Abstract
Latency-associated transcript (LAT) sequences regulate herpes simplex virus (HSV) latency and reactivation from sensory neurons. We found a HSV-2 LAT-related microRNA (miRNA) designated miR-I in transfected and infected cells in vitro and in acutely and latently infected ganglia of guinea pigs in vivo. miR-I is also expressed in human sacral dorsal root ganglia latently infected with HSV-2. miR-I is expressed under the LAT promoter in vivo in infected sensory ganglia. We also predicted and identified a HSV-1 LAT exon-2 viral miRNA in a location similar to miR-I, implying a conserved mechanism in these closely related viruses. In transfected and infected cells, miR-I reduces expression of ICP34.5, a key viral neurovirulence factor. We hypothesize that miR-I may modulate the outcome of viral infection in the peripheral nervous system by functioning as a molecular switch for ICP34.5 expression.
Keywords: HSV, latency-associated transcript, latency, reactivation, human
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) are closely related human herpes viruses. HSV-1 and HSV-2 establish lifelong incurable latency in and reactivate preferentially from trigeminal ganglia and dorsal root ganglia to cause oro-facial and genital herpes, respectively. Although infections are usually mild, these viruses can cause severe disease including encephalitis and neonatal herpes, and HSV-2 infection is a risk factor for HIV acquisition. The only readily detectable viral transcript during latency of both HSV-1 and HSV-2 is the noncoding latency-associated transcript (LAT), which is transcribed from within the long repeats of the viral genome (Fig. 1) (1). A ≈8-kb primary LAT is spliced, yielding a stable ≈2-kb LAT intron (2). Deletion of the LAT promoter in both HSV-1 and HSV-2 reduces the efficiency of reactivation (3–5), and substitution of HSV-1 LAT for native HSV-2 LAT sequences confers an HSV-1 reactivation phenotype (6). The HSV-1 LAT is currently believed to act in part by increasing the establishment or maintenance of latency, likely via an effect on the survival of acutely infected neurons (7), which may be mediated by inhibition of apoptosis in infected neurons (8). The molecular function of HSV-2 LAT remains largely unknown.
Fig. 1.
HSV-2 LAT region encodes a miRNA. (A) Schematic diagram of HSV-2 LAT region and HSV-2 miR-I. Restriction endonucleases used to create mutant viruses and plasmids are labeled. Stable RNAs including primary LAT, the LAT intron, ICP0, ICP34.5, ICP4, ORF-O (putative), and ORF-P (putative) are labeled based on their relative transcription-starting sites and transcription direction. The location of a TATA box in the LAT intron, which is mutated in the ΔYAT virus, is also labeled. The HSV-2 mature miRNA (bold) was identified by small-RNA cloning and maps to HSV-2 LAT exon 2 (nucleotides 569–547 and 126681–126703). The predicted anti-sense strand of miR-I is shown in italics. ≈50% of miR-I sequences cloned had one additional “C” at their 3′ end. The arrows indicate Dicer cutting sites. Mutant HSV-2 viruses and HSV-2 LAT plasmids are also shown. TRL, terminal repeat long; IRL, internal repeat long; IRS, internal repeat short; US, unique short; TRS, terminal repeat short; UL, unique long. The open boxes on ICP0 and ICP34.5 represent introns. (B) HSV-2 miR-I detection by Northern blot in 293 and HeLa cells transfected with plasmids containing full-length LAT gene but not with truncated LAT plasmids. Total RNAs from HEK 293 cells and HeLa cells transfected with or without plasmids pSSK and pCMV-SSK, which include the ICP34.5 coding region, and plasmids pSSB and pCMV-SSB, which are truncated and lack the ICP34.5 region of LAT, were blotted with 32P-labeled oligo probe for miR-I. The same membrane was stripped and reprobed with an oligonucleotide probe for the predicted anti-sense strand of miR-I and a probe for U6 small nuclear RNA (snRNA). (C) Mature miR-I was significantly reduced, but pre-miRNA increased in Dicer exon-5 disrupted cells. Wild-type (WT) and Dicer exon-5 disrupted cells (Dicer−/−) were studied with or without HSV-2 strain HG52 infection. Total RNAs were hybridized with the HSV-2 miR-I probe and the U6 probe. (D) LAT promoter is not the sole promoter for HSV-2 miR-I production in infected cell cultures. Vero cells were infected with HSV-2 strain HG52, HSV-2 strain 333, and HSV-2 mutant viruses including ΔLAT, CMV-LAT, ΔYAT, and ΔNot-SalI at 0.1 multiplicity of infection or mock-infected. miR-I, the anti-sense strand of miR-I, and U6 snRNA were detected by Northern hybridization after stripping the same membrane. (E) The sequences directly upstream of miR-I contribute to miR-I expression but to a lesser extent than LAT promoter sequences in transfected cells. HEK 293 cells and HeLa cells were transfected with pSSK and pPstI-HincII, which does not contain the LAT promoter region but contains ≈3 kb of sequence upstream of miR-I, or mock-transfected. Total RNA from these transfected cells were hybridized with HSV-2 miR-I and U6 snRNA probes.
miRNAs are a family of 21≈24-nt noncoding RNAs that regulate gene expression based on sequence similarity to their targets. Mammalian viruses including EBV, Kaposi's sarcoma-associated herpesvirus, human cytomegalovirus, and SV40 encode viral miRNAs (9). Viral encoded miRNAs were predicted for HSV-1 (10, 11) and also for HSV-2 (10). However, no miRNAs have been identified in HSV-2 or HSV-1 LAT sequences.
Results
HSV-2 LAT Exon 2 Encodes a miRNA.
To find miRNAs within the HSV-2 LAT region, a plasmid containing the LAT sequence and its promoter (pSSK) and a mutant plasmid expressing LAT under control of the CMV-IE promoter (pCMV-SSK) were transfected into 293 cells. Small RNAs isolated from the transfected cells were cloned and sequenced. A 22–23-nt HSV-2 RNA sequence (designated HSV-2 miR-I) appeared at a frequency comparable to total endogenous cellular miRNAs [supporting information (SI) Table S1]. This sequence matched a sequence in LAT exon 2, anti-sense to the viral PKR inhibitor, and neurovirulence factor ICP34.5 (12) (Fig. 1A). The sequence of miR-I represents one strand of the base of a stem-loop structure. The miR-I sequence is conserved in both HSV-2 strains for which sequence in this region is available, 333 and HG52.
We used Northern hybridization of total RNAs from transfected 293 and HeLa cells to further study miR-I (Fig. 1B). Both mature miR-I (22–23 nt) and its pre-miRNA were detected with a miR-I-specific probe in cells transfected with pSSK and pCMV-SSK, but not in cells with corresponding plasmids pSSB or pCMV-SSB, which are truncated and lack the miR-I-coding sequence. Consistent with the cloning results, cells transfected with pCMV-SSK produced more miR-I than those transfected with pSSK. Only the pre-miRNA was detected with a probe for the anti-sense strand of miR-I, indicating that miR-I is assembled into RNAi-induced silencing complex (RISC) and that the strand complementary to miR-I is degraded after unwinding.
miR-I Is Expressed in Infected Cell Cultures and Maturation of miR-I Is Dependent on Dicer.
To determine whether miR-I is expressed in productive HSV-2 infection of cell culture and if miR-I production depends on Dicer, Dicer exon-5 disrupted cell lines HCT116 Dicer−/− and RKO Dicer−/− and their corresponding wild-type cell lines were infected with HSV-2 strain HG52. In both Dicer-disrupted cell lines, ≈70% of mature cellular miRNA expression is inhibited (13). miR-I was detected with a miR-I-specific probe in both HSV-2-infected wild-type cell lines, indicating that miR-I is produced in infected cell culture. However, expression of mature miR-I in the HSV-2-infected Dicer-disrupted cells was dramatically inhibited, whereas pre-miRNA accumulated to a much higher level as compared with infected wild-type control cells (Fig. 1C), demonstrating that maturation of miR-I depends on Dicer.
HSV-2 miR-I Is Expressed from the LAT and Other Promoters During Productive Viral Infection in Vitro.
To study transcriptional regulation of miR-I in infected cell culture, Vero cells were infected with HSV-2 wild-type virus strains 333 and HG52 and a series of mutant viruses bearing mutations in the LAT promoter and LAT intron region. All of the wild-type and mutant viruses expressed miR-I, implying that none of the mutated sequences alone are required for miR-I expression in productive infection (Fig. 1D). The LAT-promoter-deletion mutant ΔNot-SalI expressed a reduced level of miR-I (Fig. S1). Deletion of a TATA box within the LAT intron (in ΔYAT) had no obvious effect on miR-I expression. Thus, in productive infection, although the LAT promoter influences miR-I expression, other potential or unidentified promoters also appear to play a role.
The Sequences Immediately Adjacent to miR-I Contribute to miR-I Expression, but to a Lesser Extent than LAT-Promoter-Region Sequences in Transfected Cells.
Because there are ≈7 kb between miR-I and the LAT promoter, we hypothesized that adjacent sequences upstream of miR-I, including the putative ORF-O,P promoter, a pre-α promoter in HSV-1 (14), may also contribute to miR-I expression. To study the contribution of other potential promoter sequences in the absence of other viral gene expression, a transient expression system in transfected cell cultures was used. We detected miR-I in both 293 and HeLa cells transfected with pPstI-HincII (which includes miR-I sequences and ≈3 kb of upstream sequences) (Fig. 1E), implying that sequences more immediately upstream of miR-I can contribute to its expression under some circumstances. In this system, the contribution of the directly upstream sequences to miR-I expression is much less than the farther upstream sequences that contain the LAT promoter (Fig. 1E), suggesting LAT-promoter sequences are responsible for the majority of miR-I expression even in transfected nonneuronal cells.
HSV-2 miR-I Is Highly Expressed in Guinea Pig Dorsal Root Ganglia Latently and Acutely Infected with HSV-2 Wild-Type but Not LAT-Promoter-Deletion Virus.
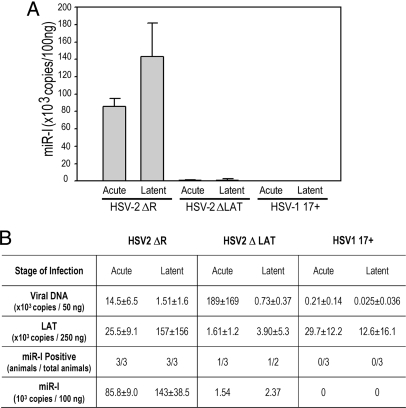
We next studied in vivo miR-I expression in an in vivo model of HSV-2 latency. A Taqman PCR assay was adapted to detect miR-I in RNA isolated from infected guinea pig dorsal root ganglia. The Taqman-PCR miRNA detection system uses a miRNA-specific stem-loop RT primer and miRNA-specific PCR primers to enable the specific detection of the mature 22-nt miR-I in a small amount of RNA sample. Guinea pigs were inoculated intravaginally with ΔLAT (a virus that lacks the LAT promoter and potential downstream enhancer sequences and has an impaired reactivation phenotype in the guinea pig genital model) (5), ΔR (the ΔLAT rescuant, which is genotypically and phenotypically indistinguishable from wild-type HSV-2) (5), or HSV-1. We used the Taqman assay to analyze miR-I in guinea pig ganglia acutely and latently infected with these viruses. miR-I was detected in samples from all animals latently and acutely infected with HSV-2 (Fig. 2). No miR-I was detected in HSV-1-infected ganglia (Fig. 2). A very low amount of miR-I was detected in acutely and latently infected animals in the ΔLAT group, but the level of the signal was nearly 100-fold lower than in wild-type HSV-2 (ΔR)-infected animals, implying that the LAT promoter is primarily responsible for the production of miR-I in sensory ganglia in vivo.
Fig. 2.
HSV-2 miR-I is highly expressed in guinea pig ganglia during latency, and the LAT promoter accounts for production of miR-I in both acutely and latently infected dorsal root ganglia. Total RNA was prepared from dorsal root ganglia of guinea pigs infected with DLAT acutely (n = 3) and latently (n = 2), HSV-2 ΔR (ΔLAT rescuant virus) acutely (n = 3) and latently (n = 3), or with HSV-1 17syn+ acutely (n = 3) and latently (n = 3). miR-I-specific real-time PCR was used to detect miR-I. A synthetic single-stranded miR-I was used as a standard. All animals acutely or latently infected with HSV-2 ΔR showed high levels of miR-I expression. One animal acutely and one animal latently infected with ΔLAT gave very low miR-I signals, and the others were under the detectable level. miR-I copy numbers in different groups are indicated in panel (A). The copy numbers of LAT RNA, virus DNA, and miR-I are shown in panel (B). HSV-1 viral DNA and LAT copies were measured with HSV-1 specific primers and Taqman probes.
miR-I Is Expressed in Human Sacral Dorsal Root Ganglia Latently Infected with HSV-2.
Four coded human autopsy sacral dorsal root ganglia samples (H1, H2, H3, and H4) from males aged 32- to 62-years were studied for miR-I expression by miR-I-specific Taqman PCR. H2 had the highest miR-I expression level, H1 and H3 had lower levels compared to H2, and H4 was miR-I negative (Table 1 and Fig. S2). When the code was broken, it was found that H1 and H3 were from humans seropositive for HSV-2. H4 was from a seronegative patient, and serology information was unavailable for sample H2. H2 had the highest HSV-2 viral-DNA load as determined by Taqman PCR. H1 and H3 had lower HSV-2 DNA loads, and H4 was HSV-2-DNA negative. miR-I detection results were consistent with the serological and viral DNA detection data. These results indicate that miR-I is expressed in human ganglia latently infected with HSV-2 and imply that miR-I likely plays an important role in natural HSV-2 latency and reactivation.
Table 1.
Detection of miR-I in human sacral dorsal root ganglia latently infected with HSV-2
| Clinical samples | H1 | H2 | H3 | H4 |
|---|---|---|---|---|
| miR-I (×103 copies per 100 ng of RNA) | 1.17 | 51.53 | 11.11 | 0 |
| HSV-2 serology | + | n/a | + | − |
| HSV-2 DNA (copies per 500 ng of DNA) | 138 | 808 | 46 | 0 |
| Ganglia tested | RS1 | RS2 | RS1 | LS1 |
n/a, not available; RS, right sacral ganglia; LS, left sacral ganglia.
miR-I Specifically and Efficiently Reduces the Expression of ICP34.5 Through a siRNA Mechanism.
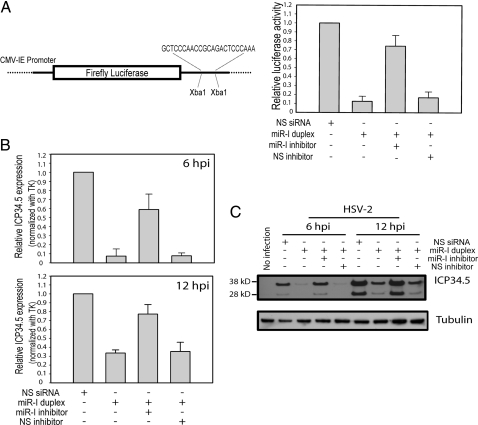
Because miR-I is located on the anti-sense strand of ICP34.5, a key viral neurovirulence factor (15), it is reasonable to hypothesize that ICP34.5 is a natural target of miR-I. To test this hypothesis, we constructed a luciferase reporter with a sequence completely complementary to miR-I cloned downstream of the firefly luciferase gene. Synthetic miR-I duplex efficiently and specifically silenced the expression of this luciferase reporter construct in transfected 293 cells (Fig. 3A) and HeLa cells (data not shown). The silencing of reporter activity was sequence specific and was rescued by a miR-I-specific inhibitor (Fig. 3A). We next tested whether miR-I can reduce the expression of ICP34.5 in infected cell culture. miR-I duplex was transfected into U2OS cells 18 h before infection with HSV-2. Total RNA was collected 6 or 12 h after infection. ICP34.5 was detected by real-time PCR with two PCR primers flanking the miR-I cutting site and a Taqman probe overlapping the miR-I cutting site to guarantee that the assay detected only the full-length ICP34.5 and not miR-I-degraded ICP34.5. miR-I efficiently and specifically silenced ICP34.5 expression [by 93% at 6 h postinfection (hpi) and by 68% at 12 hpi] but not that of thymidine kinase (Fig. 3B). The specific miR-I inhibitor, but not the nonspecific miRNA inhibitor, inhibited the specific silencing. We also used Northern blot to detect HSV-2-ICP34.5 expression. Two major bands corresponding to monocistronic ICP34.5 mRNA (1.4 kb) and bicistronic ICP34.5-ICP0 mRNA (4.4 kb) were detected with ICP34.5 exon-1-specific probes and showed an increased level at 12 hpi compared with 6 hpi (Fig. S3A). In pretransfected cells, miR-I knocked down the ICP34.5 monocistronic mRNA expression level by ≈50% and gave rise to a new band of ≈250 nucleotides, which does not hybridize with downstream ICP34.5-specific sequences (Fig. S3B), implying that this represents a 5′ ICP34.5 degradation product by miR-I-programmed RISC. Use of a HSV-2-ICP34.5-specific antibody to detect ICP34.5 protein showed that ICP34.5 protein expression was also efficiently silenced by miR-I at both 6 hpi and 12 hpi (Fig. 3C).
Fig. 3.
miR-I can efficiently silence target gene expression. (A) HSV-2 miR-I efficiently silences the expression of a firefly luciferase reporter with miR-I target sequence in its 3′ UTR. (Left) Shown is a diagram of the HSV-2 miR-I reporter. (Right) HEK 293 cells were cotransfected with the firefly luciferase reporter plasmid with miR-I target sequence, Renilla luciferase plasmid, and either 2 nM NS-siRNA control or 2 nM miR-I duplex, 2 nM miR-I plus 30 nM miR-I inhibitor, or NS inhibitor. Relative luciferase activity was defined as the ratio of firefly luciferase activity to Renilla luciferase activity. miR-I, but not the nonspecific siRNA control (NS-siRNA), efficiently silenced the target reporter expression, whereas the activity of cotransfected Renilla luciferase was unaffected by miR-I. miR-I-specific inhibitor, but not NS inhibitor, rescued the inhibition of luciferase reporter activity by miR-I. The figure is a summary of four independent experiments. (B) miR-I efficiently knocks down ICP34.5 expression in infected cell culture detected by real-time PCR. Forty nanomolar of miR-I duplex or nonspecific NS-siRNA was transfected with or without 100 nM miR-I inhibitor or NS inhibitor into U2OS cells 18 h before infection with HSV-2 strain 333. After 6 and 12 hpi, total RNAs were extracted, and the uncut ICP34.5, and thymidine kinase (TK) were detected by real-time PCR. Relative ICP34.5 expression was defined as the ratio of ICP34.5 to TK. The figure represents a summary of four independent experiments. (C) miR-I efficiently knocks down ICP34.5 protein expression in infected cell culture. Twenty nanomolar miR-I duplex or nonspecific NS-siRNA was transfected with or without 100 nM miR-I inhibitor or NS inhibitor as described B, and HSV-2 ICP34.5 was detected by Western blot. The same membrane was stripped and reblotted with an anti-β-tubulin antibody as the internal loading control.
HSV-1 LAT Exon-2 Encodes a miRNA in a Location Similar to HSV-2 miR-I.
An HSV-1 sequence located in LAT exon 2 and also overlapping HSV-1 ICP34.5 in an anti-sense direction was found to have 77% homology to miR-I. Together with neighboring sequences, this homologous sequence can be folded into a pre-miRNA-like stem-loop structure. However, we did not detect a mature form of this HSV-1 miR-I homolog, although we detected a pre-miRNA-like signal similar in size to miR-I pre-miRNA (Fig. S1).
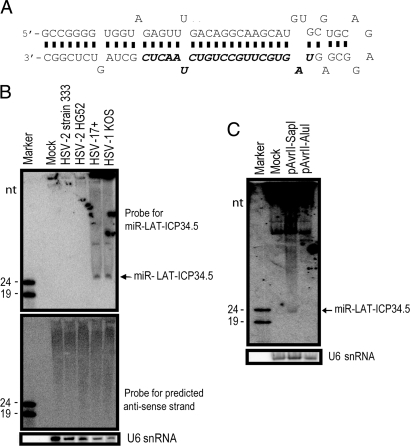
We further screened the HSV-1 LAT-ICP34.5 region for other possible miRNA secondary structures with M-Fold and found another miRNA candidate, which we designated as miR-LAT-ICP34.5 (Fig. 4A). This region was predicted to encode miRNAs (10, 11), but no experimental validation was published. We confirmed the presence of miR-LAT-ICP34.5, but not its anti-sense strand, in HSV-1-infected cells by Northern blot with a miR-LAT-ICP34.5-specific probe (Fig. 4B). miR-LAT-ICP34.5 was also detected in cells transfected with an HSV-1 subclone plasmid, pAvrII-SapI, containing the entire HSV-1 LAT sequence and its promoter but not with pAvrII-AluI, which is truncated and lacks miR-LAT-ICP34.5 sequences (Fig. 4C). The presence of HSV-1 miR-LAT-ICP34.5 indicates that miRNAs encoded by HSV-1 and HSV-2 LATs are evolutionarily conserved in their locations rather than in their sequences, implying that the mechanism by which LAT functions in trans as miRNAs in regulating ICP34.5 expression is shared in HSV-1 and HSV-2.
Fig. 4.
HSV-1 also encodes a miRNA in LAT exon 2, overlapping the anti-sense strand of the ICP34.5 gene. (A) Diagram of HSV-1 miR-LAT-ICP34.5. miR-LAT-ICP34.5 sequence (nucleotides 429–449 and 125942–125922) is shown in bold italics. (B) Detection of HSV-1 miR-LAT-ICP34.5 in HSV-1-infected cells. HSV-1 miR-LAT-ICP34.5 was detected by Northern blot in cells infected with either HSV-1 strains 17syn+ or KOS but not HSV-2 strains. The anti-sense strand of miR-LAT-ICP34.5 was not detected when hybridized with anti-sense strand probe. (C) Detection of miR-LAT-ICP34.5 in cells transfected with HSV-1 LAT plasmids. miR-LAT-ICP34.5 was detected in 293 cells transfected with pAvrII-SapI, which contains the entire HSV-1 LAT promoter and LAT sequences, but not in cells transfected with pAvrII-AluI, which is truncated and lacks miR-LAT-ICP34.5 sequences.
Discussion
Before this report, LAT was the only abundant viral product reported during HSV latency. The mechanism by which LAT sequences influence establishment of and reactivation from latency is poorly understood. In this study, we identify a latently expressed HSV-2 viral miRNA encoded in LAT exon 2. miR-I is a viral miRNA identified in HSV-2. Although highly expressed, miR-I was not previously predicted, although several other miRNA were predicted for HSV-2 (10). Interestingly, the sequence of miR-I has only 53% GC content, but it is embedded in an extremely high (over 90%) GC region. The miRNA is found both in transfected and infected cells in vitro and in acutely and latently infected guinea pigs in vivo. We also detected miR-I in human sacral dorsal root ganglia latently infected with HSV-2. Because humans are the natural host of HSV-2, this finding strongly suggests that miR-I has important biological functions in natural HSV-2 latency. We further showed that the expression of miR-I was controlled by the LAT promoter in a guinea pig latency model and that it reduced expression of ICP34.5 in vitro. A similar miRNA encoded by HSV-1 LAT exon 2 was expressed in HSV-1 infection, suggesting a conserved mechanism.
miR-I was the only mature miRNA that we found in the entire HSV-2 LAT region. The LAT was originally hypothesized to control lytic gene expression via an anti-sense interaction with ICP0 (1). In this experiment, we found no evidence of an HSV-2 LAT-encoded miRNA anti-sense to ICP0. The total number of miR-I clones from the sequenced plasmids almost equaled that of total endogenous cellular miRNAs (Table S1), indicating that miR-I was highly expressed and efficiently processed by Drosha and Dicer. miR-I meets all of the criteria used to identify miRNAs (16). Thus, it is clear that the HSV-2 primary LAT is a primary miRNA gene, which may explain why spliced LAT is very difficult to detect as compared to the LAT intron, even though the LAT promoter is highly active during latency.
Unlike other viral-encoded miRNAs, miR-I is regulated by several different promoters in different stages of the viral life cycle. During productive infection in cell culture and in cell transfection experiments, the LAT promoter is not the sole determinant of miR-I expression. Several other promoters, potentially including those for putative HSV-2 homologs of ORF-O and ORF-P (Fig. 1) (17), could contribute to miR-I expression. The viral transactivator ICP4 represses expression from the HSV-1 LAT (18), ORF-O, and ORF-P (17, 19) promoters, suggesting an additional means by which miR-I expression may be regulated during early infection and reactivation. In ganglia acutely and latently infected with HSV-2, miR-I is primarily expressed from the LAT promoter, which is the only viral promoter known to be highly active during latency (1), and this result also implies that miR-I may play a role in functions previously attributed to the LAT.
We predicted a potential miR-I homolog in HSV-1. We detected the pre-miRNA of an HSV-1 miR-I homolog that is the same size as the HSV-2 miR-I pre-miRNA but were not able to detect the mature form in infected cell culture (Fig. S1). However, we do not rule out the possibility that the HSV-1 miR-I homolog is processed into its mature form during latency in vivo. We predicted and confirmed a HSV-1 miRNA, designated as HSV-1 miR-LAT-ICP34.5, located ≈120 bp downstream of the miR-I homolog and overlapping the anti-sense strand of the 5′ UTR of ICP34.5. This miRNA was identified in HSV-1 LAT sequences. Whether the different nature of viral-encoded miRNAs between HSV-1 and HSV-2 contributes to the differences in behavior between HSV-1 and HSV-2 remains to be studied.
Although there may be additional targets for these miRNAs, the fact that HSV-2 miR-I, the HSV-1 miR-I homolog, and HSV-1 miR-LAT-ICP34.5 are more conserved in location than in sequence suggests that the location of miR-I may be more important than its sequence for its function. We found that miR-I reduces ICP34.5 expression both in reporter systems and in a viral cell-culture infection system (Fig. 3), strongly suggesting that at least one miR-I function is to silence the expression of ICP34.5. ICP34.5 is required for efficient viral replication in neuronal cells in vivo (20). Recent studies indicate that in addition to influencing innate immune responses by inhibiting PKR (21), ICP34.5 may also influence neurovirulence through other mechanisms, such as targeting the Beclin 1 autophagy protein (22). The LD50 of HSV-1 ICP34.5 mutant viruses is reduced as much as 106-fold in the brain (15). ICP34.5-deletion mutants show significantly decreased replication in the human brain (23–25). These data suggest that regulation of ICP34.5 expression could help to control viral replication in neurons, including viral spread among neurons, thus affecting establishment of latency and the likelihood of subsequent reactivation. We hypothesize that control of ICP34.5 expression in individual infected neurons by these LAT-encoded miRNAs affects the outcome (i.e., productive infection vs. latency) of infection in those neurons, either leading to spreading to other neurons or to direct establishment of latency, respectively. Control of ICP34.5 expression may also alter the outcome of viral infection in other cell types, influencing viral spreading. Studies of a miR-I-negative mutant virus that maintains the wild-type ICP34.5-protein-coding sequence will help to further delineate the function of the miRNA in vivo.
Experimental Procedures
Primers, Oligonucleotides Probes, Plasmids, GFP and Luciferase Reporters, Cells, Viruses, Antibodies, and RNA Synthesis.
Please see SI Materials and Methods and Tables S2–S10 for further details. The HSV-2 strain HG52 (GenBank accession no. NC_001798) and HSV-1 strain 17syn+ (GenBank accession no. NC_001806) genome sequences were used as reference sequences. The sense and anti-sense strands of HSV-2 miR-I were chemically synthesized (Dharmacon) and annealed in 1× annealing buffer to make miR-I duplex. 2′-O-methyl modified RNA oligonucleotides completely complementary to the sense strand of miR-I (used as miR-I inhibitor) or completely complementary to HSV-1 miR-LAT-ICP34.5 (used as NS miRNA inhibitor) were also synthesized by Dharmacon. Rabbit polyclonal anti-HSV-2 ICP34.5 antibody was raised against synthetic peptides PGAPAVPRPGAPAVP and SAPAASSLLRRWLLV corresponding to the N-terminal HSV-2 ICP34.5 exon 1 sequence.
Cloning of HSV-2 LAT-Region-Encoded miRNA and Detection of miRNAs by Northern Blot.
pSSK or pCMV-LAT (see Fig. 1A) was transfected into 293 cells by using Lipofectamine 2000 (Invitrogen). Total RNAs were purified at 24 h after transfection. Small RNAs were then gel purified and cloned by using the adapter ligation method (26). Approximately 100 μg of total RNA from transfected or infected cells was separated on a 12% denaturing PAGE gel before transfer to a membrane and hybridization with 32P-labeled oligonucleotide probes as described previously (26).
Genital Infection of Guinea Pigs with HSV.
Female Hartley guinea pigs (250–350 g, Charles River Breeding Laboratories) were inoculated intravaginally with 2 × 105 pfu of HSV-1 strain 17syn+, the HSV-2 LAT-promoter-deletion mutant ΔLAT, or its rescuant HSV-2 ΔR as described previously (27). Lesion severity was similar among the three groups, and recurrence patterns were as expected for these viruses, with reduced recurrence frequency for HSV-1 and ΔLAT viruses compared with wild-type HSV-2. Three animals in each group were killed on day 8 for analysis of acute miR-I expression. For analysis during latency, animals infected with HSV-1 (n = 3) and HSV-2 ΔLAT (n = 2) were killed on day 28, and animals infected with HSV-2 ΔR (n = 3) were killed on day 42 after inoculation. Lumbosacral dorsal root ganglia (L1 through S2) were collected from each animal immediately after death and snap frozen on dry ice.
Human Ganglia and Serum Samples.
Human sacral dorsal root ganglia and blood samples were collected at autopsy. The former were snap frozen on dry ice and stored at −80°C, and the latter were processed to obtain serum. HSV-2 DNA load was quantified by real-time PCR as described previously (28). Titers of species-specific human serum antibodies against HSV-2 were determined as described previously (29).
Detection of miR-I by Taqman Real-Time PCR.
Total RNA from tissues or cells was reverse-transcribed with a miR-I-specific stem-loop reverse transcription primer. A real-time-PCR detection method was established by using a miR-I-specific primer and Taqman probe in an ABI 7900 real-time PCR system (Applied Biosystems). Please see SI Materials and Methods for further details, including Tables S9 and S10.
Detection of HSV-2 ICP34.5 by miR-I Cutting Site-Specific Real-Time PCR.
An HSV-2 ICP34.5 real-time PCR system with primers flanking the miR-I site and a Taqman probe overlapping the miR-I cutting site was set up to detect the full-length ICP34.5 with the One Step RT-PCR kit (Applied Biosystems).
Supplementary Material
Acknowledgments.
We thank all members of Dr. Philip R. Krause's laboratory and Jerry Weir and Nancy Markovitz for critical review. We also thank Dr. Stephen E. Straus for lifelong mentorship and discussions that laid the groundwork for this study. We also thank Dr. Stephen E. Straus for lifelong mentorship and discussions that laid the groundwork for this study. This study was supported by the intramural research programs of the Center for Biologics Evaluation and Research and of the National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801845105/DCSupplemental.
References
- 1.Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 2.Farrell MJ, Dobson AT, Feldman LT. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA. 1991;88:790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner I, et al. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989;8:505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leib DA, et al. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause PR, et al. Expression of the herpes simplex virus type 2 latency-associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J Exp Med. 1995;181:297–306. doi: 10.1084/jem.181.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshikawa T, et al. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend on the latency-associated transcript region. J Exp Med. 1996;184:659–664. doi: 10.1084/jem.184.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson RL, Sawtell NM. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J Virol. 2001;75:6660–6675. doi: 10.1128/JVI.75.14.6660-6675.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perng GC, et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 9.Cullen BR. Viruses and microRNAs. Nat Genet. 2006;38(Suppl):S25–30. doi: 10.1038/ng1793. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer S, et al. Identification of microRNAs of the herpesvirus family. Nat Methods. 2005;2:269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 11.Cui C, et al. Prediction and identification of herpes simplex virus 1-encoded microRNAs. J Virol. 2006;80:5499–5508. doi: 10.1128/JVI.00200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummins JM, et al. The colorectal microRNAome. Proc Natl Acad Sci USA. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohenzky RA, Papavassiliou AG, Gelman IH, Silverstein S. Identification of a promoter mapping within the reiterated sequences that flank the herpes simplex virus type 1 UL region. J Virol. 1993;67:632–642. doi: 10.1128/jvi.67.2.632-642.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 16.Ambros V, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randall G, Lagunoff M, Roizman B. The product of ORF O located within the domain of herpes simplex virus 1 genome transcribed during latent infection binds to and inhibits in vitro binding of infected cell protein 4 to its cognate DNA site. Proc Natl Acad Sci USA. 1997;94:10379–10384. doi: 10.1073/pnas.94.19.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell MJ, Margolis TP, Gomes WA, Feldman LT. Effect of the transcription start region of the herpes simplex virus type 1 latency-associated transcript promoter on expression of productively infected neurons in vivo. J Virol. 1994;68:5337–5343. doi: 10.1128/jvi.68.9.5337-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagunoff M, Roizman B. The regulation of synthesis and properties of the protein product of open reading frame P of the herpes simplex virus 1 genome. J Virol. 1995;69:3615–3623. doi: 10.1128/jvi.69.6.3615-3623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson RL, Stevens JG. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983;131:171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- 21.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orvedahl A, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Rampling R, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 24.Markert JM, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 25.McKie EA, et al. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours—Evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74:745–752. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang S, Tao M, McCoy JP, Jr, Zheng ZM. Short-term induction and long-term suppression of HPV16 oncogene silencing by RNA interference in cervical cancer cells. Oncogene. 2006;25:2094–2104. doi: 10.1038/sj.onc.1209244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertke AS, Patel A, Krause PR. Herpes simplex virus latency-associated transcript (LAT) sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J Virol. 2007;81:6605–6613. doi: 10.1128/JVI.02701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pevenstein SR, et al. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.