Abstract
Changes in organ morphology have been essential to the evolution of novel body forms and in permitting organisms to invade new ecological niches. Changes in the arrangement of cells and tissues and in the regulation of morphological movements are fundamental to evolutionary transitions of organ shape and function. However, little is known about the genetic and developmental control of these changes. We use interspecific differences in the migration and extension of the nematode hermaphrodite gonadal arms to study the generation of morphological novelty. We show that the extending Pristionchus pacificus gonadal arms display a ventral migration that is unique to the Diplogastridae in comparison to the Rhabditidae, including Caenorhabditis elegans, and other nematodes. This results in the distal gonad residing along the ventral side of the body in P. pacificus in contrast to lying on the dorsal side of the body as in C. elegans. We show that at the cellular level this morphogenetic movement is regulated by signals from the developing vulva and the sister gonadal arm. We further show that in P. pacificus Wnt signaling is essential for this regulation. We show genetic and molecular evidence that suggest the Wnt ligands Ppa-mom-2 and Ppa-cwn-2 are components of the signaling mechanism. Supporting these findings, the hermaphrodite gonad of Ppa-bar-1 mutant animals mimics the shape of the C. elegans hermaphrodite gonad; the arms fail to extend ventrally. Thus, this genetic analysis of gonad migration provides insight into the mechanisms underlying the generation of morphological novelty and organ shape.
Keywords: development, gonad, nematode
The evolution of new organ morphologies among extant species had major impacts in the rise of different taxa, speciation, and the colonization of new ecological niches. One example of this is the homology of insect wings, crustacean gills, and spider spinnerets, all of which are likely to have evolved from a common ancestral organ (1, 2). A second example is the modification of the vertebrate heart in progressively more derived vertebrates, i.e., fish to mammal (3, 4). The mammalian heart has more chambers and is more specialized in comparison to that of a more basal vertebrate. It has been hypothesized that the progressive changes to the vertebrate heart contributed to the colonization of land as well as many other ecological expansions. Despite the importance of these evolutionary transitions, the genetic and molecular changes associated with the evolution of different organ morphologies remain largely unknown.
Nematodes and the nematode gonad are ideal for comparative investigations into changes at the genetic and molecular levels that give rise to new morphologies. Nematode systems offer the advantages of complete cellular and genetic analysis with complex yet experimentally tractable anatomical differences. For example, the nematode gonad often comprises the majority of the worm on a cellular basis and a large proportion by volume, making it easy to observe (Fig. 1). Additionally, the gonad exhibits a wide range of morphologies that likely reflects different reproductive strategies (5). Last, the nematode gonad is an excellent system in which to look for genetic and molecular causative changes that result in morphological novelty; the nematode reproductive system, i.e., the gonad and the vulva, is an exemplar of the genetic and cellular control of development and is studied in a number of phenotypically diverse nematode groups (6).
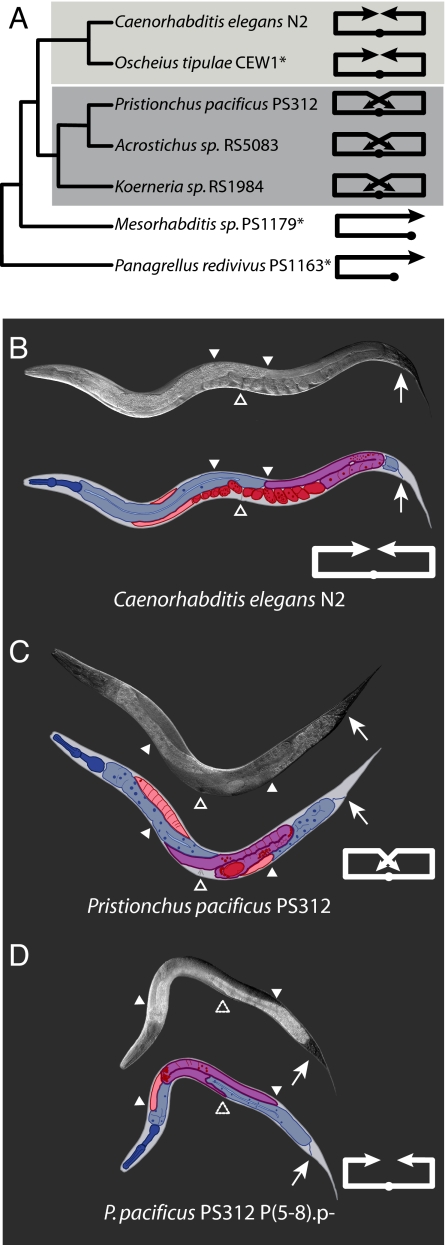
Fig. 1.
The gonadal arms of P. pacificus hermaphrodites have a novel ventral arm extension in comparison to C. elegans. (A) Cladogram of nematode species (34). The asterisk indicates data taken for O. tipulae CEW1 from ref. 9, for Mesorhabditis sp. PS1179 from ref. 10, and for P. redivivus PS1163 from ref. 11. Line diagrams indicate the path of hermaphrodite gonadal arm extension, and the solid dot indicates the position of the vulva. The ventral gonadal arm extension is unique to the Diplogastridae. Light gray shading indicates the Rhabditidae family. Medium gray shading indicates the Diplogastridae family. With the exception of Mesorhabditis sp. and P. redivivus, which have monodelphic gonads, all strains are didelphic. However, even in Mesorhabditis sp. and P. redivivus the gonadal arm passes the position of the vulva and does not make a ventral gonadal arm extension. (B–D Upper) Nomarski photographs of animals. (B–D Lower) Color-coded cartoons of the photographs to identify individual tissues: The somatic gonad and germ line are outlined and shaded in tones of purple and red. Purple, one gonadal arm in focus from uterus to distal tip; light red, the other gonadal arm that is mostly out of the plane of focus; red, embryos; small dark red amyboid-like shapes, sperm; solid dark red ovals, representative germ cell nuclei. The digestive tract is outlined and shaded in blue. Dark blue, pharynx; blue, gut; light blue, the lumen of the intestine; navy blue ovals, representative gut nuclei. Solid white triangles denote the distal tip of the gonadal arms. Solid open triangles denote the position of the vulva, i.e., the ventral side of the animal. Dashed open triangles represent the homologous position where the vulva would reside in a wild-type animal; i.e., there is no vulva. Small arrows indicate the position of the anus, i.e., the ventral side of the animal. Line diagrams represent the path of gonadal arm elongation. (B) Wild-type fully adult C. elegans N2 hermaphrodite. (C) Wild-type fully adult P. pacificus PS312 hermaphrodite. (D) P. pacificus PS312 young adult in which the entire vulva has been laser-ablated; i.e., P(5–8).p were killed immediately after hatching. Animals without a vulva must be scored at young adult stage before they fill with embryos and the internal morphology is disturbed.
Pristionchus pacificus is a nematode that has been established as a model system in evolutionary biology to allow detailed comparative studies with Caenorhabditis elegans (7). The full development of P. pacificus, like C. elegans, is amenable to microscopic analysis; the embryo and hatching larvae are transparent, and the cell lineage is invariant, making it ideal for cell ablation studies. P. pacificus also has a sequenced genome and an integrated genetic map. Thus, like C. elegans, P. pacificus is highly amenable to genetic manipulation by mutation and mating experiments. The development of the vulva and the gonad is well studied at the cellular level and is being elucidated further through mutant analysis (6, 8).
The hermaphrodite gonad of C. elegans consists of two gonadal arms, i.e., two bilaterally symmetric, reflexed, epithelium-like tubes. Each tube extends from a central anterior–posterior point along the ventral body wall of the developing animal (Fig. 1B). The developing arms first extend along the ventral side of the animals, then turn to extend to the dorsal body wall. Last, they extend along the dorsal side back to the anterior–posterior center of the animal. Thus, each tube adopts a U-like shape and contains the primordial germ cells. There are two components to gonadal arm elongation. First, arm elongation is guided by the migration of the distal tip cells (DTCs); cell ablation of the DTCs results in no gonadal arms. Second, the force of a proliferating and growing germ line is also needed as a driving force for full extension. In the absence of the germ line the DTCs migrate along the same approximate path of arm elongation to form the two U-shaped tubes. However, the DTCs fail to migrate fully back to the anterior–posterior center, resulting in short and thin arms. Each tube represents a single ovo-testis where gametes are produced in an assembly line manner. The first gametes produced are sperm and are stored in the spermatheca. Subsequently, there is a switch, and all further gametes differentiate as oocytes. Oocytes enter the spermatheca and are fertilized, and zygotes from both gonadal arms empty into a common uterus where they wait to be expelled into the outside environment via the vulva, the egg-laying organ of the animal.
The hermaphrodite gonad of P. pacificus is homologous to that of C. elegans; i.e., it consists of two reflexed gonadal arms (Fig. 1C). The somatic gonadal tissues that form the tubes and the underlying germ line differentiate into homologous tissues organized in a similar linear fashion along the length of the arms in comparison with C. elegans. However, there are many differences between the two gonads at a gross morphological level, at a tissue level, at the level of the cells, and in subcellular architecture (8). The extending P. pacificus gonadal arms display a major difference, a unique ventral migration that results in the distal gonad residing along the ventral side of the body, thus giving rise to a very different gross morphology in comparison to C. elegans. Here we demonstrate that a signal from the vulva and Wnt signaling are essential for this unique morphogenetic movement and the generation of novel organ morphology.
Results
Ventral Gonadal Arm Extension in P. pacificus Hermaphrodites Represents an Evolutionary Novelty.
The most obvious difference in gross morphology between P. pacificus and C. elegans is due to differing paths of gonadal arm extension (Fig. 1 A–C). The majority of the extending gonadal arms of P. pacificus hermaphrodites (≈70%) migrate ventrally as they come back toward the anterior–posterior center of the animal (Table 1, first row). Thus, the gonad of P. pacificus adopts a pretzel-like shape as opposed to the smooth extended U-shaped arms of C. elegans. This ventral migration of the hermaphrodite gonadal arms appears to be a novelty of the Diplogastridae family, e.g., P. pacificus PS312, Acrostichus sp. RS5083, and Koerneria sp. RS1984 (Fig. 1A). In the Rhabditidae, in species where the gonad has been examined, i.e., C. elegans N2 and Ocheius tipulae CEW1 (9), the gonadal arms do not have this late ventral migration. Likewise, more basal nematodes, such as Mesorhabditis sp. (10) and Panagrellus redivivus (11), also do not have this migration. Thus, this late ventral gonadal arm extension is a synapomorphy for the Diplogastridae and represents an evolutionary novelty.
Table 1.
The vulva signals the gonadal arms to extend ventrally
| Tissue ablated | Cells ablated | Developmental time, h after hatching | No. of gonadal arms scored | % of gonadal arms that fail to extend ventrally |
|---|---|---|---|---|
| None | None | N/A | 260 | 31 |
| A gonadal arm | Z1 or Z4 | 1–2 | 42 | 76 |
| Vulva | P(5–7).p + P8.p | 1–2 | 20 | 100 |
| Vulva | P(5–7).p | 1–2 | 68 | 100 |
| Vulva | P(5–7).p | 18–20 | 38 | 92 |
| Vulva | P(5–7).pxx* | 26–28 | 32 | 81 |
| Vulva | P(5,6).p, P(5,7).p, P(5,8).p, P(6,7).p, P(6,8).p, or P(7,8).p | 1–2 | 136 | 54 |
| Vulva | P5.p, P6.p, P7.p, or P8.p | 1–2 | 88 | 27 |
| Neurons | P(5–8).a | 1–2 | 32 | 28 |
| Muscles | M cell | 1–2 | 40 | 33 |
| Somatic gonad | Z1.p and Z4.a | 8–10 | 36 | 28 |
*By the time of the ablation the VPCs P(5–7).p have undergone between one and two cell divisions. As a result, it is the two to four descendants from each VPC that were killed.
The Vulva and the Two Gonadal Arms Are Required for Ventral Gonadal Arm Extension.
Two possibilities exist for the control of the ventral gonadal arm extension in P. pacificus. First, the migration may be preprogrammed into the leading DTCs; i.e., the migration is cell-autonomous. Alternately, the leading DTCs may respond to a signal from cells in the neighborhood that either instructs them to migrate ventrally or allows them to respond to a preexisting cue and turn ventrally. A classical method to look for cell interactions in nematodes is through laser ablation of cells. Because the gonadal arms extend ventrally as they approach the center of the animal, we hypothesized that the two gonadal arms may signal to each other or that cells associated with the vulva or the somatic gonad may be a source for such a signal. To that end, we ablated a single gonadal arm, Z1 or Z4; all surviving ventral epidermal cells, P(5–8).p; the vulval precursor cells (VPCs), P(5–7).p; the neuronal sister cells of the VPCs, P(5–7).a; the vulval muscles, the M cell; or the majority of the somatic gonadal tissues, Z1.p and Z4.a (Table 1).
The vulva signals to the extending gonadal arms late in development to induce ventral migration. Ablation of all three VPCs at the J2-larval stage immediately after birth results in gonadal arms failing to extend ventrally; 0% of gonadal arms extend ventrally in ablated animals (Fig. 1D and Table 1, third and fourth rows). Ablation of the descendants of the VPCs after they had divided once or twice yielded similar results to the ablation of the VPCs at birth (Table 1, fifth and sixth rows). We conclude that the developing vulva signals to the elongating gonadal arms as they approach the anterior–posterior center of the body and directs the ventral extension. This putative diffusible signal is likely received by the leading DTCs.
The signal from the vulva to the extending gonadal arms is quantitative. Only the cell ablation of all existing ventral epidermal cells or the three VPCs resulted in 100% of hermaphrodite gonadal arms failing to extend ventrally (Table 1, third and fourth rows). Cell ablation of any two VPCs or any VPC and P8.p results in an intermediate percentage of gonadal arms that fail to extend ventrally (Table 1, seventh row). Cell ablation of any single ventral epidermal cell has no affect on the percentage of gonadal arms that extend ventrally (Table 1, eighth row).
The DTCs may also signal each other in P. pacificus. Cell ablation of either Z1 or Z4 at the J2-larval stage completely eliminates one of the gonadal arms. In such animals the remaining arm frequently fails to extend ventrally (Table 1, second row). Because ablation of the majority of the somatic gonad does not have an effect on the ventral gonadal arm extension (Table 1, 11th row), each DTC is a putative source of a signal to the other DTC. Alternatively, the germ cells that underlie the DTC maybe the source of the signal. Thus, only ablation of an entire gonadal arm (Table 1, second row) or the vulva strongly affected the ventral gonadal arm migration (Table 1, third through sixth rows). Ablations of the other tissues do not have an affect on the ventral gonadal arm extension (Table 1, ninth through 11th rows). Therefore, our results indicate that ventral migration by the DTCs responds to a nonautonomous mechanism involving cell–cell interactions between the two gonadal arms and between the vulva and the gonadal arms.
Mutations in the Hox Gene Ppa-lin-39 Can Phenocopy Ablation of the VPCs.
The ablation of the vulva can be genetically phenocopied by mutations in the Hox gene Ppa-lin-39. Like in C. elegans, the P. pacificus Hox gene lin-39 specifies the cell fates of anterior–posterior midbody cells. Expression of lin-39 singles out four cells, P(5–8).p, from the other ventral epidermal cells, P(1–4).p and P(9–11).p, and prevents them from undergoing programmed cell death (PCD) at the J1-larval stage like these other cells (12). Reduced Ppa-LIN-39 function in a weak Ppa-lin-39 loss-of-function mutant (tu29) results in animals where various combinations of the epidermal cells, P(5–8).p, die by PCD. In animals where no VPCs survive there is no vulva and the gonadal arms always fail to migrate ventrally [supporting information (SI) Table S1, fourth row]. In animals with intermediate numbers of surviving VPCs an intermediate percentage of arms fail to extend ventrally, mimicking the quantitative nature of the VPC ablation results (Table S1, first through third rows). Therefore, Ppa-lin-39 mutants have reduced ventral arm extension because VPCs/vulval cells are reduced or absent.
Ppa-BAR-1/β-Catenin Is Essential for the Ventral Gonadal Arm Extension.
Ppa-BAR-1, a homologue of β-catenin, is essential for the ventral gonadal arm extension. In Ppa-bar-1 homozygous mutants the VPCs fail to differentiate and form a vulva. Instead, the VPCs linger and ultimately fuse to the hypodermis (13). In all 138 gonadal arms scored in adult Ppa-bar-1 homozygous animals, no ventral extension was observed (Fig. 2A and Table 2, second row). Thus, strong loss-of-function mutations in Ppa-bar-1 mimic the effect of ablating the vulva on the gonadal arms. This leaves two possibilities for the effect of Ppa-bar-1 on the ventral gonadal arm migration. Ppa-bar-1 acts directly within the DTCs to process a Wnt signal putatively from the vulva or indirectly by making the uninduced VPCs unable to signal. Consistent with some signaling function for Ppa-BAR-1 in the DTC, we can observe Ppa-BAR-1 in the nuclei of DTCs (Fig. S1).
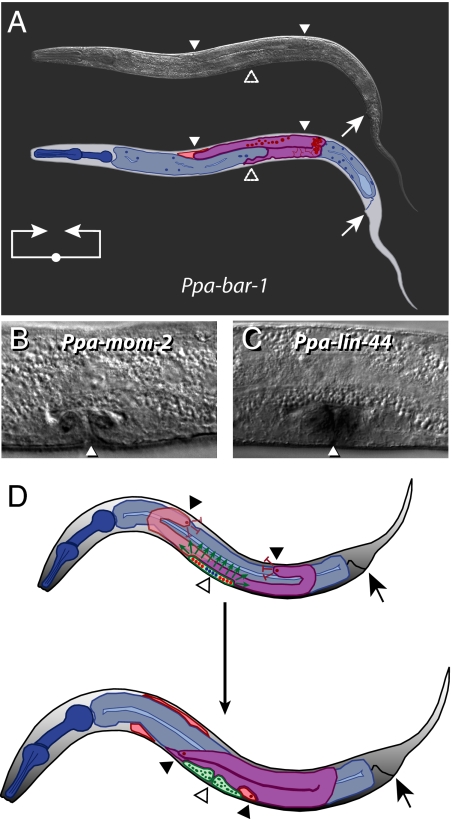
Fig. 2.
The ventral gonadal arm extension is regulated by the Wnt signaling pathway. (A) A Ppa-bar-1 homozygous mutant animal. For a description of cartoon color coding and line diagram see the Fig. 1 legend. Homozygous Ppa-bar-1 animals grow more slowly, they are smaller, their intestines are not as darkly colored, and their intestines do not fluoresce as their wild-type siblings. (B and C) Photographs of J4-stage wild-type P. pacificus PS312 animals for Wnt ligand RNA in situ hybridizations. Note the dark staining in the vulva, indicated by a solid triangle. (B) Ppa-mom-2 is expressed in a subset of the descendants of the secondary cells P5.p and P7.p (C) Ppa-lin-44 is expressed in multiple vulval cells. (D) Putative model for the cellular mechanism for signaling ventral gonadal arm extension. Color coding and symbols are the same as for the other cartoons with the following exceptions: Solid dark red circles at the tip of each gonadal arm represent the DTC nuclei. Vulval tissue is outlined and shaded in green; solid blue circles represent the nuclei of the descendants of P6.p that form the center of the vulva; solid red circles represent the nuclei of the descendants of P5.p and P7.p, respectively, that form the periphery of the vulva; solid green ovals represent vulval nuclei at a later stage in development. The black/white gradient within the body of the animal represents a proposed gradient in Ppa-UNC-6/Netrin along the dorsal–ventral body wall. (Upper) Late J3-stage/early J4-stage P. pacificus hermaphrodite. Both gonadal arms are shown; the arm normally hidden behind the gut is partly transparent. Green arrows indicate the production of a signal, putatively including Ppa-MOM-2 and Ppa-CWN-2, plus additional Wnt ligands. Red inhibitory arrows indicate the production of a signal from the extending gonadal arms. These signals are likely to be processed by the DTCs to carry out the novel P. pacificus ventral migration. By analogy to C. elegans, this is likely done by expressing Netrin receptors in the DTCs that can interpret the Netrin gradient. (Lower) Late J4-stage/young-adult-stage P. pacificus hermaphrodite. Note the resulting pretzel-like gonad morphology of the adult as a result of novel developmental signaling in P. pacificus in comparison to C. elegans.
Table 2.
The P. pacificus Wnt pathway affects the novel ventral gonadal arm extension
| Strain | Vulval induction* | No. of gonadal arms scored | % of gonadal arms that fail to extend ventrally |
|---|---|---|---|
| A. PS312 | + | 260 | 31 |
| B. bar-1 | −† | 138 | 100 |
| C. groucho | +‡ | 207 | 23 |
| D. bar-1; groucho | +§ | 104 | 50 |
| E. mom-2 | + | 242 | 50 |
| F. cwn-2 | + | 250 | 55 |
| G. mom-2, 2VPC(-) | +¶ | 44 | 95 |
| H. egl-20 | + | 252 | 25 |
| I. lin-17 | + | 246 | 20 |
| J. lin-18 | + | 220 | 28 |
| K. mom-2 cwn-2 | ND | ND | ND |
| L. mom-2 egl-20 | + | 164 | 41 |
| M. cwn-2 egl-20 | + | 264 | 23 |
| N. lin-17; egl-20 | + | 212 | 34 |
| O. mom-2 egl-20; lin-18 | + | 74 | 24 |
| P. mom-2 egl-20; lin-18 | −† | 56 | 75 |
| Q. cwn-2 egl-20; lin-18 | + | 102 | 25 |
| R. lin-17; lin-18; egl-20 | + | 56 | 25 |
| S. lin-17; lin-18; egl-20 | −† | 30 | 53 |
ND, not done. We have not been able to construct the Ppa-mom-2 Ppa-cwn-2 double mutant.
*A plus sign (+) indicates a normal vulva in which all the vulval precursor cells P(5–7).p are induced and differentiate into a vulva. A minus sign (−) indicates that at least one VPC was uninduced.
†No VPCs are induced in the Ppa-bar-1 mutant, an average of 0.5 ± 0.7 VPCs are induced in the Ppa-mom-2 Ppa-egl-20; Ppa-lin-18 triple mutant and an average of 1.7 ± 1.3 are induced in the Ppa-lin-17; Ppa-lin-18; Ppa-egl-20 triple mutant.
‡In Ppa-groucho (tu102) mutant animals, P3.p and P4.p always survive and are often induced in addition to P(5–7).p, i.e. 61% and 71% of the time, respectively (22).
§In Ppa-bar-1; Ppa-groucho-1 double mutant animals each cell, P(3–7).p, has an ≈30% chance of being induced (13).
¶P(5,6).p, P(6,7).p, or P(5,7).p were laser-ablated 24 h after hatching at 20°C.
Mutations in Ppa-groucho suppress the effect of mutations in Ppa-bar-1 on the ventral gonadal arm migration. Ppa-bar-1; Ppa-groucho double mutants show an intermediate percentage of arms that migrate ventrally (Table 2, fourth row). In the Ppa-bar-1; Ppa-groucho double mutant vulval tissue is ectopically induced from P(3–7).p. Hence, one interpretation of the suppression of the Ppa-bar-1 ventral migration defect is that the presence or absence of induced vulval tissue in the double mutant is on its own sufficient to affect ventral gonadal arm migration. This would imply that Ppa-bar-1 has only an indirect effect on the observed signaling. However, in rare Ppa-bar-1; Ppa-groucho adult animals where no induced vulval tissue was observed, the gonadal arms still sometimes migrated ventrally. Thus, suppression of the Ppa-bar-1 ventral migration phenotype by Ppa-groucho does not absolutely correlate with the presence of induced vulval tissue. As a result, the interpretation of the suppression of Ppa-bar-1 by Ppa-groucho is ambiguous in its implications for either a direct or indirect role for Ppa-bar-1 in the signaling that regulates ventral gonadal arm extension.
Several Wnt Ligands Are Candidates for Components of the Vulval Signal for Ventral Gonadal Arm Extension.
Do upstream members of the Wnt signaling pathway affect ventral gonadal arm extension? The P. pacificus genome encodes five Wnt ligands, four Frizzled receptors, and one Ryk/Derailed tyrosine kinase-related receptor (13). To date mutants have been generated in one Frizzled receptor, Ppa-lin-17, one Ryk/Derailed tyrosine kinase-related receptor, Ppa-lin-18, and three Wnt ligands, Ppa-cwn-2, Ppa-egl-20, and Ppa-mom-2 (13, 14). Analysis of the gonadal arms in homozygous single mutant animals suggests that the Wnt ligands Ppa-mom-2 and Ppa-cwn-2 are components of a putative Wnt signal to the DTCs. Ppa-mom-2 and Ppa-cwn-2 homozygous animals have a normal vulva; however, they have an effect on ventral gonadal arm extension, though not as strong as that of Ppa-bar-1 (Table 2, fifth and sixth rows). These reduced phenotypes likely reflect redundancy in the ligand/receptor signaling system, a finding also well known from C. elegans Wnt signaling (15, 16). Unfortunately, double mutant analysis of gonadal arm extension in Ppa-mom-2 Ppa-cwn-2 has not been possible. Ppa-mom-2 and Ppa-cwn-2 are in a region of linkage group IV (LGIV) that does not recombine; to date we have been unable to achieve a recombination event with any markers in this region.
Two additional observations are consistent with the possibility of a complex Wnt signal from the vulva instructing the migration of the DTCs. First, ablation of two VPCs in a Ppa-mom-2 mutant background results in the failure of almost all gonadal arms to migrate ventrally (Table 2, seventh row). This is consistent with the loss of additional WNT signal due to laser ablation of the VPCs (Table 1, seventh row, and Table 2, fifth and seventh rows). Second, Wnt ligands are expressed in the vulva. RNA in situ hybridization of whole-mount mixed-stage worms indicates that the Wnt ligands Ppa-mom-2 and Ppa-lin-44 are expressed in the developing vulva at the J4 stage when the ventral gonadal arm extension is initiated (Fig. 2 B and C).
Triple mutant analysis of upstream components of the Wnt pathway, i.e., ligands and receptors, suggests an indirect effect of Wnt signaling on gonadal arm extension. First, Ppa-mom-2 Ppa-egl-20; Ppa-lin-18 and Ppa-lin-17; Ppa-lin-18; Ppa-egl-20 triple mutant animals often fail to induce the VPCs; consequently, many of these animals lack a vulva. The hermaphrodite gonadal arms in triple mutant animals that have a normal vulva have a normal ventral gonadal extension (Table 2, 15th and 18th rows). The hermaphrodite gonadal arms in triple mutant animals that do not have a normal vulva often fail to extend ventrally (Table 2, 16th and 19th rows). Thus, uninduced VPCs do not signal as well as true vulval tissue.
However, triple mutant analysis of the Wnt pathway also suggests a direct role for Wnt signaling. The gonadal arms of Ppa-mom-2 Ppa-egl-20; Ppa-lin-18 and Ppa-lin-17; Ppa-lin-18; Ppa-egl-20 triple mutant animals that lack a vulva can extend ventrally. This is in contrast to the Ppa-bar-1 phenotype where we have never observed a ventral gonadal arm extension. Thus, VPCs that show no signs of induction, i.e., no cell division and no formation of an invagination later in development, are still capable of signaling. Because there is no reason to assume the uninduced VPCs in the Ppa-bar-1 homozygous mutants are any different in their behaviors or properties from the uninduced VPCs in these triple mutants, a plausible explanation for the full penetrance of the Ppa-bar-1 ventral gonadal extension phenotype is that Ppa-BAR-1 is directly involved in transducing the Wnt signal within the DTCs. Thus, the effects of the Wnt pathway on the ventral gonadal arm extension are pleiotropic and are likely due to direct involvement in the signal from the vulva to the DTCs, in addition to the effect seen due to the uninduced vulva.
A role for Ppa-egl-20 in the suppression of other Wnt pathway members is suggested by the double mutant analysis of the remaining available Wnt ligand mutations. Ppa-mom-2 Ppa-egl-20 and Ppa-cwn-2 Ppa-egl-20 double mutants are less penetrant in their effects on the ventral gonadal arm extension than the Ppa-mom-2 and Ppa-cwn-2 single mutants (Table 2, 12th and 13th rows). In fact, they are almost indistinguishable from wild-type animals in the percentage of gonadal arms that extend ventrally. Ppa-egl-20 has been shown to suppress the vulval phenotypes of other members of the P. pacificus Wnt pathway, e.g., Ppa-lin-17 (13). Antagonism between Wnt pathways members has also been demonstrated for Wnt signaling in C. elegans (17–19).
Discussion
Organogenesis is a complex developmental process that requires coordination among cells, tissues, and other organs. Changes in the coordination of different developmental processes at these various levels are essential to the evolution of body form and the ability to adapt to new environments. As our knowledge progresses, changes in the regulation of many basic processes of cellular biology will be found to be fundamental to the advent of morphological novelty (20). This case study in comparative analysis of gonadogenesis between P. pacificus and C. elegans suggests that one principal way to bring about morphological change is through novel cell–cell interactions among the different parts of the reproductive system, in particular the gonad and the vulva. This is likely to be a lesson that can also be applied to the evolution of other organs leading to the generation of morphological novelties. One such case maybe the vertebrate heart, which is also likely to have descended through modification from a rather unspecialized epithelium-like tube (3, 4).
At the molecular level, the cell–cell interactions between the P. pacificus gonad and vulva involve Wnt signaling. Wnt signaling is one of the oldest conserved pathways in metazoans (21). It has been implicated as an ancestral mode for establishing a body axis, is involved at many levels in organogenesis throughout animal phyla, and also provides a mechanism to establish alternative lineages in sister cells during development. Nevertheless, few of the molecular and genetic changes that resulted in the pleiotropic uses of Wnt signaling during metazoan evolution are known. The phylogenetic roots between the species where Wnt pathway function has been studied at a molecular level are often too deep to allow the formation and testing of hypotheses. Here we show that the Wnt pathway is involved in the generation of a morphogenetic novelty during organogenesis in a system that allows the investigation of molecular and genetic changes. The evolution of a Wnt-based organizer to signal migration and morphogenesis of the P. pacificus gonad may offer an intriguing parallel to the evolution of an organizer for axis formation, i.e., establishment of a diffusible signal, regulation of the receipt of the signal, and transducing the signal to downstream patterning genes.
The expression of the Wnt pathway within the gonad and vulval lineages may be ancestral to the Pristionchus and Caenorhabditis clade. The lineages of the gonad and the vulva appear to be homologous between P. pacificus and C. elegans. In this study we show that Wnt signaling affects the hermaphrodite gonad of P. pacificus. It has previously been shown that the Wnt pathway is involved in the generation of the P. pacificus vulva (13, 14, 22). In a similar fashion, in C. elegans, Wnt signaling is also required at different times in development to generate both a functional gonad and a functional vulva (15, 16, 23–26). Although the exact roles in specific cell-fate decisions within these lineages are likely to be quite different between P. pacificus and C. elegans, it is tempting to speculate that alterations in the timing and spatial expression of Wnt ligands or Frizzled receptors in vulval and gonadal lineages, respectively, may be responsible for the change in organ shape that we describe here. Indeed, changes in the regulation of gene expression have proven to be one major mode of evolutionary advances (27).
Regardless of the causative changes leading to altered Wnt signaling, we demonstrate that the cooption of Wnt signaling played a crucial role in the acquisition of cell–cell interactions between vulva and gonad. In the acquisition of a late ventral migration in the Diplogastridae clade, either a novel signal has evolved in the vulva that the extending gonadal arms can recognize or the extending gonadal arms have obtained the ability to respond to a preexisting signal from the vulva, possibly involving Ppa-MOM-2 and Ppa-CWN-2 (Fig. 2D). This results in the arms performing an extension back to the ventral side of the animal to adopt a unique morphology. Emerging lessons from C. elegans may be suggestive that signaling via the Wnt pathway is a common mechanism to regulate cell migrations (28). Future studies are needed to address whether the cross-talk between sister gonadal arms also involves Wnt signaling.
It remains to be determined whether Wnt signaling is instructive or merely permissive and allows the DTCs to recognize preexisting guidance cues (Fig. 2D). In C. elegans, most dorsal–ventral migrations require the Netrin guidance pathway, a pathway conserved from C. elegans to humans (29, 30). Netrin is expressed as a gradient along the dorsal ventral axis of C. elegans. Many of the genetic lesions that alter migrations along the dorsal–ventral axis result in the misregulation of Netrin receptors that are required to read the Netrin gradient (31–33). Similar to C. elegans, the Pristionchus clade has UNC-6/Netrin homologues and homologues of the DCC class of Netrin receptors. Surprisingly, to date, no homologues of the UNC-5 family of Netrin receptors have been identified in any Pristionchus clade member with available genome and cDNA sequence information (D.R. and R.J.S., unpublished observation). Therefore, future studies might reveal that changes in molecular environmental cues or the ability to recognize those cues will also prove a plausible and perhaps emerging paradigm for the advent of evolutionary morphological change.
The observed stochastic variation seen in P. pacificus PS312 ventral arm migration allows the extension of the genetic analysis described in this study to population-level processes. In comparison to P. pacificus, representative strains of several nematode groups basal to the Diplogastridae, such as Pelodera icosiensis RS5097 and Rhabditoides inermis SB328, show a great deal of variation in the way the gonadal arms are packaged in the body (data not shown). Also, intriguingly, the hermaphrodite gonadal arms in the close relative of P. pacificus Acrostichus sp. RS503 invariably make a dorsal-to-ventral migration. Therefore, the observed variation in PS312 may represent an intermediate stage in the canalization of a trait. There are currently >105 independent wild isolates of P. pacificus (M. Herrmann, J. Werner, S. Kienle, and R.J.S., unpublished observations), which represent a library of genetic and morphological trait variation. These strains show differences in the expressivity of the ventral gonadal arm migration and offer the potential to look for the changes leading to fixation and possible canalization of a morphological trait. Because the majority of the isolated P. pacificus strains interbreed well, this also opens the opportunity to initiate a quantitative trait loci analysis of ventral gonadal arm migration. Based on the seeming quantitative nature of the signal we have identified, such investigations will uncover selection on the expression of genetic loci that modulate and regulate Wnt signaling.
Materials and Methods
Genetic Analysis.
All strains were maintained at 20°C on plates seeded with Escherichia coli (OP50) using standard culture techniques (35). P. pacificus strain PS312 was used for laser ablation. The mutations were as follows: Ppa-bar-1 (tu362) LGI, Ppa-groucho (tu102) LGV, Ppa-cwn-2 (tu373) LGIV, Ppa-egl-20 (tu364) LGIV, Ppa-lin-17 (tu108) LGV, Ppa-lin-18 (tu359) LGI, Ppa-lin-39 (tu29) LGIII, and Ppa-mom-2 (tu363) LGIV.
A gonadal arm was scored as extending ventrally if the DTC touched the ventral body wall after reflexing. All gonadal arms counted were visible by Nomarski microscopy throughout the center of the animal. During mutant analysis, arms that were too small and failed to extend back to the center of the animal and arms that took a wandering course were discarded.
Laser Ablations.
To stage animals, embryos from PS312 cultures were isolated and allowed to hatch for 2 h. Larvae were transferred for ablation experiments immediately or were transferred to seeded plates and allowed to develop at 20°C until they reached the correct stage (Table 1).
Standard ablation techniques were done by using a MicroPoint Ablation Laser System from Laser Science (36). For ablation, animals were transferred to a 5% agar pad that contained 10 mM sodium azide. Ablation experiments were repeated at least twice as independent sets. When possible, animals were checked 12–24 h later by Nomarski for the absence of ablated tissues.
Anti-BAR-1 Stainings.
Adult hermaphrodites were picked into 0.25 mM levamisole in PBS and decapitated. Extruded gonads were transferred to 2% PFA in PBS (30 min), washed three times in PBS (15 min each), washed in −20°C methanol (5 min), washed in −20°C acetone (5 min), and rehydrated twice in PBS (30 min each). Gonads were blocked three times in 1% BSA in PBS (30 min each) and incubated in a solution of 1% BSA in PBS with affinity-purified anti-BAR-1 polyclonal rabbit antibodies overnight at 4°C (13). Subsequently, the gonads were washed three times with 1% BSA in PBS (15 min each), incubated for 4 h in 1% BSA in PBS containing 1 μg/ml DAPI and goat anti-rabbit secondary antibodies conjugated to Cy3 (111-165-045, Jackson ImmunoResearch), and washed three times in 1% BSA in PBS (15 min each). Stained gonads were mounted in VectaShield mounting medium (H-100; Vector Laboratories) on a 5% noble agar pad for viewing.
Wild-type gonads were stained on three occasions, and >40 arms were scored per experiment. DTC nuclei stained in 10–30% of dissected arms depending on the experiment. The nuclei of oocytes and some germ cells also stained in 20–60% of the gonadal arms. Ppa-bar-1 mutant animals were concurrently stained twice alongside the wild-type preparations, and >30 arms were scored per experiment. Nuclear staining of Ppa-BAR-1 was never observed. In all preparations high uniform background staining was seen in the germ line and gut. The luminal surface of the spermathecal corridor and the cell border of some somatic gonadal cells stained unspecifically as well.
RNA In Situ Hybridization.
RNA in situ hybridization was performed as in ref. 13.
Supplementary Material
Acknowledgments.
We thank B. Schlager (Max Planck Institute for Developmental Biology) for the affinity-purified anti-Ppa-BAR-1 rabbit polyclonal antibody. We also thank D. Bumbarger, C. Eckmann, M. Harris, R. Hong, and K. Siegfried for critically reading the manuscript. This work was supported by the Max Planck Institute. D.R. was funded by a Ruth L. Kirschstein National Research Service Award from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800597105/DCSupplemental.
References
- 1.Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385:627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- 2.Damen WG, Saridaki T, Averof M. Diverse adaptations of an ancestral gill: A common evolutionary origin for wings, breathing organs, and spinnerets. Curr Biol. 2002;12:1711–1716. doi: 10.1016/s0960-9822(02)01126-0. [DOI] [PubMed] [Google Scholar]
- 3.Bishopric NH. Evolution of the heart from bacteria to man. Ann NY Acad Sci. 2005;1047:13–29. doi: 10.1196/annals.1341.002. [DOI] [PubMed] [Google Scholar]
- 4.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitwood BG, Chitwood MB. Introduction to Nematology. Baltimore: University Park; 1974. [Google Scholar]
- 6.Rudel D, Sommer RJ. The evolution of developmental mechanisms. Dev Biol. 2003;264:15–37. doi: 10.1016/s0012-1606(03)00353-1. [DOI] [PubMed] [Google Scholar]
- 7.Hong RL, Sommer RJ. Pristionchus pacificus: A well-rounded nematode. BioEssays. 2006;28:651–659. doi: 10.1002/bies.20404. [DOI] [PubMed] [Google Scholar]
- 8.Rudel D, Riebesell M, Sommer RJ. Gonadogenesis in Pristionchus pacificus and organ evolution: Development, adult morphology and cell-cell interactions in the hermaphrodite gonad. Dev Biol. 2005;277:200–221. doi: 10.1016/j.ydbio.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Félix M-A The C. elegans Research Community, editor. Oscheius tipulae. WormBook. 2006 Aug 16; doi: 10.1895/wormbook.1.119.1. Available at www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felix MA, Sternberg PW. Symmetry breakage in the development of one-armed gonads in nematodes. Development. 1996;122:2129–2142. doi: 10.1242/dev.122.7.2129. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg PW, Horvitz HR. Gonadal cell lineages of the nematode Panagrellus redivivus and implications for evolution by the modification of cell lineage. Dev Biol. 1981;88:147–166. doi: 10.1016/0012-1606(81)90226-8. [DOI] [PubMed] [Google Scholar]
- 12.Eizinger A, Sommer RJ. The homeotic gene lin-39 and the evolution of nematode epidermal cell fates. Science. 1997;278:452–455. doi: 10.1126/science.278.5337.452. [DOI] [PubMed] [Google Scholar]
- 13.Tian H, Schlager B, Xiao H, Sommer RJ. Wnt signaling induces vulva development in the nematode Pristionchus pacificus. Curr Biol. 2008;18:142–146. doi: 10.1016/j.cub.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 14.Zheng M, Messerschmidt D, Jungblut B, Sommer RJ. Conservation and diversification of Wnt signaling function during the evolution of nematode vulva development. Nat Genet. 2005;37:300–304. doi: 10.1038/ng1512. [DOI] [PubMed] [Google Scholar]
- 15.Gleason JE, Szyleyko EA, Eisenmann DM. Multiple redundant Wnt signaling components function in two processes during C. elegans vulval development. Dev Biol. 2006;298:442–457. doi: 10.1016/j.ydbio.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, et al. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Zinovyeva AY, Forrester WC. The C. elegans Frizzled CFZ-2 is required for cell migration and interacts with multiple Wnt signaling pathways. Dev Biol. 2005;285:447–461. doi: 10.1016/j.ydbio.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Forrester WC, Kim C, Garriga G. The Caenorhabditis elegans Ror RTK CAM-1 inhibits EGL-20/Wnt signaling in cell migration. Genetics. 2004;168:1951–1962. doi: 10.1534/genetics.104.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan CL, et al. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell. 2006;10:367–377. doi: 10.1016/j.devcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Lecuit T, Le Goff L. Orchestrating size and shape during morphogenesis. Nature. 2007;450:189–192. doi: 10.1038/nature06304. [DOI] [PubMed] [Google Scholar]
- 21.Pires-daSilva A, Sommer RJ. The evolution of signalling pathways in animal development. Nat Rev Genet. 2003;4:39–49. doi: 10.1038/nrg977. [DOI] [PubMed] [Google Scholar]
- 22.Schlager B, Roseler W, Zheng M, Gutierrez A, Sommer RJ. HAIRY-like transcription factors and the evolution of the nematode vulva equivalence group. Curr Biol. 2006;16:1386–1394. doi: 10.1016/j.cub.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 23.Myers TR, Greenwald I. Wnt signal from multiple tissues and lin-3/EGF signal from the gonad maintain vulval precursor cell competence in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:20368–20373. doi: 10.1073/pnas.0709989104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegfried KR, Kidd AR, III, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;166:171–186. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegfried KR, Kimble J. POP-1 controls axis formation during early gonadogenesis in C. elegans. Development. 2002;129:443–453. doi: 10.1242/dev.129.2.443. [DOI] [PubMed] [Google Scholar]
- 26.Lam N, Chesney MA, Kimble J. Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Curr Biol. 2006;16:287–295. doi: 10.1016/j.cub.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prud'homme B, Gompel N, Carroll SB. Emerging principles of regulatory evolution. Proc Natl Acad Sci USA. 2007;104:8605–8612. doi: 10.1073/pnas.0700488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silhankova M, Korswagen HC. Migration of neuronal cells along the anterior-posterior body axis of C. elegans: Wnts are in control. Curr Opin Genet Dev. 2007;17:320–325. doi: 10.1016/j.gde.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Wadsworth WG. Moving around in a worm: Netrin UNC-6 and circumferential axon guidance in C. elegans. Trends Neurosci. 2002;25:423–429. doi: 10.1016/s0166-2236(02)02206-3. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann R. Cell migration in invertebrates: Clues from border and distal tip cells. Curr Opin Genet Dev. 2001;11:457–463. doi: 10.1016/s0959-437x(00)00217-3. [DOI] [PubMed] [Google Scholar]
- 31.Su M, et al. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development. 2000;127:585–594. doi: 10.1242/dev.127.3.585. [DOI] [PubMed] [Google Scholar]
- 32.Mak HY, Ruvkun G. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development. 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- 33.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 34.Kiontke K, et al. Trends, stasis, and drift in the evolution of nematode vulva development. Curr Biol. 2007;17:1925–1937. doi: 10.1016/j.cub.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 35.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein HF, Shakes DC. Caenorhabditis elegans: Modern Biological Analysis of an Organism. San Diego: Academic; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.