Abstract
Chronic cholestasis often results in premature death from liver failure with fibrosis; however, the molecular mechanisms contributing to biliary cirrhosis are not demonstrated. In this article, we show that the death signal mediated by TNF-related apoptosis-inducing ligand (TRAIL) receptor 2/death receptor 5 (DR5) may be a key regulator of cholestatic liver injury. Agonistic anti-DR5 monoclonal antibody treatment triggered cholangiocyte apoptosis, and subsequently induced cholangitis and cholestatic liver injury in a mouse strain-specific manner. TRAIL- or DR5-deficient mice were relatively resistant to common bile duct ligation-induced cholestasis, and common bile duct ligation augmented DR5 expression on cholangiocytes, sensitizing mice to DR5-mediated cholangitis. Notably, anti-DR5 monoclonal antibody-induced cholangitis exhibited the typical histological appearance, reminiscent of human primary sclerosing cholangitis. Human cholangiocytes constitutively expressed DR5, and TRAIL expression and apoptosis were significantly elevated in cholangiocytes of human primary sclerosing cholangitis and primary biliary cirrhosis patients. Thus, TRAIL/DR5-mediated apoptosis may substantially contribute to chronic cholestatic disease, particularly primary sclerosing cholangitis.
Keywords: cholangitis, primary sclerosing cholangitis
TNF and TNF-receptor superfamilies play indispensable roles in immunity and inflammatory responses (1, 2). TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L binds two death receptors [TRAIL-R1/death receptor 4 (DR4) and TRAIL-R2/death receptor 5 (DR5)] (3), and has been recently identified as a key molecule in immune regulation of T cells [memory CD8 T cell control by CD4 T cells (4, 5) and Th2 cell response (6)]. TRAIL has been also demonstrated as a critical molecule in extrinsic tumor suppression by the immune system (3, 7, 8), and thus TRAIL is of great interest to clinical oncologists (3, 9). Recombinant TRAIL and anti-DR4 or DR5 monoclonal antibodies (mAbs) were reported to be substantially tumoricidal in mice without apparent toxicity (10–14), and have now started to be applied in cancer therapy (3, 9, 15, 16). Furthermore, TNF/TNF-receptor superfamilies contribute to a number of diseases, and TNF- and Fas ligand-induced hepatocyte death have particularly been shown to play a critical role in hepatic disorders (17). Some preparations of recombinant TRAIL or agonistic mAbs specific for DR4 or DR5 were reported as cytotoxic against cultured hepatocytes (18–20) and diseased human liver (21). Moreover, the first clinical data on anti-DR5 mAb (lexatumumab) in patients with advanced solid cancers indicated dose-limiting toxicity, including elevations of serum amylase, transaminases, and bilirubin (16). Still, the pathophysiological mechanisms underlying the toxicity of the TRAIL/DR5 pathway in liver disease remain to be revealed. In this article we show that agonistic anti-DR5 mAb treatment resulted in primary sclerosing cholangitis (PSC)-like cholestatic liver injury in a mouse strain-specific manner, and TRAIL/DR5-mediated apoptosis substantially contributed to chronic cholestatic disease. Chronic cholestasis often results in premature death from liver failure with fibrosis (22), thus the pathogenic contribution of the TRAIL/DR5-pathway to biliary cirrhosis may be key to provide an efficacious means to treat this resilient disease (22–24).
Results
Anti-DR5 mAb Treatment Causes Cholestatic Liver Damage in B6, but Not BALB/c Mice.
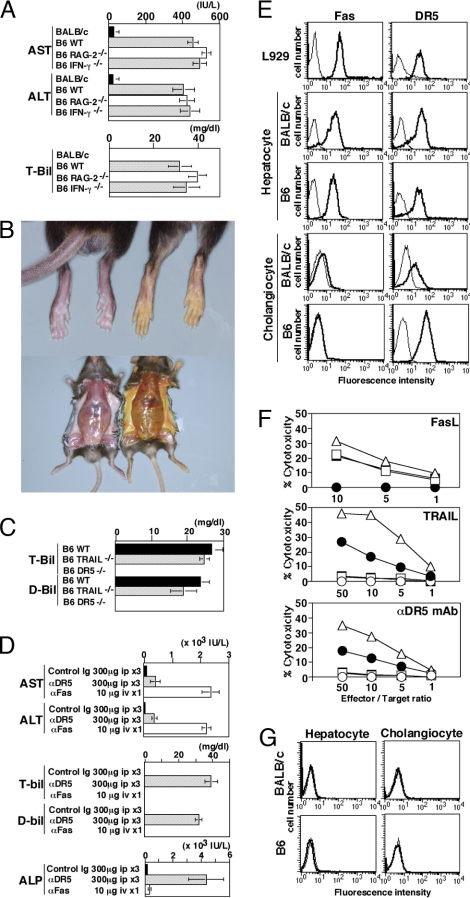
No signs of significant hepatotoxicity or systemic toxicity was observed in BALB/c mice after agonistic anti-DR5 mAb administration (Fig. 1A), as we have previously reported (14). However, in a sharp contrast, weight loss and ruffled fur were observed in C57BL/6 (B6) mice given the same regime, and B6 mice developed severe jaundice and high serum bilirubin levels (Fig. 1 A and B). Once serum bilirubin levels were elevated, B6 mice did not recover and had to be killed with severe liver failure. Adaptive immunity and interferon (IFN)-γ played no role in this disease, because B6 RAG-2−/− or B6 IFN-γ−/− mice developed similar jaundice and liver failure (Fig. 1A). Resistance of B6 DR5−/− mice to anti-DR5 mAb-induced cholestasis clearly demonstrated that DR5 was indispensable (Fig. 1C). Notably, increased levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) after anti-DR5 mAb treatment were significant (Fig. 1A), but minor compared with mice treated with anti-Fas mAb (Fig. 1D). By contrast, anti-DR5 mAb treatment elevated serum alkaline phoshatase (ALP) and bilirubin levels, while anti-Fas mAb did not (Fig. 1D). These results suggested that anti-DR5 mAb treatment specifically induced cholestatic liver injury rather than direct hepatocyte injury observed after anti-Fas mAb treatment in B6 mice.
Fig. 1.
Cholestatic liver injury in anti-DR5 mAb-treated B6 mice and sensitivity of cholangiocytes to TRAIL/DR5-induced apoptosis. (A) Serum AST, ALT, and total bilirubin (T-bil) levels of indicated mice treated with intraperitonial injections of anti-DR5 mAb (n = 10 in each group). (B) Jaundice induced by anti-DR5 mAb injections (Right), but not control Ig treatment (Left). (C) Serum T-bil and direct bilirubin (D-bil) levels after anti-DR5 mAb injections in B6 WT, DR5−/−, and TRAIL−/− mice (n = 10 in each group). (D) Serum AST, ALT, T-bil, D-bil, and ALP levels of B6 mice after repeated anti-DR5 mAb or single anti-Fas mAb injection (n = 10 in each group). (E) DR5 and Fas expression on freshly isolated B6 and BALB/c hepatocytes and cholangiocytes. Bold lines indicate the staining with anti-Fas or anti-DR5 mAb; thin lines indicate the staining with isotype-matched control Ig. (F) Sensitivity of L929 cells (triangles) and freshly isolated hepatocytes (squares) and cholangiocytes (circles) from B6 mice (filled circles) or BALB/c mice (open circles) to cytotoxicity by FasL-transfected cells, TRAIL-transfected cells, or anti-DR5 mAb-induced cytotoxicity. (G) TRAIL expression on freshly isolated B6 and BALB/c hepatocytes and cholangiocytes. Bold lines indicate the staining with anti-TRAIL mAb; thin lines indicate the staining with isotype-matched control Ig.
TRAIL/DR5-Induced Cell Death in Freshly Isolated B6, but Not BALB/c Cholangiocytes.
Hepatocytes freshly isolated from both B6 and BALB/c mice expressed Fas and DR5, and both were equally sensitive to FasL-induced cytotoxicity, but resistant to TRAIL or anti-DR5 mAb (Fig. 1 E and F). Cholangiocytes isolated from B6 mice expressed higher levels of DR5 than those from BALB/c mice and were sensitive to TRAIL/DR5-induced apoptosis (Fig. 1 E and F). Comparatively, cholangiocytes isolated from both strains were resistant to FasL-induced apoptosis (Fig. 1F). TRAIL was not detected on cholangiocytes and hepatocytes isolated from either B6 or BALB/c mice (Fig. 1G).
Cholangitis Induction by Anti-DR5 mAb Treatment in B6, but Not BALB/c Mice.
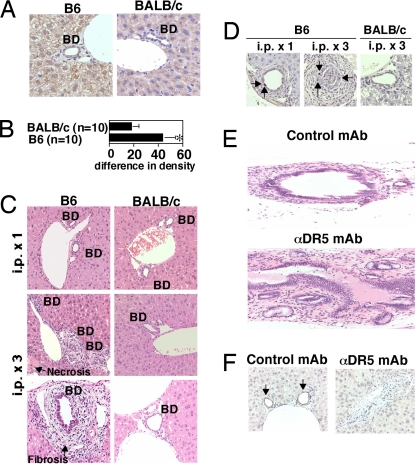
Immunohistochemical examination demonstrated significantly higher expression of DR5 on the cholangiocytes of B6 mice when compared with BALB/c mice in vivo (P < 0.05) (Fig. 2 A and B). Few dying hepatocytes and the normal features of bile ducts were observed in anti-DR5 mAb-treated BALB/c mice (Fig. 2C). In contrast, anti-DR5 mAb injection caused fibrosis around bile ducts, diffuse multifocal biliary obstruction, and induction of focal death of hepatocytes in B6 mice, although hepatic injury was not extensive (Fig. 2C). TUNEL staining indicated apoptosis in cholangiocytes of anti-DR5 mAb-treated B6 mice, but not BALB/c mice (Fig. 2D). The extrahepatic bile duct was thickened and indurated with fibrosis and inflammatory cell infiltration in anti-DR5 mAb-treated B6 mice (Fig. 2E). Notably, immunohistochemical staining for cytokeratin 19 (25) demonstrated the disappearance of intrahapatic bile ducts after anti-DR5 mAb treatments (Fig. 2F). These results suggested that anti-DR5 mAb induced apoptosis in cholangiocytes and caused cholangitis with bile duct loss and biliary obstruction in B6 mice.
Fig. 2.
Cholangitis induced by anti-DR5 mAb treatment in B6 mice but not BALB/c mice. (A) Immunohistochemical examination of DR5 expression in livers of B6 and BALB/c WT mice. BD, bile duct. (B) Quantification of DR5 expression in immunohistochemistry. Sections were analyzed by using optimal densitometric mean value (MEAND), and data are represented as the mean ± SD (increased % intensity) of plural sections from indicated numbers of mice in each group. ∗, P < 0.05 compared with BALB/c mice. (C) Histological examination of livers in anti-DR5 mAb-treated B6 and BALB/c mice 4 days after one or three injections of anti-DR5 mAb. BD, bile duct. (D) Apoptosis of biliary epithelial cells demonstrated by TUNEL staining. The arrows indicate apoptotic cholangiocytes. (E) Histological examination of extrahepatic bile duct in anti-DR5 mAb- and control Ig-treated B6 mice 4 days after three injections. (F) Cholangiocytes indicated by immunohistochemical staining for cytokeratin 19. The arrows indicate intact bile ducts. Original magnification: ×20 on E and ×40 on others.
TRAIL/DR5 Regulates Cholestatic Disease Induced by Common Bile Duct Ligation.
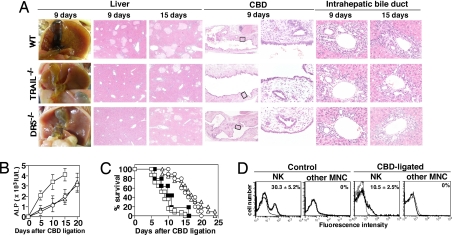
To define the role of endogenous TRAIL/DR5 in cholestasis, we next examined the fate of B6 WT, TRAIL−/−, and DR5−/− mice after common bile duct (CBD) ligation. Weight loss and ruffled fur were obvious in WT mice one day after CBD ligation, but less so in TRAIL−/− and DR5−/− mice (data not shown). Massive hepatocyte damage and inflammation around the CBD and intrahepatic bile ducts were more obvious in WT, compared with TRAIL−/− or DR5−/−, mice 9 days after CBD ligation (Fig. 3A). Fifteen days after CBD ligation, liver injury was comparable among WT, TRAIL−/−, and DR5−/− mice; however, inflammation around intrahepatic bile ducts proceeded in WT mice and some small bile ducts were obstructed (Fig. 3A). Consistently, serum ALP levels were more rapidly elevated in WT mice (Fig. 3B), and TRAIL−/− and DR5−/− mice survived significantly longer than WT mice after CBD ligation (P < 0.001) (Fig. 3C). These results indicate that TRAIL/DR5-mediated signals play an important role in cholangitis, accelerating the onset of hepatic injury and reducing the survival after CBD ligation.
Fig. 3.
TRAIL/DR5 impairment delayed the onset of CBD ligation-induced cholestic disease. (A) Macroscopic and microscopic features of liver and bile duct in B6 WT, TRAIL−/−, and DR5−/− mice at the indicated days after CBD ligation. Original magnifications: ×5 in liver, ×2.5 and ×40 in CBD, and ×40 in intrahepatic bile duct. (B) Time kinetics of serum ALP levels in WT (squares), DR5−/− (circles) and TRAIL−/− (triangles) mice after CBD ligation. (C) Survival rate of WT (open squares), DR5−/− (open circles), TRAIL−/− (open triangles), and anti-asialo GM1 Ab-treated WT mice (filled squares) after CBD ligation. (D) TRAIL expression on liver MNC in control and CBD-ligated mice. Bold lines indicate the staining with anti-TRAIL mAb; thin lines indicate the staining with isotype-matched control Ig.
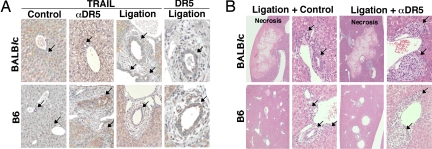
The population of liver natural killer (NK) cells expressing TRAIL was reduced after CBD ligation compared with that observed in control mice, whereas as we previously reported (7), other cells within the mononuclear cells (M-NC) did not express TRAIL (Fig. 3D). NK cell depletion by anti-asialo GM1 Ab did not significantly prolonged survival after CBD ligation, indicating that TRAIL expressed on liver NK cells did not appear to play a major role in CBD ligation-induced cholestatic disease (Fig. 3C). Rather, TRAIL expression was induced on cholangiocytes in CBD-ligated mice, albeit a weaker expression level when compared with that induced on cholangiocytes of obstructed bile ducts in anti-DR5 mAb-treated B6 mice (Fig. 4A). Thus, cholestasis may induce TRAIL expression on mouse cholangiocytes, and that might play a role in cholangitis induced by anti-DR5 mAb and CBD ligation, leading to the irreversible progression toward liver failure.
Fig. 4.
DR5 and TRAIL expression on cholangiocytes in CBD-ligated and anti-DR5 mAb-treated mice. (A) TRAIL and DR5 expression on cholangiocytes of B6 and BALB/c mice 3 days after CBD ligation, or anti-DR5 mAb-treated mice. Original magnification: ×40. (B) Cholangitis was induced with a single injection of anti-DR5 mAb in CBD-ligated mice. Original magnifications: ×5 in the images on the left and ×40 in the images on the right. The arrows indicate bile ducts.
Sensitization of Anti-DR5 mAb-Induced Cholangitis by Common Bile Duct Ligation.
CBD ligation induced necrosis of hepatocytes, fibrosis, and inflammatory cell infiltration around the bile ducts, and vasodilatation, but not biliary obstruction, in both WT B6 and BALB/c mice (Fig. 4B). Notably, in addition to TRAIL expression, DR5 expression on cholangiocytes was augmented by CBD ligation in both mouse strains, particularly in BALB/c mice (Fig. 4A). Single injection of anti-DR5 mAb induced biliary obstruction in CBD-ligated B6 and BALB/c mice, which was reminiscent of that observed in B6 mice repeatedly treated with anti-DR5 mAb; however, anti-DR5 mAb treatment did not significantly augment the hepatocyte necrosis (Fig. 4B).
Contribution of Apoptosis Related Molecules to Anti-DR5 mAb-Induced Cholangitis.
The mitochondrial pathway of apoptosis appeared to play a limited role in anti-DR5 mAb-induced cholestatic liver injury, because disease onset was only partly delayed in B6 Bid−/− mice that are resistant to Fas-induced hepatocellular apoptosis (26) [supporting information (SI) Fig. S1A]. Thus, DR5-induced cholangitis is mediated through distinct signaling pathways to Fas-mediated hepatitis, consistent with its differential regulation of events downstream of truncated Bid (27).
We observed anti-DR5 mAb-induced jaundice in B6, C3H, (B6 × BALB/c) F1, and A/J mice, but not in BALB/c, CB17 SCID, DBA/2, or NOD mice (Fig. S1B). Quantitative PCR analysis demonstrated higher DR5 expression in intrahepatic biliary tree preparation containing cholangiocytes of B6, C3H, and (B6 × BALB/c) F1 mice compared to that of BALB/c mice (Fig. S1C). Moreover, Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein (FLIP) mRNA was reduced in B6 mice, and Myc mRNA expression was higher in C3H mice compared with BALB/c mice (Fig. S1C), both alterations may sensitize these strains to TRAIL/DR5-mediated apoptosis (28). Overall, these results suggested that higher DR5 expression and altered expression of apoptosis-related molecules resulted in a significant increase in the sensitivity of mouse cholangiocytes to DR5-mediated apoptosis.
Significant TRAIL Expression on Cholangiocytes in Human Chronic Cholestatic Diseases.
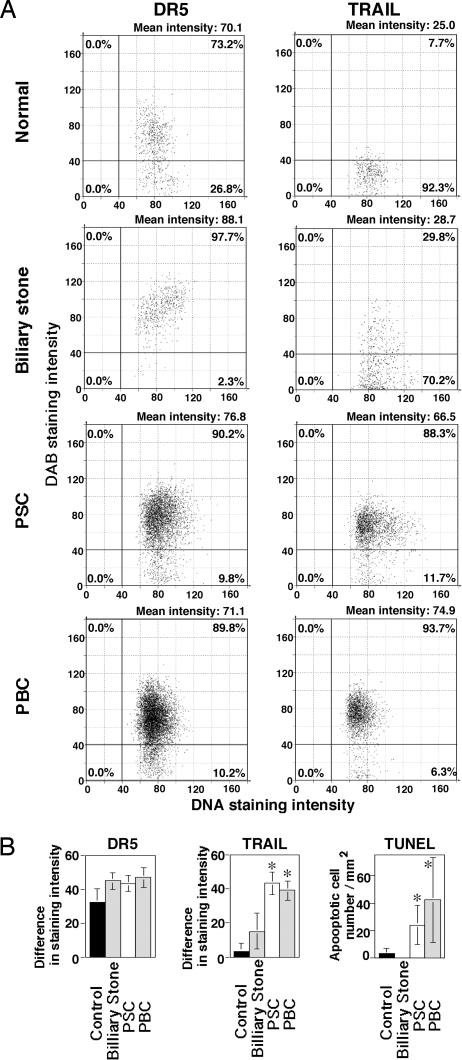
Fibrosis with the appearance of myofibroblasts was observed around intrahepatic bile ducts in anti-DR5 mAb-treated B6 mice and CBD-ligated B6 WT mice, but not anti-DR5 mAb-treated BALB/c mice or CBD-ligated B6 TRAIL−/− or DR5−/− mice (Fig. S2). Notably, anti-DR5 mAb-induced cholangitis was characterized by a typical “onion-skin” histological appearance with the disappearance of biliary epithelial cells and fibrosis (Fig. 2 C, E, and F and Fig. 4B). These features mimic the characteristic histological picture observed in human PSC (23, 24); therefore, we finally examined TRAIL and DR5 expression in normal and diseased human samples. DR5 was similarly expressed on both cholangiocytes and hepatocytes and TRAIL was not expressed on hepatocytes in all of the samples (Fig. 5 and Fig. S3). TRAIL was not expressed on cholangiocytes in normal or CBD-obstructed patients, indicating that in human beings, TRAIL would likely be induced on cholangiocytes by cholestasis alone. However, TRAIL expression on cholangiocytes was significant in all PSC patients (Fig. 5 and Fig. S3). Interestingly, cholangiocytes significantly expressed TRAIL in all primary biliary cirrhosis (PBC) patients, characterized by progressive destruction of bile ducts (24), and apoptosis was observed in cholangiocytes of both PSC and PBC patients (Fig. 5 and Fig. S3). These results raised the possibility that TRAIL/DR5-mediated signaling may be involved in the pathogenesis and pathological features of chronic cholestatic diseases in human beings, particularly in the diseases with progressive destruction of bile ducts.
Fig. 5.
Increased TRAIL expression and apoptosis in cholangiocytes of PSC and PBC patients. (A) Quantification of TRAIL and DR5 expression on cholangiocytes. In the representative sections (presented in Fig. S3) of each group, 324 to 6,313 of cholangiocytes were analyzed using TissueFAXS, and the results were presented as scatter grams using HistoQuest. (B) TRAIL and DR5 expression and number of apoptotic cells in cholangiocytes. Sections were analyzed using MEAND, and data are represented as the mean ± SD (increased % of intensity) of sections from plural patients in each group. ∗, P < 0.05 compared with normal and patients with CBD obstruction caused by biliary stones.
Discussion
Recombinant TRAIL and mAbs against DR4 and DR5 are attractive anticancer drugs currently being applied in cancer therapy, despite the fact that the hepatotoxicity of these reagents is still controversial (29–31). In this article, we demonstrate in a mouse strain-specific manner that agonistic anti-DR5 mAb induced apoptosis of cholangiocytes, resulting in cholangitis and subsequent hepatic injury. Moreover, impaired TRAIL/DR5-mediated signaling alleviated cholangitis and delayed the onset of cholestatic disease after CBD ligation. Thus, DR5-mediated signals play a substantial role in induction of cholangiocyte cell death and cholangitis, and that these events critically contribute to cholestatic disease with fibrosis and subsequent hepatic injury.
It has been reported that bile acid enhances DR5 expression and TRAIL sensitivity of hepatocytes (32), and hepatocyte death induction by TRAIL- and NK1.1-expressing cells has been recently reported under CBD-ligation conditions (33). However, we observed that hepatic damage in TRAIL−/− and DR5−/− mice was similar to that in WT mice 15 days after CBD ligation. Moreover, depletion of TRAIL-expressing NK cells did not prolong survival after CBD ligation. Importantly, cholangitis was greater in WT mice than that observed in TRAIL−/− or DR5−/− mice after CBD ligation, and anti-DR5 mAb treatment induced bile duct obstruction, but not hepatic injury, in CBD-ligated mice. Hence, the endogenous TRAIL/DR5 pathway predominantly contributes to cholangitis rather than hepatocyte death. It is more likely that hepatocytes undergo apoptosis by a combination of TRAIL, FasL, and TNF after cholestasis caused by CBD-ligation or anti-DR5 mAb-induced bile duct obstruction.
CBD ligation sensitized BALB/c mice to anti-DR5 mAb-induced jaundice by enhancement of DR5 expression on cholangiocytes, and we have occasionally (approximately <1%) observed WT BALB/c mice suffering from jaundice after repeated anti-DR5 mAb treatment. Administration of human recombinant TRAIL induced jaundice in a small proportion of B6 mice under some experimental conditions (data not shown), suggesting that decoy receptors might protect cholangiocytes from TRAIL-mediated apoptosis. Moreover, we observed weak FLIP expression in cholangiocytes in some PSC samples (data not shown). Thus, sensitivity to TRAIL/DR5-mediated apoptosis of cholangiocytes may be regulated by both genetic differences and unknown environmental factors not only in mice but also in human beings.
The histopathology observed in anti-DR5 mAb-induced cholangitis in mice is characteristic of human PSC (23, 24), and TRAIL expression in cholangiocytes was significant in both PSC and PBC patients. PBC is presumed to be an autoimmune disease (24); thus, TRAIL might be induced on cholangiocytes responding to cytokines produced during autoreactive immune responses. The pathogenesis of PSC is largely unknown, although immune response has been proposed to play a role (23, 24, 34). Possible mechanisms may include the following: activated gut T cells emigrating to the liver (35) or immune responses against normal flora or their metabolites (23) may play a pathogenic role to produce type I IFNs or IFN-γ (36) that induce TRAIL expression and augment the TRAIL sensitivity (8, 37–39); or autoantibodies reactive with the cholangiocytes in PSC patients (40) may include cell death-inducing anti-DR5 Ab. Anti-DR5 Ab-induced apoptosis is not inhibited by decoy receptors for TRAIL (3) and activation of Fcγ receptor-expressing cells by Ab (14) might facilitate cholangitis as compared with that induced by TRAIL itself. TRAIL has been reported to promote the migration and invasion of cholangiocarcinoma cells (41), and this might be the reason for a higher risk of developing cholangiocarcinoma in PSC patients (23, 24). The prevalence of PSC is significantly greater than previously estimated (23) and PSC currently results in irreversible liver damage, ultimately leading to biliary cirrhosis and premature death unless liver transplantation is performed (23, 24). Thus, genetic, molecular, and cellular investigations into the mechanisms of TRAIL/DR5-mediated cholangitis in extensive clinical studies with greater numbers of subjects will now be required to reveal the exact role of TRAIL/DR5-mediated signaling in the pathogenesis of PSC. Moreover, these investigations will aid the safe application of TRAIL or anti-DR5 mAb therapies to cancer patients and possibly improve the therapeutic index of the TRAIL-DR5 pathway.
Methods
For additional details, see SI Methods.
Mice.
All mice were obtained as described in SI Methods and maintained under specific pathogen-free conditions and used in accordance with the institutional guidelines of Juntendo University and the Peter MacCallum Cancer Centre.
Human Samples.
Tissue specimens were obtained with consent from patients at Juntendo University Hospital and Kanazawa University Hospital. This study was reviewed and approved by the ethical review committee of our universities and had the informed consent from all patients.
Antibody Treatment in Vivo.
Anti-mouse DR5 mAb (MD5–1) and control hamster Ig (UC8–1B9) were prepared as previously described (14). Mice were intraperionially injected with anti-DR5 mAb or control Ig (300 μg per mouse) four times at 3-day intervals. The sera were collected from individual mice 5 days after the last injection. Ten micrograms of purified anti-mouse Fas mAb (Jo2) (BD PharMingen) was injected intravenously into B6 mice, and sera were collected from individual mice 4 h after injection. Serum transaminase (ALT and AST), T-bil, D-bil, and ALP were measured by the standard photometric method using Hitachi type 7350 automatic analyzer (Tokyo, Japan). Data are represented as the mean ± SD of 5 to 10 mice in each group.
Tumor Cell Lines.
FasL- and TRAIL-sensitive L929, mouse TRAIL-transfected 2PK-3, mouse Fas ligand (FasL)-transfected L5178Y, and Fc receptor-expressing P815 cells have been prepared as described previously (14).
Preparation for Primary Hepatocytes, Biliary Epithelial Cells, and Intrahepatic Biliary Tree.
Mice hepatocytes, biliary epithelial cells, or intrahepatic biliary tree was isolated as previously reported (25, 42, 43).
Flow Cytometric Analysis.
Preparation of liver MNC, immunofluorescent staining with phycoerythrin (PE)-conjugated anti-Fas (CD95) mAb (Jo2)(BD Phramingen), PE-conjugated anti-DR5 mAb (MD5–1) (eBioscience), or PE-conjugated anti-TRAIL mAb (N2B2) (e Bioscience), and flow cytometric analyses were performed as previously described (14).
Cytotoxicity Assay.
Cytotoxic activity was tested by a 4-h 51Cr release assay as previously described (14). Data are represented as the mean ± SD of triplicate samples.
CBD Ligation.
CBD ligation was performed in 6- to 8-week-old mice as previously described (32). In some experiments, 3 days after ligation, mice were intraperitonially treated with 300 μg of anti-DR5 mAb or control Ig, and the liver was removed 2 days after the mAb treatment. Some mice were treated intraperitonially with anti-asialo GM1 Ab (Wako Pure Chemical) on days −2, 2, 6, 10, and 14 as previously described (14).
Quantitative Analysis of Immnohistochemical Samples.
Immunohistochemistry was performed as described previously (14). Immunostained sections were quantified with a KS400 image analysis system (Carl Zeiss Imaging Solutions) for optical MEAND. Sections were also analyzed using a TissueFAXS (TissueGnostics) image cytometer, and specific diaminobensidine density on cholangiocytes was analyzed by the analysis software HistoQuest (TissueGnostics).
Reproducibility.
All presented results show the representative data from 3 to 10 independent experiments using 5 to 20 mice in each group. Macroscopic and microscopic photographs of the representative objects are presented.
Statistical Analysis.
Statistical analysis was performed by two-sample t test for the cytotoxicity and quantitative PCR analysis. P values <0.05 were considered as significant.
Supplementary Material
Acknowledgments.
We thank Dr. Allen Wensky and Dr. Astar Winoto for B6 DR5−/− mice; Dr. Stanley J. Korsmeyer for Bid−/− mice. This work was supported by the Ministry of Education, Science, and Culture, Japan (K.T., K.I., K.H., H.I., H.Y., Y.N., and Ko Okumura) the National Health and Medical Research Council of Australia Program Grant and Research Fellowship, and the Cancer Council of Victoria Project Grant (to J.S.); and the Wilhelm-Sander-Stiftung Grant 2005.144.1 (to S.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802702105/DCSupplemental.
References
- 1.Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC. The role of TNF and its family members in inflammation and cancer: lessons from gene deletion. Current Drug Targets. 2002;1:327–341. doi: 10.2174/1568010023344571. [DOI] [PubMed] [Google Scholar]
- 2.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 4.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 6.Weckmann M, et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat Med. 2007;13:1308–1315. doi: 10.1038/nm1660. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, et al. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195:161–169. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ. Targeting death-inducing receptors in cancer therapy. Oncogene. 2007;26:3745–3757. doi: 10.1038/sj.onc.1210374. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walczak H, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 12.Chuntharapai A, et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J Immunol. 2001;166:4891–4898. doi: 10.4049/jimmunol.166.8.4891. [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa K, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolcher AW, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 16.Plummer R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 17.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 18.Jo M, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence D, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 20.Mori E, et al. Human normal hepatocytes are susceptible to apoptosis signal mediated by both TRAIL-R1 and TRAIL-R2. Cell Death Differ. 2004;11:203–207. doi: 10.1038/sj.cdd.4401331. [DOI] [PubMed] [Google Scholar]
- 21.Volkmann X, et al. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498–1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 22.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YM, Kaplan MM. Management of primary sclerosing cholangitis. Am J Gastroenterol. 2002;97:528–534. doi: 10.1111/j.1572-0241.2002.05585.x. [DOI] [PubMed] [Google Scholar]
- 24.Levy C, Lindor KD. Current management of primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol 38 Suppl. 2003;1:S24–S37. doi: 10.1016/s0168-8278(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 25.Cho WK, Mennone A, Boyer JL. Isolation of functional polarized bile duct units from mouse liver. Am J Physiol Gastrointest Liver Physiol. 2001;280:G241–G246. doi: 10.1152/ajpgi.2001.280.2.G241. [DOI] [PubMed] [Google Scholar]
- 26.Yin XM, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 27.Werner AB, de Vries E, Tait SWG, Bontjer I, Borst J. TRAIL receptor and CD95 signal to mitochondria via FADD, caspase-8/10, Bid, and Bax but differentially regulate events downstream from truncated Bid. J Biol Chem. 2002;277:40760–40767. doi: 10.1074/jbc.M204351200. [DOI] [PubMed] [Google Scholar]
- 28.Ricci MS, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest. 2004;113:58–64. doi: 10.1172/JCI200419255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao C, et al. TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Res. 2004;64:8502–8506. doi: 10.1158/0008-5472.CAN-04-2599. [DOI] [PubMed] [Google Scholar]
- 31.Gores GJ, Kaufmann SH. Is TRAIL hepatotoxic? Hepatology. 2001;34:3–6. doi: 10.1053/jhep.2001.25173. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi H, Bronk SF, Taniai M, Canbay A, Gores GJ. Cholestasis increases tumor necrosis factor-related apoptotis-inducing ligand (TRAIL)-R2/DR5 expression and sensitizes the liver to TRAIL-mediated cytotoxicity. J Pharmacol Exp Ther. 2002;303:461–467. doi: 10.1124/jpet.102.040030. [DOI] [PubMed] [Google Scholar]
- 33.Kahraman A, et al. TRAIL mediated liver injury by the innate immune systems in the bile duct-lagated mice. Hepatology. 2008;47:1317–1330. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaRusso NF, et al. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–764. doi: 10.1002/hep.21337. [DOI] [PubMed] [Google Scholar]
- 35.Eksteen B, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baccala R, Kono DH, Theofilopoulos AN. Interferons as pathogenic effectors in autoimmunity. Immunol Rev. 2005;204:9–26. doi: 10.1111/j.0105-2896.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 37.Kayagaki N, et al. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fulda S, Debatin KM. IFNγ sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- 39.Harada K, et al. Innate immune response to double-stranded RNA in biliary epithelial cells is associated with the pathogenesis of biliary atresia. Hepatology. 2007;46:1146–1154. doi: 10.1002/hep.21797. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, Broome U, Ericzon BG, Sumitran-Holgersson S. High frequency of autoantibodies in patients with primary sclerosing cholangitis that bind biliary epithelial cells and induce expression of CD44 and production of interleukin 6. Gut. 2002;51:120–127. doi: 10.1136/gut.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–G136. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- 42.Musallam L, Ethier C, Haddad PS, Bilodeau M. Role of EGF receptor tyrosine kinase activity in antiapoptotic effect of EGF on mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1360–G1369. doi: 10.1152/ajpgi.2001.280.6.G1360. [DOI] [PubMed] [Google Scholar]
- 43.Katayanagi K, Kono N, Nakanuma Y. Isolation, culture and characterization of biliary epithelial cells from different anatomical levels of the intrahepatic and extrahepatic biliary tree from a mouse. Liver. 1998;18:90–98. doi: 10.1111/j.1600-0676.1998.tb00133.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.