Abstract
Interaction within groups exploiting a common resource may be prone to cheating by selfish actions that result in disadvantages for all members of the group, including the selfish individuals. Kin selection is one mechanism by which such dilemmas can be resolved This is because selfish acts toward relatives include the cost of lowering indirect fitness benefits that could otherwise be achieved through the propagation of shared genes. Kin selection theory has been proved to be of general importance for the origin of cooperative behaviors, but other driving forces, such as direct fitness benefits, can also promote helping behavior in many cooperatively breeding taxa. Investigating transitional systems is therefore particularly suitable for understanding the influence of kin selection on the initial spread of cooperative behaviors. Here we investigated the role of kinship in cooperative feeding. We used a cross-fostering design to control for genetic relatedness and group membership. Our study animal was the periodic social spider Stegodyphus lineatus, a transitional species that belongs to a genus containing both permanent social and periodic social species. In S. lineatus, the young cooperate in prey capture and feed communally. We provide clear experimental evidence for net benefits of cooperating with kin. Genetic relatedness within groups and not association with familiar individuals directly improved feeding efficiency and growth rates, demonstrating a positive effect of kin cooperation. Hence, in communally feeding spiders, nepotism favors group retention and reduces the conflict between selfish interests and the interests of the group.
Keywords: kin selection, social spiders, Stegodyphus, tragedy of the commons, nepotism
The evolution of altruism and cooperation is a long-standing paradox (1). Cooperation within larger groups that exploit a finite resource can be especially prone to cheating (2, 3). In such a situation, the selfish interests of individuals result in disadvantages for all members of the group (3). This dilemma can be resolved or reduced if cooperation is enforced through mechanisms such as reciprocity or punishment (4). Alternatively, kin or multilevel selection resolves social dilemmas if group members interact with related individuals and compete with other such groups in a population (5). Cooperative group members then benefit indirectly because individuals that help relatives gain fitness by passing on shared genes to the next generation (6, 7). Creating and exploiting a common resource among kin may, however, not completely resolve a social dilemma if local kin competition reduces the benefits of cooperation (8). Forces other than kin selection can promote cooperation. For example, helping decisions in cooperatively breeding birds may be driven by ecological constraints, direct fitness benefits, or simply group membership rather than kinship, and relatively few studies unambiguously document kin selection as the driving force underlying helping behavior (9). Whether kin-directed cooperation facilitates the initial spread of helping is therefore a particularly interesting problem for understanding the origin of cooperation. To identify the selective forces behind the evolution of cooperation, the use of facultatively cooperative or transitional systems may be especially illuminating (10), because once established, cooperation may underlie novel selection regimes that override initial selection pressures (5, 11). Here we investigate whether kin selection reduces the negative effects of competition or selfish actions in a communally hunting spider described as a transitory species toward permanent sociality.
Communally feeding spiders are ideal to investigate costs and benefits of cooperation because of their feeding mode. Subsocial and social spiders are known to hunt cooperatively; not only do they build and share a common capture web, but they also share large prey items (11). After successful capture of a large prey, spiders will not divide up the carcass and go their way to finish off their share. Spiders digest externally, and many do that by injecting their digesting enzymes and sucking up the liquidized prey content without destroying the exoskeleton of the prey item (12). Hence, communally feeding spiders all spit into the same carcass and thereby exploit a common resource that was jointly created. Such a system is prone to cheating because each feeder can either invest in the digestion process by contributing enzymes or may cheat by sucking up the fluids with little prior investment. Feeders do compete heavily, which is evidenced by a general reduction in feeding efficiency of groups as compared to single feeders (13–15). The outcomes of such conflicts are easily quantified through measuring feeding efficiency and weight gain. Growth and body size are tightly linked to fitness in spiders because mating success and fecundity are generally positive functions of body size (16–18).
Cooperation in spiders is extremely rare, occurring only in species with extensive maternal care and a cooperative period in juvenile stages (subsocial spiders) or in permanent group-living species with cooperative foraging and breeding (permanent social spiders) (11). Social spiders do not show division of labor but communally care for the young, build and maintain the capture web, and hunt and feed together on large prey items. Phylogenetic analyses suggest several independent origins of permanent sociality from subsocial species (19, 20). The transition occurs through philopatry and the elimination of breeding dispersal, leading to the unusual situation of complete inbreeding among group members (11). It was suggested that ecological constraints favored philopatry and group formation (21, 22), creating communally foraging societies and the potential for local competition among relatives. This system is therefore powerful for investigating the role of kin cooperation in the transition to permanent sociality.
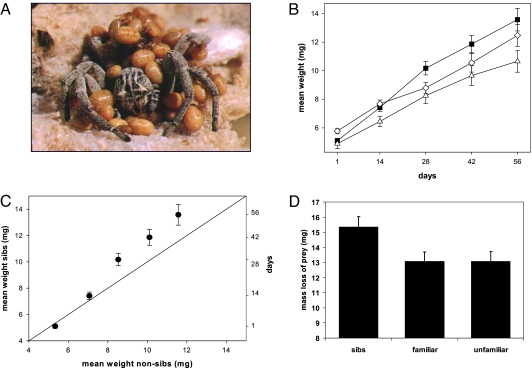
Our study species, the subsocial spider Stegodyphus lineatus (Eresidae) occurs in arid habitats of circum-Mediterranean distribution (23). The species is semelparous, and females invest maximally in their single brood (17). A mother feeds her young with digested fluids stored in her body and is eventually completely consumed by her offspring (matriphagy; Fig. 1A). The young remain together in the natal nest for 1–2 months after the death of the mother and continue to feed communally. The genus Stegodyphus contains 15 subsocial and 3 permanently social derived species, which represent convergent transitions to sociality (20).
Fig. 1.
In the subsocial spider Stegodyphus lineatus, cooperative feeding among sibs results in elevated growth rates and higher feeding efficiency than feeding among non-kin. (A) Stegodyphus lineatus second-instar young consuming their mother (matriphagy). (B) Effect of relatedness on growth. Growth curves (mean ± SE) of spider groups in three treatments: sibs (squares), familiar unrelated (triangles), and unfamiliar unrelated (diamonds). Sib groups had significantly higher growth rates than familiar and unfamiliar nonkin groups, which did not differ from each other. (C) Mean (± SE) weight of sib groups plotted against that of familiar and unfamiliar nonkin groups (pooled, see Results and Discussion for explanation). Dots above the line of equality indicate that mean weights of sib groups are higher than weights of nonkin groups. (D) The effect of relatedness on feeding efficiency. Mean (and SE) mass (mg) extracted by groups of sib and nonsib spiders (based on a mean of 5.7 feeding trials per group over a period of 8 weeks); kin groups extracted significantly more mass from their prey than did groups of unrelated spiders.
Here we experimentally demonstrate benefits of kin cooperation in the transitional spider Stegodyphus lineatus. We used a split brood design to separate genetic relatedness (kin) from familiarity (learned association) (24) and compared growth and feeding efficiency in spiders that feed communally. We show that communal feeding among kin results in elevated feeding efficiency and growth rates. Our data clearly demonstrate that genetic similarity among group members facilitates cooperation and points to kin selection as a driving force in the transition to sociality. We compared three treatment groups: sibs, unfamiliar nonsibs, and familiar nonsibs. The latter were unrelated but familiar spiders created in foster experiments (see Materials and Methods) to control for nongenetic learned associations that could erroneously be interpreted as kin-selected effects (25). With this experimental design, possible nepotistic interactions would take place only in sib groups and not in familiar nonsib groups. All groups of spiders were raised under standardized laboratory conditions in groups by mothers that actively fed them and were eventually consumed by the young. Experimental data showed that under these conditions, fostered young perform as well as siblings, suggesting equal investment in maternal care by the semelparous spiders once they are in the reproductive stage (26). We measured growth as weight gained over an experimental period of 8 weeks and measured feeding efficiency of the groups by quantifying the mass extracted from prey in repeated 2-hour assays of cooperative feeding.
Results and Discussion
Sib groups gained significantly more weight than unrelated spider groups (both familiar and unfamiliar) over the experimental period of 8 weeks [linear mixed effect model restricted maximum likelihood (REML); treatment × time interaction (random effect): F1,234 = 9.31, P = 0.0025 (Fig. 1B); relatedness (fixed effect): F1,58 = 4.39, P = 0.04; time (random effect): F1,234 = 300.3, P < 0.0001]. We present results from the reduced model because statistical comparison of the full and reduced models revealed that familiar and unfamiliar nonsib groups followed similar growth curves. By chance, nonsib unfamiliar spider groups had a higher start weight than the two other groups (sibs = familiar < unfamiliar, F1,57 = 6.64, P = 0.012), but sib groups overtook the unfamiliar group by following a significantly steeper growth trajectory (Fig. 1C). Thus, we demonstrate a direct benefit of feeding with kin as opposed to feeding with nonkin in terms of body weight. This appears to be based on higher feeding efficiency of sib groups compared with nonsib groups, as kin groups extracted significantly more mass from their prey than did groups of unrelated spiders during a fixed feeding duration (Fig. 1D; linear mixed effect model REML; relatedness (fixed effect): F1,58 = 8.91, P = 0.004; number of feeding spiders in group (covariate): F1,294 = 238.14, P < 0.0001). The nonsignificant interaction term was excluded in the reduced model (relatedness × number of feeding spiders; F2,292 = 1.06, P = 0.34). Thus, a more efficient utilization of prey by kin groups translates into higher growth rates compared with unrelated foraging groups.
Groups of sibs and nonsibs did not differ in the average number of spiderlings that fed on a prey item (F2,57 = 0.49, P = 0.62); therefore, their inclination to feed cooperatively was equal. The average number of spiderlings that fed on a prey item was 3.4 ± 0.67 (n = 60, Shapiro–Wilk W test for normal distribution, W = 0.98, P = 0.71). After 8 weeks, within-group mortality of spiders was marginally smaller in sib groups than in unrelated groups [generalized linear model (GLM) binomial, logit link, P = 0.06; mean number of dead spiders (SD) for sibs = 0.55 (0.88); familiar = 0.94 (1.1); unfamiliar = 0.84 (1.25)], indicating that there may also be a survival benefit of living with genetic siblings.
Spiders are externally digesting predators that inject their digestive enzymes into the body of the prey. Consequently, when sharing prey, spiders share not only the available biomass but also the effort for digesting the food. Producing digestive enzymes is costly (27), so that a particular individual feeding in a group will likely benefit by abusing the investment of others, causing each individual to retain most of its enzymes to avoid such exploitation. Jointly feeding spiders withholding digestive enzymes may explain the surprising finding that groups of another social spider, Stegodyphus mimosarum, take longer to digest prey than single feeders (14). Hence group feeding is less beneficial than individual feeding even for the permanently social spiders, but our results show that these disadvantages of competition are much higher when cooperative feeding occurs among nonkin, perhaps through a much reduced willingness to share extracellular digestive enzymes among unrelated group members (28). In the transitional species as investigated here, mechanisms that reduce negative effects of competition when a communal resource is shared, here in the form of nepotism among genetic kin, is likely to facilitate the transition of sociality.
Our design allowed us to separate the effect of genetic relatedness from familiarity. Unrelated spiders that were raised together did not show a higher feeding efficiency than unfamiliar nonsibs. Therefore, the possibility that the measured effects are mediated through familiarity or learned associations is unlikely. Our results imply that the spiders must be able to identify kinship directly and invest differentially in cooperative feeding depending on the genetic relatedness of group members. Kin recognition could be mediated through self-referent phenotype matching (29) or phenotype matching through the learning of cues from the neighbors or the mother (30). Another possibility is that cooperation relies on the variance in genetic cues within the group, such that groups with nonrelatives would have a large variance and those with relatives would have a low variance (31, 32).
The ability of S. lineatus to recognize kin was also suggested by a previous study. Under extreme shortage of prey, cannibalism can occur in these spiders, and cannibalism was mostly directed toward unrelated group members (33). Cannibalism avoidance does not provide an alternative explanation for our results here because we show that similar numbers of spiders were feeding cooperatively across all experimental groups. In addition, spiders were not food limited in our experiments and no cannibalism occurred. Finally, we found that feeding rate (extracted fluids from prey per hour) differed across treatment groups and thus cooperative feeding per se, and not behavioral avoidance or group-member rejection, explains our results.
Kin-mediated benefits of cooperation confer benefits of philopatry and facilitate the retention of siblings in the natal nest, matching the subsocial route to sociality, which has been proposed for social spiders (34) and for the evolution of eusociality in insects (35). The offspring have a direct fitness interest in remaining in the nest associated with kin, and moreover, this interest coincides with the mother's interest in sacrificing her own body to carriers of her genes. Our data clearly demonstrate that genetic similarity among siblings facilitates cooperation; it reduces the negative effects of competition and points to kin selection as a driving force in the transition to sociality.
Materials and Methods
Female Stegodyphus lineatus with egg sacs were collected with their nests on the Greek island of Karpathos in June 2006. Egg sacs hatched in the laboratory and were allocated to three treatments after a predetermined schedule: sibs, unfamiliar nonsibs, and familiar nonsibs. All broods were handled 1 or 2 days after hatching: the young were removed from the nest and counted. Young predestined to be used in the sib and unfamiliar nonsib treatment were returned to their mothers, and young for the familiar nonsib treatment were mixed and redistributed to foster mothers such that each foster female received genetically mixed broods of young originating from 10 unrelated families. After matriphagy and the subsequent molt into the first independent instar (easy to distinguish because the spiderlings become hairy for the first time), 60 groups of 6 spiderlings each were formed and placed in Petri dishes (60-mm diameter). Twenty groups consisted of sibs (genetically related), 20 groups consisted of unrelated spiderlings from the fostered broods (nonsibs, familiar) that grew up in the same nest as a “family” group, and the remaining 20 groups were formed with unrelated spiderlings (nonsibs, unfamiliar), each of which came from a different family. It was not necessary to cross-foster the sibling groups because females are known not to discriminate between their own and foreign young as long as they are in the right reproductive stage (26).
The feeding experiments started as soon as the spiderlings had produced silk inside the Petri dishes. A Calliphora fly was anesthetized with CO2, weighed to the nearest microgram by using an electronic balance, and introduced into the center of the Petri dish. As the first spiderling attacked the prey, the number of feeding spiderlings was determined every 10 min for 2 hours, when the fly was removed and weighed again. The mean number of spiderlings feeding was entered in the analysis as covariate. Feeding assays were replicated up to eight times per group (mean of 5.7 assays per group, SD = 1.41) over a period of 8 weeks. Time intervals between feeding assays were the same for all groups. In several cases an assay had to be terminated because the fly had destroyed the web or the spiderlings did not react. Assays were also excluded if only a single spiderling fed on the fly. A mean was calculated for each group over the mass extracted from the fly (weight after trial − weight before the trial) and the number of spiderlings that fed together. In addition to the weekly assays, groups were fed twice a week with six Drosophila to standardize food availability. Individual spiders were weighed every second week to obtain growth curves. Variances of response variables were homogenized to comply with assumptions of parametric analyses and analyzed with linear mixed effect models fitted by REML, coding group ID and time (growth experiment) as random variable using R (R Development Core Team, www.R-project.org).
Acknowledgments.
We thank J. Helms, T. Dirks, A. Taebel-Hellwig, N. Schilling, and C. Tuni for collecting data and spiders and S. Pekar for help with the statistical analysis. R. Bshary, M. Herberstein, Y. Lubin, I. Pen, S. West, and especially J. J. Boomsma and P. d′Ettorre provided valuable comments on the manuscript. This work was supported by grants from the Carlsberg Foundation (95091549) and the EU (MC MEIF-CT-2006-023645) to T.B. and the Deutsche Forschungsgemeinschaft (Schn 561/6-1 to J.M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Pennisi E. How did cooperative behavior evolve. Science. 2005;309:93558. doi: 10.1126/science.309.5731.93. [DOI] [PubMed] [Google Scholar]
- 2.Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- 3.Rankin DJ, Bargum K, Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol Evol. 2007;22:643–651. doi: 10.1016/j.tree.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Sigmund K. Retaliation and collaboration among humans. Trends Ecol Evol. 2007;22:593–600. doi: 10.1016/j.tree.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Keller L. Levels of Selection. Princeton, NJ: Princeton Univ Press; 1999. [Google Scholar]
- 6.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7:1–1652. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 8.West SA, Pen I, Griffin AS. Cooperation and competition between relatives. Science. 2002;296:72–75. doi: 10.1126/science.1065507. [DOI] [PubMed] [Google Scholar]
- 9.Cockburn A. Evolution of helping behavior in cooperatively breeding birds. Annu Rev Ecol Syst. 1998;29:141–177. [Google Scholar]
- 10.West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17:R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Lubin Y, Bilde T. The evolution of sociality in spiders. Adv Study Behav. 2007;37:83–145. [Google Scholar]
- 12.Foelix RF. Biology of Spiders. New York: Oxford Univ Press; 1996. [Google Scholar]
- 13.Schneider JM. Survival and growth in groups of a subsocial spider (Stegodyphus lineatus) Insect Soc. 1995;42:237–248. [Google Scholar]
- 14.Ward PI, Enders MM. Conflict and cooperation in the group feeding of the social spider Stegodyphus mimosarum. Behaviour. 1985;94:167–182. [Google Scholar]
- 15.Whitehouse MEA, Lubin Y. Competitive foraging in the social spider Stegodyphus dumicola. Anim Behav. 1999;58:677–688. doi: 10.1006/anbe.1999.1168. [DOI] [PubMed] [Google Scholar]
- 16.Maklakov AA, Bilde T, Lubin Y. Sexual selection for increased male body size and protandry in a spider. Anim Behav. 2004;68:1041–1048. [Google Scholar]
- 17.Schneider JM, Lubin Y. Does high adult mortality explain semelparity in the spider Stegodyphus lineatus (Eresidae)? Oikos. 1997;79:92–100. [Google Scholar]
- 18.Head G. Selection on fecundity and variation in the degree of sexual size dimorphism among spider species (class Araneae) Evolution. 1995;49:776–781. doi: 10.1111/j.1558-5646.1995.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 19.Agnarsson I, Aviles L, Coddington JA, Maddison WP. Sociality in Theridiid spiders: Repeated origins of an evolutionary dead end. Evolution. 2006;60:2342–2351. [PubMed] [Google Scholar]
- 20.Johannesen J, Lubin Y, Smith DR, Bilde T, Schneider JM. The age and evolution of sociality in Stegodyphus spiders: A molecular phylogenetic perspective. Proc R Soc London Ser B. 2007;274:231–237. doi: 10.1098/rspb.2006.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilde T, et al. Survival benefits select for group living in a social spider despite reproductive costs. J Evol Biol. 2007;20:2412–2426. doi: 10.1111/j.1420-9101.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 22.Bilde T, Lubin Y, Smith D, Schneider JM, Maklakov AA. The transition to social inbred mating systems in spiders: Role of inbreeding tolerance in a subsocial predecessor. Evolution. 2005;59:160–174. [PubMed] [Google Scholar]
- 23.Kraus O, Kraus M. The genus Stegodyphus (Arachnida, Araneae) sibling species, species groups, and parallel evolution of social living. Verh Naturwiss Ver Hamburg. 1988;30:151–254. [Google Scholar]
- 24.Mateo JM, Holmes WG. Cross-fostering as a means to study kin recognition. Anim Behav. 2004;68:1451–1459. [Google Scholar]
- 25.Sherman PW, Reeve HK, Pfennig DW. Recognition Systems. In: Krebs JR, Davis NB, editors. Behavioural Ecology: An Evolutionary Approach. London: Blackwell; 1997. pp. 69–96. [Google Scholar]
- 26.Schneider JM. Reproductive state and care giving in Stegodyphus (Araneae: Eresidae) and the implications for the evolution of sociality. Anim Behav. 2002;63:649–658. [Google Scholar]
- 27.Secor SM. Gastric function and its contribution to the postprandial metabolic response of the Burmese python Python molurus. J Exp Biol. 2003;206:1621–1630. doi: 10.1242/jeb.00300. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser D. Coupling cell movement to multicellular development in myxobacteria. Nat Rev Microbiol. 2003;1:45–54. doi: 10.1038/nrmicro733. [DOI] [PubMed] [Google Scholar]
- 29.Hauber ME, Sherman PW. Self-referent phenotype matching: Theoretical considerations and empirical evidence. Trends Neurosci. 2001;24:609–616. doi: 10.1016/s0166-2236(00)01916-0. [DOI] [PubMed] [Google Scholar]
- 30.Getz WM, Smith KB. Honey bee kin recognition: Learning self and nestmate phenotypes. Anim Behav. 1986;34:1617–1626. [Google Scholar]
- 31.Boomsma JJ, et al. Informational constraints on optimal sex allocation in ants. Proc Natl Acad Sci USA. 2003;100:8799–8804. doi: 10.1073/pnas.1430283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meunier J, West SA, Chapuisat M. Split sex ratios in the social Hymenoptera: A meta-analysis. Behav Ecol. 2008;19:382–390. [Google Scholar]
- 33.Bilde T, Lubin Y. Kin recognition and cannibalism in a subsocial spider. J Evol Biol. 2001;14:959–966. [Google Scholar]
- 34.Kullmann EJ. Evolution of social behavior in spiders (Araneae - Eresidae and Theridiidae) Am Zool. 1972;12:419–426. [Google Scholar]
- 35.Queller DC. Does population viscosity promote kin selection? Trends Ecol Evol. 1992;7:322–324. doi: 10.1016/0169-5347(92)90120-Z. [DOI] [PubMed] [Google Scholar]