way and the ensuing perceptual decisions depend upon the prestimulus context in which they occur.
Keywords: fusiform face area, ongoing activity, BOLD fMRI, prestimulus activity, visual perception
Since the earliest neurophysiological recordings, two issues have puzzled brain researchers: why do, trial by trial, cortical responses to identical stimuli vary so much (1), and what is the functional significance of spontaneous neural activity, i.e., activity that cannot be accounted for by the experimental manipulation and that is thus usually discarded as unexplained variance? The two issues have in part been tied together by relating response variability to fluctuations in ongoing prestimulus activity (2, 3). In anesthetized animals, an excellent prediction of the actual response evoked in each trial was achieved by assuming a fixed stimulus-driven or task-related response from averaging and adding, trial by trial, this response to baseline activity (2). Evidence for the perceptual relevance of ongoing neural activity comes from monkey electrophysiology (4) and more recently human imaging studies (5) where periliminal stimuli were perceived when prestimulus activity was at higher levels, thus resembling effects from experimentally instructed allocation of attention. This all-or-none effect of ongoing activity on detection of periliminal stimuli could correspond to the well known behavioral effect of arousal and alertness on perceptual thresholds (6). Here, we address whether ongoing activity only impacts whether something can be perceived or whether it also influences what is perceived. We therefore investigated whether spontaneously occurring cortical activity variations in the seconds before input processing bias subsequent perceptual decisions on a suprathreshold but ambiguous visual input. We then addressed whether observed responses were simply the sum of evoked responses plus the baseline activity or whether baseline activity affected the evoked response, which would indicate an interaction between baseline activity and evoked components).
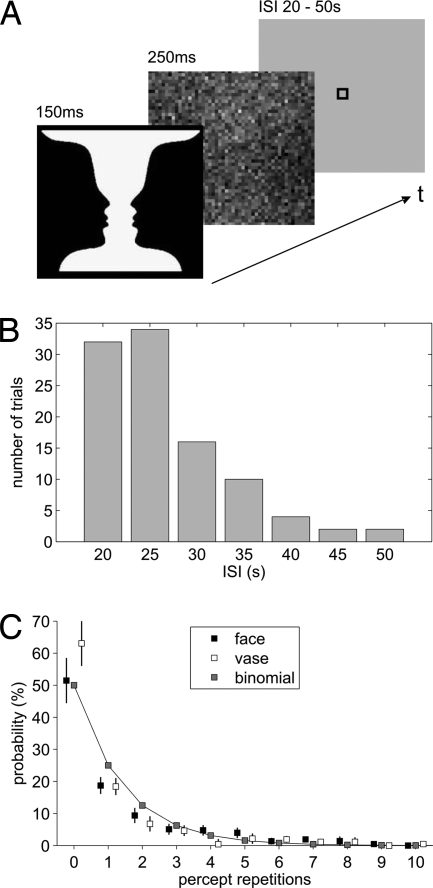
Using functional magnetic resonance imaging (fMRI), we pursued this issue by asking subjects what they perceived each time they were shown the ambiguous vase-faces stimulus introduced by Rubin (7). We chose this stimulus because, different from binocular rivalry, it induces instantaneous dominance of one of the two competing percepts (8) and because faces are arguably associated with the strongest categorical neural selectivity recorded by single-cell studies as well as functional neuroimaging (9, 10). We hoped that this latter property would allow us to read out from local neuroimaging signals a potential bias in favor of one of the two percepts, whereas for the majority of perceptual ambiguities such a signal would be beyond the reach of a noninvasive functional imaging approach in humans. Instead of continuously presenting this stimulus as in previous work (11), we implemented a very sparse event-related fMRI design (Fig. 1) with highly variable and ≥20-s-long interstimulus intervals (ISIs). For each stimulus presentation, we obtained a forced-choice classification for vase or faces. No instruction or reward was associated with reporting one over the other percept or their ratio of overall presentations.
Fig. 1.
Experimental paradigm and analysis of percept repetitions. (A) During fMRI, subjects were repeatedly shown Rubin's ambiguous figure followed by a noise mask. In each trial, subjects reported whether they had perceived a vase or faces. (B) Distribution of ISIs across trials. (C) The incidence of repetitions for either percept averaged across all ISIs can very well be approximated by a binomial distribution (goodness-of-fit R2 = 0.97, for faces percepts, R2 = 0.93, for vase percepts).
Results
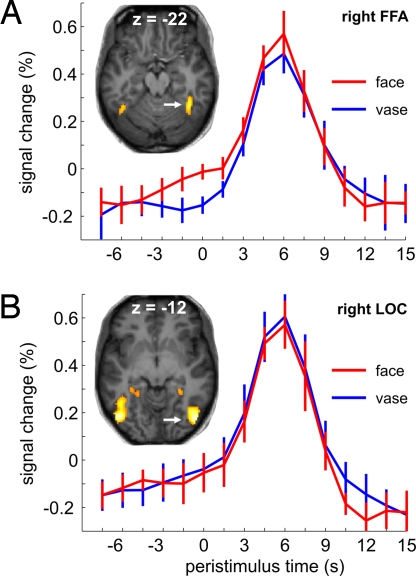
In a group of 12 subjects, we tested the hypothesis that faces percepts compared with vase percepts would be associated with higher ongoing activity levels immediately before stimulus presentation in the fusiform face area (FFA), an extrastriate visual region specialized for face processing (12, 13). We hence localized the FFA on a subject-by-subject basis as a region of interest that responded more to faces than objects in a separate session. We then analyzed local fMRI signal time courses during presentation of the vase-faces ambiguity and assigned trial-by-trial the time-courses to two populations as a function of which of the two percepts had been reported. We tested for percept-dependent differences in two epochs, covering the stimulus-driven response as well as the latest time point of the prestimulus baseline period that could not yet contain stimulus-driven signal. Greater stimulus-driven FFA responses occurred whenever brief stimulus presentation yielded a faces rather than a vase percept (Fig. 2A). This result is in line with previous findings from sustained presentation of the Rubin ambiguity (11, 14, 15), where perception is bistable and periods of face dominance are associated with greater FFA activity.
Fig. 2.
Peristimulus fMRI signal time courses from right FFA (A) and right LOC (B), averaged across subjects as a function of percept. Significant percept-dependent signal differences were found in the right FFA at time points −1.5 s (t11 = 2.88, P = 0.015), 0 s (t11 = 3.34, P = 0.007), and 6 s (t11 = 2.76, P = 0.019) (two-sided paired t tests). Error bars represent ± SEM. The images show, for a single representative subject, the two main regions of interest identified in the localizer experiment.
Confirming our critical hypothesis, we also found a significant right-FFA activity difference of similar magnitude to the stimulus-related effect already present in immediate prestimulus periods and thus related to neural activity several seconds before (Fig. 2A). That we could not obtain in other regions, such as lateral occipital complex (LOC) (Fig. 2B), a significant symmetric result with greater signal in trials with object percepts was to be expected from previous findings with this stimulus (14, 15). Yet, we had two initial concerns regarding our result: spatial and temporal specificity.
We tested spatial specificity by analyzing time courses throughout a large set of control regions. These regions were identified subject-by-subject as those that responded during stimulus presentation and the reported perceptual decision, covering various brain areas that have been shown to be involved in visual perception, attention, and decision making [supporting information (SI) Fig. S1]. None of these occipital, temporal, parietal, or frontal regions showed significant signal deviations between conditions before stimulus presentation, as might have been the case if, for instance, the perceptual decision depended on preceding levels of arousal or selective attention.
We also analyzed time courses from both occipital face-sensitive areas (OFA) where, as in the left FFA, we found nonsignificant trends for a face bias (Fig. S1) similar to the nonsignificant trends for a vase bias in object-sensitive regions. Of note, we are not proposing that the vase-faces ambiguity is fully resolved at a single cortical locus as the FFA. Presumably, the perceptual decision both in face and vase trials involves integration across several regions in the ventral visual stream as well as interaction with higher-order structures (16–19). However, we exploited the categorical selectivity of face-sensitive areas to trace mechanisms by which prestimulus activity in a specialized sensory area translates into a directed bias toward one of the two percepts when performing a perceptual decision on an ambiguous sensory input. That the right FFA should exert a stronger influence on face perception than the left FFA and the fusiform more than the occipital face-sensitive regions is in accord with a large body of findings for the pathology and physiology of face perception (13). In other words, the strength of the readout of a face bias in the prestimulus baseline was directly related to the degree of face sensitivity of these regions.
The effect in the prestimulus epoch is based on a retrospective assignment of single trials to either percept upon subsequent stimulation but it arises during time points that belong to prolonged periods of rest or baseline and that would indiscriminately be modeled as such in a conventional analysis. Sensory input in our experiment was sparse, not cued, included catch trials with upside-down versions of the stimuli, and separated by randomized variable intervals in the range of duration that is commonly used for baseline epochs separating different experimental conditions (Fig. 1B). Accordingly, stimulus presentation resulted in deactivations in brain regions that are known to be more active during such baseline periods (Fig. S2). There was no difference in the prestimulus signal in these regions between subsequent reports of vase or faces percepts. These results suggest that, between stimuli, subjects were in a resting state and that despite the overarching task context, the effect we found arises from task-unrelated spontaneous activity variations in specifically face-sensitive regions (20).
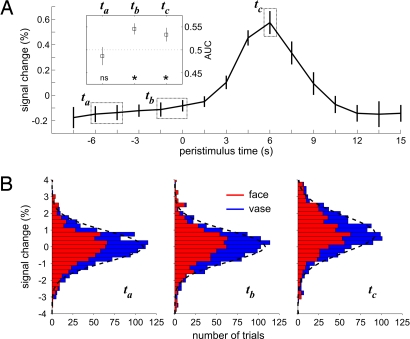
Regarding our second concern, temporal specificity of the effect for the immediate prestimulus period was established by comparing this period with an earlier epoch of baseline activity (Fig. 3). Across all trials and, hence, without considering the association with condition, neural activity at the prestimulus time points where we found the effect on subsequent perception seemed to behave the same way as at an earlier time point during baseline. Yet the association of signal intensity with subsequent perception differed between the two time points. This observation was confirmed by testing the interaction of time point (ta, tb) with condition (vases, faces) across subjects. This interaction was significant (F1,11 = 10.04, P < 0.01) and thus showed that the epoch immediately preceding the stimulus was more predictive than the earlier epoch. The only possible explanation for this observation is that along individual trials sufficient signal changes occurred between these two time points and that these spontaneous fluctuations resulted in the redistribution of condition-dependent signal intensity. Temporal specificity of the effect for the prestimulus epoch was further corroborated by calculating choice probability functions (21) for different time points (Fig. 3A and Fig. S3). These analyses also established that FFA activity from the baseline period that precedes stimulation carries as much information about the upcoming decision as the epoch that contains the evoked response and the subjects' overt report of their decision.
Fig. 3.
Time-dependent analysis of activity levels in right FFA. (A) BOLD signal time course averaged across subjects and percepts. The inserted plot shows average choice probabilities (expressed as AUC) for time points ta, tb, and tc. Asterisks mark choice probabilities significantly larger (P < 0.05, two-sided paired t test) than 0.5 in epochs tb (prestimulus baseline) and tc (peak response), indicating a relationship between higher activity levels and the perceptual decision for faces (individual data shown in Fig. S3). Note that there is no significant correlation between percept and signal at the earlier time point ta. (B) Stacked distributions of activity levels at time points ta, tb, and tc with the proportion of trials leading to faces and vase percepts indicated in red and blue, respectively. Distributions of FFA signal at ta and tb are very similar, but within this distribution face trials are reshuffled to higher signal intensities only at baseline time point tb as well as during the evoked response in tc. Fitted Gaussian distributions are indicated by dashed lines.
Finally, we asked whether stimulus-evoked responses were modulated by or interacted with variations in baseline signal. One simple account of our results, compatible with previous findings (2), would be that higher FFA-response peaks during faces percepts result from elevated prestimulus activity onto which sensory input adds a fixed stimulus-driven amplitude. It can be seen from Fig. 2 that the difference is greatest before stimulus onset, reduces during the intervening few seconds, and then increases again at the peak response. That the difference in activity between vase and faces trials changes over peristimulus time is consistent with a modulation of evoked responses by preceding levels of baseline activity and suggests an interaction between baseline activity and the evoked response. This observation contrasts with a simple additive effect of the evoked component, which would produce faces-vase differences that are conserved throughout peristimulus time.
We tested for this interaction in the following simple way: If there is a linear effect without interaction, then the stimulus-related response should just be superimposed in an additive way onto the baseline (a vertical shift in the response profile associated with the faces vs. vase percept). However, if there is an interaction, the shape of the response should change with baseline. This second possibility was established by an F test from a repeated-measure ANOVA of the scan-specific hemodynamic responses over peristimulus time (time-bins 0–6 s, corresponding to the hemodynamic response slope) and condition (faces vs. vase percepts). This test showed a main effect of condition (F1,11 = 5.27, P = 0.042) and of time (F4,44 = 64.01, P < 0.001) but also an interaction of condition with time that pinpoints nonlinearity in the relation of evoked to ongoing activity (F4,44 = 3.43, P = 0.032). Our analyses of the response waveform hence revealed a picture that differs from a simple mechanistic account. Although on average, both ongoing and evoked FFA-activity levels were correlated with faces perception, they did not reflect a single effect propagated from baseline through to the response peak.
Discussion
Our experiment illustrates neural and perceptual consequences of spontaneous variations in ongoing neural activity, but, as with previous studies on similar issues (5, 22), it cannot clarify their functional origin or cognitive connotation. This uncertainty is in fact a constituting element of any neural activity that is considered spontaneous because it cannot be related to concomitant input or output. The effect we describe arises from ongoing activity because nothing, apart from our post hoc sorting of trials, differentiates activity at the time point where this effect can be shown from that at earlier time points. The effect we describe is necessarily related to spontaneous fluctuations because it varies between trials and cannot be found at earlier prestimulus time points, thus discriminating our paradigm from those with cued attention and induced mind set (23–25).
Current views on the origin of spontaneous activity fluctuations as seen in functional imaging studies propose that these fluctuations group together neural processes occurring at several layers, ranging from intrinsic activity patterns that even persist in sleep or anesthesia (26, 27) to conscious mental processes involving thoughts that in turn can be stimulus-unrelated (mind-wandering) (28) or stimulus-related (context-oriented) (29). Most imaging studies have addressed spontaneous fluctuations by recording brain activity during prolonged periods of rest (29). Although the analytical tools that are used in resting state studies target the detection of spatially distributed patterns (30–32), it is important to note that the effect that we show manifests rather locally. It therefore remains unclear whether the variations in the ongoing activity of FFA signals reported here are akin to slow fluctuations as they have been described in the form of resting-state networks. In our experiment, the perceptual consequences of ongoing activity in face-sensitive areas can be dissociated from that of its cortical neighbors even though all of these areas belong to an overall system that displays coherent activity modulations at rest (33, 34).
The specific question we addressed by our experiment is whether ongoing activity fluctuations impact subsequent perceptual decisions. The effect that we found cannot be predicted or modeled from purely paradigm-related properties or behavioral parameters. A classic evoked-response model of our imaging experiment, as opposed to the “neural history” record we provide, would therefore fail to account for an effect that we discovered to be systematic, to affect evoked response shape, and to reflect future stimulus-driven perception. The two latter aspects highlight that discarding the information content of ongoing activity removes a part of the variance that not only adds onto but also interacts with the stimulus-driven neural and behavioral responses in the explicit experimental paradigm.
Our findings show that ongoing slow activity fluctuations contribute a functionally relevant signal that impacts on how we make up our mind during subsequent perceptual decisions on sensory input (35). Yet, the interaction of perceptual condition with the response shape that we found suggests that more complex mechanisms come into play than a mere additive relation, different from what has been suggested by other recent functional neuroimaging findings in the human motor system (36). Both prestimulus signal and peak response were correlated with perceptual outcome, but this correlation did not arise from a single main effect. In other words, it was not merely a higher baseline signal propagated all of the way to the response peak that decided how the stimulus would be perceived. Instead, the interaction we found points at independent or even complementary contributions to whether individual trials were reported as faces.
This result is in agreement with a well established tenet in models of perceptual decisions according to which two independent sources of variability impact on behavior (37). Whereas one source of variability is linked to the slope with which the accumulation of stimulus-related sensory evidence drifts toward a threshold criterion, the other source of variability is linked to the initial state of the system before sensory input. Our data show that this initial state is subject to fluctuations that are slow enough to be detected by hemodynamic signals and separated from evoked responses. We cannot exclude the presence of more rapid frequencies nor of even slower frequencies in the apparently broad-band dynamics that determine the initial state. However, it is interesting to note that, in electrophysiological studies, the effects from focal electrical microstimulation have never been shown for earlier time periods than those that already contain stimulus-driven signal. One might therefore speculate that microstimulation effects are constrained to that part of the perceptual variability that is driven by response-gain changes. A reason why earlier microstimulation (38) has no effect might be that it provides an input of nonphysiological structure, which in turn would suggest that the effect that we report here is grounded in a meaningful structure that undergoes slow fluctuations. Slow fluctuations are ubiquitous in behavioral data and can be demonstrated with very high trial numbers. Their presence has been linked to a mnemonic mechanism that operates within the mental set and contributes to the formation of representations, i.e., a meaningful structure (39). Variations in the trace of representations could be important in determining how a given sensory input is processed and, accordingly, one of the appealing proposals on the role of spontaneous brain activity fluctuations has been that they might reflect dynamic predictions (29).
What would be the impact of dynamic predictions on subsequent evoked responses? Our observation of a subadditive or attenuated evoked FFA response, in the context of a high baseline activity during face perception, is consistent with models of perceptual inference that relate evoked responses to prediction error or free-energy suppression (40). In these models, prior hypotheses determine the level of prediction error for a given stimulus and more or less well “explain away” bottom-up sensory evidence. In the present context, one might understand the prestimulus FFA signal in terms of an endogenous prior representation for a face that explains away sensory evidence from the ambiguous stimulus, resulting in less prediction error, a bias toward face perception, and a reduced evoked FFA response. In short, high baseline activity suppresses the error-related evoked response, accounting for the interaction that we observed. Conversely, in trials where a faces percept emerges despite a low baseline signal, this would be associated with a strong error signal and thus a high evoked response amplitude. Hence, and in accordance with our experimental observation, both baseline and peak signal would become associated with face perception but because of different mechanisms on individual trials.
The findings of several functional neuroimaging studies in recent years have been interpreted in terms of predictive coding or related models (41, 42), but these studies addressed the relationship between spatially and functionally distinct sites belonging to different levels in the visual processing hierarchy. The experiment reported here illustrates that this top-down framework of prediction can be extended to the dynamical processes that occur over time at a given site of the brain. Regarding these dynamics, it is important to note the presence and functional importance of slow signal components that can be monitored by low-pass filtered hemodynamic recordings of neural activity as provided by fMRI. Our findings hence might present a neurophysiological counterpart of slow fluctuations detected in behavior that have been interpreted as a memory structure in mental set (39). Such time-varying functional properties readily lend themselves to a description by computational models from theoretical physics, for instance self-organized criticality and related frameworks. In these models, dissipative systems (like the brain) generate intrinsic fluctuations that transpire into complex and unpredictable behavior and thus account for unexplained yet functionally relevant variance in neural and behavioral recordings (43).
Materials and Methods
Subjects.
Seventeen subjects (12 female; ages 20–29) gave written informed consent before participation. Data from two subjects were discarded because of excessive fMRI motion artifacts and from another because of failure of the localizer experiment. Two further subjects were excluded from analysis because of an insufficient number (<10/90) of vase or faces percepts, respectively. The remaining 12 subjects (9 female) were right-handed and had normal or corrected-to-normal visual acuity. The principal investigator (A.K.) had ethics committee approval for this study.
Stimuli and Experimental Paradigm.
Stimuli were presented by using Eprime software (Psychology Software Tools) and back-projected from an LCD projector onto a screen attached to the head coil at a viewing distance of ≈20 cm. Target stimuli were 150-ms presentations of the Rubin vase-faces stimulus for 90 trials and of an upside-down version of the Rubin stimulus for 10 catch trials. Stimuli were presented in two sessions of 50 trials each. Targets were followed without any delay by a noise mask of identical overall contrast and 250-ms duration (Fig. 1A). Target and mask subtended ≈14 × 14° of visual angle. Interstimulus intervals between target stimuli ranged unpredictably from 20 to 50 s according to the distribution shown in Fig. 1B. In psychophysical piloting, these settings had been found to provide roughly equivalent frequencies of faces and vase percepts and to prevent perceptual switching within single trials.
In accord with the well known face-inversion effect, we knew from psychophysical piloting that, albeit not impossible, percepts of the upside-down Rubin stimuli as faces would be less likely. Indeed, during scanning, the inverted stimuli yielded vase percepts nearly twice as often as the noninverted stimuli. Because our paradigm precluded determining accuracy values, we considered this increase in vase percepts as behavioral evidence that subjects did not predetermine their perceptual decision but awaited the sensory input for which they could anticipate neither timing nor type.
The only stimulus-related instruction to subjects was to report as quickly and accurately as possible after each presentation by left- or right-hand key presses whether they had perceived a vase or faces, whatever the stimulus (upright or upside-down). Subjects were further instructed to maintain their gaze within the boundaries of a line-drawn box of ≈1° side length that was centered on a medium gray background screen throughout the entire session.
Behavioral Data Analysis.
The absolute frequencies of vase and faces responses were calculated separately for target stimuli and catch trials. For each response category and each subject, mean reaction times (RTs) were determined. Even after excluding subjects with trial numbers for one of the percepts that were insufficient for statistical analysis, the remaining 12 subjects still showed considerable interindividual perceptual variability. Overall, the stimulus was perceived approximately as often as a vase or faces across trials and subjects (58% faces, range across subjects 17–74%). The individual vase-faces ratio was consistent across the two sessions. Importantly, RTs were comparable for both percepts (faces: 812 ± 72 ms; vase: 842 ± 75 ms; t11 = 0.88, P = 0.4; two-sided paired t test). We found no carry-over effects between successive trials, presumably because of the brief and masked presentations. The incidence of percept repetitions was very well approximated by a binomial distribution indicating stochastic perceptual decisions and hence independence of successive trials (Fig. 1C). The probability for different percepts in two successive trials (i.e., zero repetition) was ≈50%, and this relation held across the range of ISIs that we implemented. During the training session before the experiment as well as in the debriefing interview after the experiment, no subject reported to have had more than one percept per trial.
Acquisition and Preprocessing of fMRI Data.
Functional images were acquired with a 3T MRI head scanner (Allegra, Siemens) using a T2*-weighted gradient-echo, echo-planar imaging sequence (26 slices, repetition time = 1,500 ms, echo time = 30 ms, FOV 192, voxel size 3 × 3 × 4 mm). We recorded 893 volumes for each of the two experimental sessions and 391 volumes for the localizer. Anatomical images were acquired by using a T1-weighted MPRAGE sequence (144 slices, repetition time = 2,250 ms, echo time = 26 ms, FOV 256, voxel size 1 × 1 × 1 mm).
We used SPM5 (Wellcome Department of Imaging Neuroscience, London) for image preprocessing (realignment, coregistration, normalization to MNI stereotactic space, spatial smoothing) and estimation of the statistical parametric maps. Functional images were smoothed with an isotropic Gaussian kernel of 6 mm full-width-at-half-maximum for single subject and 8 mm for group analyses.
Statistical Analysis of fMRI Signal Time Courses.
After removal of session effects and linear trends, we extracted the time course of percent signal change from peak response voxels identified by our localizer procedures. These single-trial time courses were sorted according to vase and faces percepts (i.e., dependent on subjects' behavioral responses). The onsets of the target stimuli (rounded with respect to a multiple of the repetition time) served as time markers to extract segments of the time course data starting five scans (7.5 s) before target onset and ending 10 scans (15 s) after target presentation. These segments were chosen because they were not affected by preceding or subsequent stimulations even if these occurred at the shortest (20 s) from the range of possible interstimulus intervals.
A 2 × 5 repeated-measures ANOVA (percept × time) on the average of these time course data was performed for time epochs 0–6 s relative to stimulus onset. We used two-sided paired t tests for post hoc comparisons. A further exploratory t test was performed for time point −1.5 s. For display purposes, but not statistical analyses, signal time courses were filtered with a [1 2 1] kernel.
fMRI Localizer Paradigm.
Localizer fMRI sessions were conducted to identify cortical regions that preferentially responded to objects (LOC) and faces (FFA, OFA). As a further ventral visual-control region close to the FFA, a parahippocampal cortex (PHC) region was identified. We used a standard block design with three different categories of stimuli: objects, faces, and scrambled images. Scrambled images were derived from the object and face images by randomly exchanging elements of a 20 × 20 matrix that was superimposed onto the original images. During each block, 12 items of a category were presented (500 ms per stimulus; 500 ms interstimulus intervals). Blocks were separated by 6-s blank periods and each condition was repeated over eight blocks in counterbalanced order. To maintain attention, subjects had to perform a 1-back task, i.e., indicate by a button press when two successive identical stimuli happened at variable time points twice per block.
Definition of Regions of Interest.
Object-sensitive areas were identified for each subject individually by mapping the contrast “objects > scrambled images” at P < 0.001, uncorrected. The local maximum located at the posterior part of activation in the occipito-temporal cortex was defined as LOC. To identify FFA and OFA, we mapped the contrast “faces > objects” at P < 0.001, uncorrected, and located the local maxima in the inferior temporal lobe and occipital lobe, respectively. A region in the PHC was defined by mapping the contrast “objects > faces” masked inclusively by “objects > scrambled” at P < 0.001, uncorrected.
To determine stimulus-driven and decision-related effects, we estimated an event-related model of the main experiment with the following regressors: “vase percept”, “faces percept,” and “catch trial.” For each subject we computed the contrasts of “vase > baseline” and “faces > baseline,” which were then submitted to a conjunction at P < 0.05 family-wise error rate, FWE. These maps were used to identify subject-by-subject activation foci in the right inferior frontal gyrus (IFG) and inferior parietal lobule (IPL) as well as anterior cingulate cortex (ACC), all of which activated during both perceptual choices. Table S1 summarizes the average MNI coordinates of all regions of interest.
Analysis of Choice Probabilities.
We tested the predictive power of the right-FFA signal for the perceptual decision by calculating choice probability functions for different time points (21). Choice probabilities were obtained by sorting trials according to percept and moving a criterion (i.e., threshold) across FFA-activity levels. Probability for a faces and vase trial, respectively, at a given threshold level was obtained for all threshold levels (25 steps between minimal and maximal activity). For each subject, we plotted the resulting probabilities for a faces percept against the corresponding probabilities for a vase percept, so that an area under the curve (AUC) larger than 0.5 indicates a relationship between high activity levels in right FFA and the behavioral report of a faces percept (Fig. S3). Across subjects, the mean AUC for the immediate prestimulus period tb was significantly larger than 0.5 (AUC = 0.55, t11 = 3.75, P < 0.005, paired t test), indicating a link between higher activity levels in the right FFA and the behavioral report of faces percepts (Fig. 3A). A significant result of similar size was obtained when we calculated choice probabilities for the peak response at time point tc (AUC = 0.53, t11 = 2.22, P = 0.048) but not when testing for an earlier time point, ta, in the baseline (AUC = 0.49, t11 = −0.69, not significant).
Supplementary Material
Acknowledgments.
We thank Drs. Stanislas Dehaene, Richard Frackowiak, and Anne-Lise Giraud for helpful comments on an earlier version of the manuscript. This study was funded by the Volkswagen Foundation, Germany, and the Agence Nationale de la Recherche (ANR), France.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712043105/DCSupplemental.
References
- 1.Schiller PH, Finlay BL, Volman SF. Short-term response variability of monkey striate neurons. Brain Res. 1976;105:347–349. doi: 10.1016/0006-8993(76)90432-7. [DOI] [PubMed] [Google Scholar]
- 2.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 3.Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- 4.Super H, van der Togt C, Spekreijse H, Lamme VA. Internal state of monkey primary visual cortex (V1) predicts figure-ground perception. J Neurosci. 2003;23:3407–3414. doi: 10.1523/JNEUROSCI.23-08-03407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boly M, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ergenoglu T, et al. Alpha rhythm of the EEG modulates visual detection performance in humans. Cogn Brain Res. 2004;20:376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Rubin E. Synsoplevede Figurer. Copenhagen: Gyldendalske Boghandel; 1915. [Google Scholar]
- 8.Leopold DA, Logothetis NK. Multistable phenomena: Changing views in perception. Trends Cogn Sci. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- 9.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- 11.Kleinschmidt A, Büchel C, Zeki S, Frackowiak RS. Human brain activity during spontaneously reversing perception of ambiguous figures. Proc R Soc London B. 1998;265:2427–2433. doi: 10.1098/rspb.1998.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- 13.Kleinschmidt A, Cohen L. The neural bases of prosopagnosia and pure alexia: Recent insights from functional neuroimaging. Curr Opin Neurol. 2006;19:386–391. doi: 10.1097/01.wco.0000236619.89710.ee. [DOI] [PubMed] [Google Scholar]
- 14.Hasson U, Hendler T, Ben Bashat D, Malach R. Vase or face? A neural correlate of shape-selective grouping processes in the human brain. J Cognit Neurosci. 2001;13:744–753. doi: 10.1162/08989290152541412. [DOI] [PubMed] [Google Scholar]
- 15.Andrews TJ, Schluppeck D, Homfray D, Matthews P, Blakemore C. Activity in the fusiform gyrus predicts conscious perception of Rubin's vase-face illusion. NeuroImage. 2002;17:890–901. [PubMed] [Google Scholar]
- 16.Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 17.Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 18.Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 19.Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- 20.Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 21.Dodd JV, Krug K, Cumming BG, Parker AJ. Perceptually bistable three-dimensional figures evoke high choice probabilities in cortical area MT. J Neurosci. 2001;21:4809–4821. doi: 10.1523/JNEUROSCI.21-13-04809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Sapir A, d'Avossa G, McAvoy M, Shulman GL, Corbetta M. Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci USA. 2005;102:17810–17815. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- 25.Summerfield C, et al. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314:1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- 26.Fukunaga M, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–88. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 28.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 30.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echoplanar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 33.Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. NeuroImage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Golland Y, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- 35.Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27:161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 37.Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: A timing diagram. J Neurosci. 2006;26:8965–8975. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- 39.Gilden DL. Cognitive emissions of 1/f noise. Psychol Rev. 2001;108:33–56. doi: 10.1037/0033-295x.108.1.33. [DOI] [PubMed] [Google Scholar]
- 40.Friston KJ. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinschmidt A, Büchel C, Hutton C, Friston KJ, Frackowiak RS. The neural structures expressing perceptual hysteresis in visual letter recognition. Neuron. 2002;34:659–666. doi: 10.1016/s0896-6273(02)00694-3. [DOI] [PubMed] [Google Scholar]
- 42.Murray SO, Kersten D, Olshausen BA, Schrater P, Woods DL. Shape perception reduces activity in human primary visual cortex. Proc Natl Acad Sci USA. 2002;99:15164–15169. doi: 10.1073/pnas.192579399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner G. Metastability, criticality and phase transitions in brain and its models. BioSystems. 2007;90:496–508. doi: 10.1016/j.biosystems.2006.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.