Abstract
Autism is a complex disorder that arises from the pervasive action of genetic and epigenetic factors that alter synaptic connectivity of the brain. Although GABA and glutamate receptors seem to be two of those factors, very little is known about the functional properties of the autistic receptors. Autistic tissue samples stored in brain banks usually have relatively long postmortem times, and it is highly desirable to know whether neurotransmitter receptors in such tissues are still functional. Here we demonstrate that native receptors microtransplanted from autistic brains, as well as de novo mRNA-expressed receptors, are still functional and susceptible to detailed electrophysiological characterization even after long postmortem intervals. The opportunity to study the properties of human receptors present in diseased brains not only opens new avenues toward understanding autism and other neurological disorders, but it also makes the microtransplantation method a useful translational system to evaluate and develop novel medicinal drugs.
Keywords: autism, human brain, GABA receptors, glutamate receptors
All of the highly complicated functions of the brain depend ultimately on the transmission of signals across synapses, the points of contact between neurons. Accordingly, many cerebral dysfunctions are due to problems in the process of synaptic transmission. One such synaptic disease is autism (1). The autism spectrum disorder is a multifaceted syndrome, probably due to many different causes (2–4). Although these causes are largely unknown, genetic and biochemical studies strongly implicate the GABA and glutamate neurotransmitter receptors, which are, respectively, the principal inhibitory and excitatory receptors in the mammalian brain, including that of humans (5–11). Despite the immense importance that neurotransmitter receptors have for brain functioning, relatively little is known about the functional properties of receptors of the human brain; almost nothing is known about those of the autistic brain. This dearth of information is mainly due to the difficulties inherent in experimenting with human brain cells.
We have developed two methods that allow in-depth studies of neurotransmitter receptors and ion channels of the human brain. The first, widely used method involves the heterologous expression of human receptors in Xenopus oocytes, which, because of their size, sturdiness, and availability, permit experiments that would be very difficult or impossible to carry out in the human brain. For this method, mRNA is isolated from the brain and injected into the oocytes. These cells translate the foreign messages, process and assemble the expressed receptor proteins, and insert them into their plasma membrane where they can be studied in molecular detail (12, 13).
The more recent and perhaps more powerful method entails isolating cell membranes that are then injected into the oocytes. The foreign membranes, carrying their original receptors and channels together with lipids and any associated proteins, fuse with the oocyte membrane where, again, they can be easily analyzed. This method has been used to microtransplant the receptors and channels present in membranes isolated from freshly dissected tissues or cultured cells to the oocyte membrane (14–17). Microtransplantation of receptors from brain tissues surgically resected from the brains of epileptic patients has already yielded important information on the functional properties of the “epileptic” receptors showing clearly that the microtransplantation method is very useful when fresh tissues are being used (18–20). However, fresh human brain tissues are difficult to obtain, and it was highly desirable to determine whether the frozen postmortem brain tissues that are available from brain banks all over the world could be used to study the function and structure of neurotransmitter receptors and ion channels of the human brain. Initial experiments using tissues from postmortem Alzheimer's brains have already shown that their cell membranes contain functional GABA and glutamate receptors as well as calcium channels (21, 22). However, the postmortem intervals (PMIs) of the brains used in those studies were mostly only a few hours in duration (<5.3 h). For other diseases, such as autism, the available brains have much longer PMIs (usually >10 h). Therefore, the present experiments were carried out to determine whether such postmortem brains still contained functional neurotransmitter receptors and voltage-operated ion channels that could be microtransplanted and to see whether the brain mRNAs were capable of expressing functional receptors in Xenopus oocytes. For those purposes, the autistic cerebellum and temporal cortex were chosen, because neuropathological and biochemical studies have shown abnormalities in the neuronal organization of those areas (7, 23, 24).
Results
Expression of Receptors by mRNA.
The use of the oocyte as an expression system for exogenous mRNAs is well established (12, 25). Accordingly, we began this work by using this assay to allow comparison with the more recently developed microtransplantation assay. Many studies have shown that, although some postmortem brain mRNAs are degraded by agonal factors and PMI, they are still very useful for genetic studies (26–29). However, very little is known about their expressional potency, and we have frequently seen that some human brain mRNAs with apparently good quality and integrity expressed weakly or failed to express functional receptors in Xenopus oocytes. To determine whether mRNA from the postmortem autistic brain was able to express functional neurotransmitter receptors, poly(A+)RNA was isolated from the temporal lobe (TL) of two different autistic-control, age-matched pairs (PMI range 9–17 h; see Table 1).
Table 1.
Tissue information
| Source | Brain | Dx | Age, years | Sex | PMI, h | SD, years | Tissues | Cause of death |
|---|---|---|---|---|---|---|---|---|
| ATP | B6207 | Control | 16 | M | 26.6 | 3.6 | Cb | Heart attack |
| ATP | B5666 | Autism | 8 | M | 22.16 | 5.3 | Cb | Sarcoma |
| ATP | B6076 | Control | 38 | M | 25.47 | 4.1 | Cb | Unknown |
| ATP | B6401 | Autism | 39 | M | 13.95 | 3.8 | Cb | Cardiac tamponade |
| NICHD | 4670* | Control | 4 | M | 17 | 2.8 | TL, Cb | Commotio cordis |
| NICHD | 4671* | Autism | 4 | F | 13 | 2.7 | TL, Cb | Multiple injuries |
| NICHD | 4898 | Control | 7 | M | 12 | 1.7 | TL, Cb | Drowning |
| NICHD | 4849 | Autism | 7 | M | 20 | 1.8 | TL, Cb | Drowning |
| NICHD | 1706 | Control | 8 | F | 20 | 5.3 | Cb | Rejection of cardiac transplant |
| NICHD | 4721 | Autism | 8 | M | 16 | 2.7 | Cb | Drowning |
| NICHD | 4722* | Control | 14 | M | 16 | 2.8 | TL, Cb | Multiple injuries |
| NICHD | 4899* | Autism | 14 | M | 9 | 1.7 | TL, Cb | Drowning |
Autistic-control pairs were selected according to age. ATP, Autism Tissue Program, Harvard Brain Tissue Resource Center; NICHD, National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders; Dx, diagnosis; M, male; F, female; SD, storage duration; Cb, cerebellum.
*Tissues used for mRNA preparations.
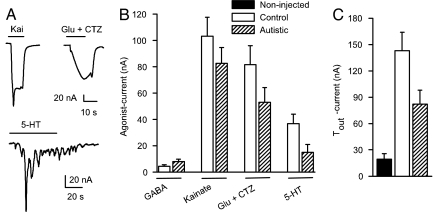
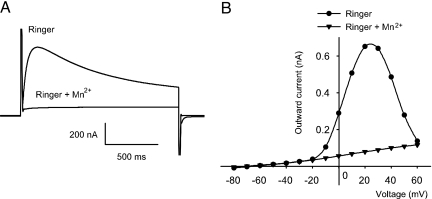
Oocytes injected with mRNA from control or autistic brains expressed receptors to GABAA, glutamate, serotonin, and acetylcholine, which generated inward currents when activated by their corresponding neurotransmitters (Fig. 1). The ionotropic GABA, glutamate, kainate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and N-methyl-d-aspartic acid receptors elicited smooth currents, whereas the metabotropic receptors to serotonin, acetylcholine, and glutamate elicited oscillatory chloride currents characteristic of those mediated by activation of the phosphoinositide cascade (30, 31). In addition to these receptors, the oocytes also expressed voltage-operated ion channels, as evidenced by a significant increase of the native Tout current (Figs. 1C and 2). This current, which is blocked by Mn2+ ions (Fig. 2), arises because depolarization of the membrane opens calcium channels and causes an influx of calcium, which in turn opens calcium-gated chloride channels (32).
Fig. 1.
Expression of human neurotransmitter receptors and calcium channels in oocytes injected with mRNAs from postmortem autistic brains. (A) Sample current responses to 100 μM kainate, 1 mM glutamate plus 10 μM CTZ, and 30 μM serotonin (5-HT) of one oocyte injected with TL mRNA from an autistic brain (case 4671, PMI = 13 h). (B) Maximum agonist-induced currents of receptors expressed in oocytes injected with TL mRNAs from two autistic and two age-matched controls (n = 17 oocytes for each column). (C) Tout currents from oocytes injected with mRNA from two autistic-control pairs. The increment in Tout current elicited by voltage pulses from −80 to 0 mV indicates the functional expression of voltage-dependent calcium channels in injected oocytes (n = 17 oocytes for each column).
Fig. 2.
Voltage dependence of the Tout current. (A) Tout current from one oocyte injected with mRNA from an autistic temporal cortex (case 4671) before and during application of Ringer plus 5 mM Mn2+. (B) Voltage–current relationship of the Tout current before and during perfusion of Mn2+.
Compared to mRNAs isolated from fresh rat or human brain tissues (13, 25, 33), these postmortem mRNAs were weaker at expressing receptors and ion channels, and there were no significant differences between autistics and controls (Fig. 1B). However, many more tissue samples need to be studied to clearly determine the expressional potencies of control and autistic spectrum disorder mRNAs and the electrophysiological and pharmacological characteristics of the heterogeneously expressed receptors and ion channels.
Microtransplantation of Receptors.
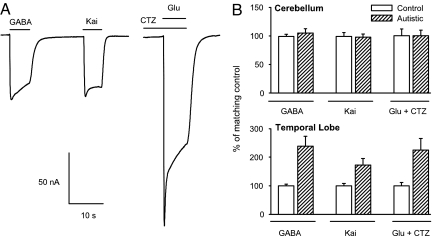
Having established the efficacy of mRNA translation, we then tested the microtransplantation assay. To see whether the original receptors present in the postmortem autistic brain were still functional, membranes were isolated from the cerebella of six different autistic-control, age-matched pairs and from the TL of three autistic-control pairs (PMI range 9–26 h). Within a few hours after injection into the oocytes, the membranes, mostly in the form of small vesicles and carrying the native human receptors (15), fuse with the oocyte membrane, and the oocytes become responsive to GABA, kainate, and glutamate. Because glutamate-currents exhibited a strong desensitization, glutamate was coapplied with cyclothiazide (CTZ) after 80 s of preincubation with CTZ to inhibit desensitization of AMPA-type glutamate receptors (34) (Fig. 3). These results clearly indicated that the postmortem receptors were indeed still functional. The short latency of the agonist responses suggests that the transplanted receptors were mostly of the ionotropic type. Occasionally, some microtransplanted oocytes with large ionotropic currents (e.g., >200 nA GABA-current) presented oscillatory responses to glutamate and acetylcholine, implying that functional metabotropic receptors were also incorporated into the oocyte's membrane and were probably coupled to the phosphoinositide-signaling pathway of the oocyte. The amplitudes of the current responses of ionotropic GABA and glutamate receptors increased progressively with time and reached a maximum 1–3 days after membrane injection. This increment probably reflects the continued fusion of human membrane vesicles with the oocyte membrane. Thereafter, the currents decayed progressively, but in many oocytes the receptors were still functional >1 week after injection.
Fig. 3.
Microtransplanted GABA and glutamate receptors from autistic and control brains. (A) Sample responses to 1 mM GABA, 100 μM kainate, and 1 mM glutamate plus 10 μM CTZ of an oocyte injected with membranes from an autistic TL (case 4671, PMI = 13 h). CTZ was preapplied for 80 s to reduce glutamate receptor desensitization. (B) Pooled agonist-induced currents elicited by native receptors in oocytes injected with membranes from TL (n = 19 oocytes for each column, 3 autistic-control pairs, 5–7 oocytes per brain), and cerebellar membranes (n = 72–96 oocytes for each column, 6 autistic-control pairs, 9–27 oocytes per brain from 1–3 preparations). Percentages are expressed relative to control.
The responses of receptors microtransplanted from autistic cerebella varied among the different cases. Of the six autistic cerebella tested, four of them (cases B6041, B5666, 4899, and 4721) (Table 1) yielded responses to GABA, kainate, and glutamate plus CTZ that were smaller than their respective paired controls. The other two cases had bigger responses to both receptor agonists (cases 4849 and 4671). Fig. 3 shows the pooled data from all autistic cerebella relative to their controls. A different panorama appeared in oocytes injected with membranes from the temporal cortex; the relative amplitudes of the kainate-, glutamate plus CTZ-, and GABA-currents of the autistic samples were larger than their controls in two of the three autistic cases (cases 4671 and 4899) (Fig. 3). Clearly, more samples and determination of the frequency distribution of the response amplitudes are needed to establish the appropriate statistical model to be used for comparing the neurotransmitter receptors from autistic and control brains and to determine the possible effects of the PMI on the amplitudes and characteristics of the currents. Nonetheless, our results already indicate that the microtransplantation assay is as robust, if not more so, than that for mRNA translation.
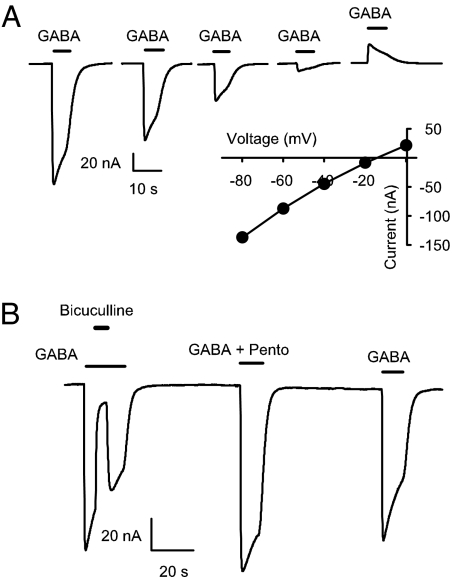
Some receptor properties were briefly examined. The glutamate-induced currents in oocytes transplanted with either autistic or control receptors demonstrated fast activation and desensitized almost completely. Preincubation with CTZ potentiated the glutamate responses, indicating that native AMPA receptors were adequately transplanted to the oocyte membrane. Moreover, native GABA receptors from autistic and control brains elicited characteristic desensitizing currents with a reversal potential of approximately −20 mV, which corresponds to the chloride equilibrium potential in Xenopus oocytes (35). Moreover, the GABA-currents elicited by receptors transplanted from postmortem control or autistic brains were blocked by bicuculline, the specific GABAA receptor antagonist, and were potentiated by pentobarbital (Fig. 4). Bicuculline (100 μM) reduced the control and autistic GABA-currents (1 mM) by 93 ± 5% and 87 ± 4%, whereas pentobarbital (100 μM) increased the GABA-currents to 121 ± 7% and 123 ± 4%, respectively (n = 5 oocytes for one autistic-control pair). These results indicate that the transplanted receptors are mainly of the GABAA type, one of the receptors thought to be the target of genetic factors associated with autism.
Fig. 4.
Properties of autistic GABA-currents. (A) Currents elicited by 1 mM GABA applied at different holding potentials to an oocyte injected with membranes from an autistic TL (case 4899, PMI = 9 h). The current/voltage relation shows an equilibrium potential close to −20 mV. (B) Effects of 100 μM bicuculline and 100 μM pentobarbital on GABA currents elicited by receptors transplanted from the TL of an autistic brain (case 4899).
Discussion
Despite some degradation, nucleic acids from postmortem autistic brains have been very useful for genetic studies. Investigations (7, 36–38) have shown a link between autism and many genes on chromosomes 15, 5, and 7, which encode GABAA and glutamate receptors and transporter subunits. Postmortem autistic brain tissues have also been used for anatomical studies that showed a decreased number of Purkinje cells in the cerebellum and small cell size and increased cell density in the hippocampus, amygdala, and entorhinal cortex (23). An increased number of neurons in the cerebral cortex has also been described (24). Furthermore, ligand-binding studies in postmortem tissues have shown that GABA receptors containing the benzodiazepine ligand-binding site are substantially reduced in the hippocampus (5, 9) and that AMPA1 receptors are reduced in the cerebellum (7).
However, almost nothing was known about the functional properties of the receptors present in the autistic postmortem brain. Our results show conclusively that, even after postmortem intervals >1 day and frozen storage of >5 years, human brain GABA and glutamate receptors, as well as calcium channels, are still functional. Moreover, these receptors and channels can be transplanted to the plasma membrane of Xenopus oocytes.
These initial experiments suggest that the TL mRNAs of autistic and control brains express approximately the same numbers of GABA and glutamate receptors (Fig. 1B), but, interestingly, the autistic mRNAs expressed fewer calcium channels, as evidenced by the smaller Tout currents of the oocytes injected with autistic mRNAs (Fig. 1C). Because the tissue sample is still low, it is premature to speculate about these findings. Nonetheless, future studies of the voltage-dependent calcium channels of the autistic brain are granted. However, the temporal cortices of the autistic brains appear to contain more functional GABA and glutamate receptors than their controls, perhaps as a result of the increased number of neurons and functional minicolumns in the autistic cerebral cortex (24). In the cerebellum, the situation seems to be more complex, with a subgroup of autistic brains having fewer GABA and glutamate receptors and another subgroup having more receptors. The average of all data from the autistic cerebella showed no overall difference (Fig. 3B). Whereas the reported decrease of Purkinje cells may account for the smaller GABA- and glutamate- currents observed in our experiments, the increased currents need further electrophysiological studies combined with neuropathological analyses of the same samples.
Because of the multiple origins and symptoms of the autism spectrum disorders, we are fully aware that it is necessary to study many more brains and tissues from many areas. However, it is already sufficiently clear that the microtransplantation of the original receptors from postmortem brains coupled with the expression of receptors by postmortem mRNAs will help to determine in great detail the type, number, and functional properties of autistic neurotransmitter receptors and channels. These procedures will help in deciphering the genetic and epigenetic mechanisms that underlie not only autism, but also other brain pathologies. Furthermore, the microtransplantation method will help to determine the mode of action of the medicines presently used to treat autism, and, perhaps more importantly, it will help to develop new medicines and to evaluate their pharmacological activity.
Materials and Methods
Isolation of mRNA.
Total RNAs from the temporal cortices of two autistic (4671 and 4789) and two control brains (4670 and 4722) were isolated by using TRIzol (Invitrogen), and the poly(A+)RNA was separated by using the Oligotex resin (Qiagen). Between 1 and 1.8 g of frozen tissue was homogenized in TRIzol reagent according to the manufacturer's instructions and by using a rotor-stator Tissue Master 240 (Omni International). The probe was thoroughly rinsed with diethyl pyrocarbonate-treated water and cleaned with a dry wipe before every preparation. Yields ranged from 679 to 1561 μg of total RNA. Each total RNA sample was dissolved in 310 μl of RNase-free water. Ten microliters was saved for quantification analysis, and the rest was diluted with 300 μl of buffer OBB from the Oligotex kit. Thirty microliters of resin was added, and the batch protocol was followed as described in the kit's booklet until the elution step, which was replaced by adding 30 μl of hot elution buffer. After 1 min at 70°C in a water bath, the sample was placed on a filter column and centrifuged for 2 min to remove the resin. The concentration of the elution was determined, and, if <1 mg/ml, it was precipitated with 1 vol of LiCl 7.5 M for 1 h, then washed with 75% ethanol, and resuspended in RNase-free water. The isolated poly(A+)RNA was dissolved in water to concentrations between 1 and 6 mg/ml and used directly or stored at −70°C until injection into Xenopus oocytes.
Preparation and Microtransplantation of Brain Membranes.
Membranes from 0.2–0.6 g of human cerebellum or TL were isolated and injected into oocytes by using procedures described in detail elsewhere (15, 21). Briefly, pieces of the frozen tissues were placed in ice-cold buffer (pH 9.0) with 200 mM glycine, 150 mM NaCl, 50 mM EDTA, 50 mM EGTA, and 300 mM sucrose and protease inhibitors (Sigma P2714). The sample was homogenized with a glass homogenizer and centrifuged (9,500 × g for 15 min at 4°C). The supernatant was ultracentrifuged in a Beckman SW41 rotor at 100,000 × g for 2 h at 4°C. The resultant pellet was resuspended in sterile distilled water and stored at −70°C. Protein concentration was determined by using a Coomassie protein assay reagent kit (Pierce). Fifty nanoliters of a membrane preparation (protein concentration 1–1.5 mg/ml) was injected into stage V–VI Xenopus oocytes (15). Injected oocytes were kept in Barth's solution [88 mM NaCl, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 1 mM KCl, 0.82 mM MgSO4, 2.4 mM NaHCO3, 10 mM Hepes (pH 7.4)] at 16–17°C until the moment of recording.
Electrophysiological Recordings.
From day 1 to day 7 after injection, membrane currents were recorded from oocytes voltage-clamped at −80 mV, using two microelectrodes filled with 3 M KCl (32). The oocytes were placed in a recording chamber (volume ≈0.1 ml) and perfused continuously (5–10 ml/min) with Ringer's solution [115 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 5 mM Hepes (pH 7.4)] at room temperature (19–21°C). Data acquisition and analyses were performed by using either custom-written Windows programs (39) or WinEDR ver 2.3.8 Strathclyde Electrophysiology software (John Dempster, Glasgow, United Kingdom). Results are expressed as mean ± SEM. Before use, the neurotransmitter receptor agonists were diluted in Ringer from stocks kept at −20°C. Kainate and CTZ were from Tocris Biosciences. Other chemicals were from Sigma.
Acknowledgments.
We thank Drs. N. C. Spitzer and E. Cherubini for help with the manuscript and Dr. J. Dempster for kindly providing the WinEDR. Human brain tissue samples were generously provided by the Autism Tissue Program via the Harvard Brain Tissue Resource Center (Belmont, MA) and by the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders (Baltimore, MD). This work was supported by American Health Assistance Foundation Grant A2006-054.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 10641.
References
- 1.Zoghbi HY. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 2.Pickles A, et al. Latent-class analysis of recurrence risks for complex phenotypes with selection and measurement error: A twin and family history study of autism. Am J Hum Genet. 1995;57:717–726. [PMC free article] [PubMed] [Google Scholar]
- 3.Belmonte M-K, et al. Autism as a disorder of neural information processing: Directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 4.Persico A-M, Bourgeron T. Searching for ways out of the autism maze: Genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Blatt G-J, et al. Density and distribution of hippocampal neurotransmitter receptors in autism: An autoradiographic study. J Autism Dev Disord. 2001;31:537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997;7:269–278. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- 7.Purcell A-E, Jeon O-H, Zimmerman A-W, Blue M-E, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- 8.Ma D-Q, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guptill J-T, et al. [3H]-Flunitrazepam-labeled benzodiazepine binding sites in the hippocampal formation in autism: A multiple concentration autoradiographic study. J Autism Dev Disord. 2007;37:911–920. doi: 10.1007/s10803-006-0226-7. [DOI] [PubMed] [Google Scholar]
- 10.Hogart A, Nagarajan R-P, Patzel K-A, Yasui D-H, LaSalle J-M. 15q11–13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao H-T, Zoghbi H-Y, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miledi R, Parker I, Sumikawa K. Transplanting receptors from brains into oocytes. In: Smith J, editor. Fidia Research Foundation Neuroscience Award Lecture Series. Vol 3. New York: Raven; 1989. pp. 57–90. [Google Scholar]
- 13.Palma E, et al. Expression of human epileptic temporal lobe neurotransmitter receptors in Xenopus oocytes: An innovative approach to study epilepsy. Proc Natl Acad Sci USA. 2002;99:15078–15083. doi: 10.1073/pnas.232574499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsal J, Tigyi G, Miledi R. Incorporation of acetylcholine receptors and Cl-channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc Natl Acad Sci USA. 1995;92:5224–5228. doi: 10.1073/pnas.92.11.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miledi R, Eusebi F, Martinez-Torres A, Palma E, Trettel F. Expression of functional neurotransmitter receptors in Xenopus oocytes after injection of human brain membranes. Proc Natl Acad Sci USA. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Martínez A, Reyes-Ruiz J-M, Martínez-Torres A, Miledi R. Functional expression in frog oocytes of human rho 1 receptors produced in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2004;101:682–686. doi: 10.1073/pnas.0307564100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miledi R, Palma E, Eusebi F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: New procedure for ion channel studies. Methods Mol Biol. 2006;322:347–355. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- 18.Palma E, et al. Phosphatase inhibitors remove the run-down of gamma-aminobutyric acid type A receptors in the human epileptic brain. Proc Natl Acad Sci USA. 2004;101:10183–10188. doi: 10.1073/pnas.0403683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ragozzino D, et al. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy. Proc Natl Acad Sci USA. 2005;102:15219–15223. doi: 10.1073/pnas.0507339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma E, et al. GABA(A)-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABA(A) “phasic” receptors. Proc Natl Acad Sci USA. 2007;104:20944–20948. doi: 10.1073/pnas.0710522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miledi R, Dueñas Z, Martinez-Torres A, Kawas C-H, Eusebi F. Microtransplantation of functional receptors and channels from the Alzheimer's brain to frog oocytes. Proc Natl Acad Sci. 2004;101:1760–1763. doi: 10.1073/pnas.0308224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernareggi A, Dueñas Z, Reyes-Ruiz J-M, Ruzzier F, Miledi R. Properties of glutamate receptors of Alzheimer's disease brain transplanted to frog oocytes. Proc Natl Acad Sci USA. 2007;104:2956–2960. doi: 10.1073/pnas.0611513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauman M-L, Kemper T-L. Neuroanatomic observations of the brain in autism: A review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Bailey A, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 25.Gundersen C-B, Miledi R, Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc London Ser B. 1983;219:103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- 26.Cummings T-J, Strum J-C, Yoon L-W, Szymanski M-H, Hulette C-M. Recovery and expression of messenger RNA from postmortem human brain tissue. Mod Pathol. 2001;14:1157–1161. doi: 10.1038/modpathol.3880451. [DOI] [PubMed] [Google Scholar]
- 27.Hynd M-R, Lewohl J-M, Scott H-L, Dodd P-R. Biochemical and molecular studies using human autopsy brain tissue. J Neurochem. 2003;85:543–562. doi: 10.1046/j.1471-4159.2003.01747.x. [DOI] [PubMed] [Google Scholar]
- 28.Mexal S, et al. Brain pH has a significant impact on human postmortem hippocampal gene expression profiles. Brain Res. 2006;1106:1–11. doi: 10.1016/j.brainres.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Atz M, et al. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309. doi: 10.1016/j.jneumeth.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gundersen C-B, Miledi R, Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc London Ser B. 1984;221:127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- 31.Parker I, Sumikawa K, Miledi R. Activation of a common effector system by different brain neurotransmitter receptors in Xenopus oocytes. Proc R Soc London Ser B. 1987;231:37–45. doi: 10.1098/rspb.1987.0034. [DOI] [PubMed] [Google Scholar]
- 32.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc London Ser B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 33.Ragsdale D-S, Miledi R. Expressional potency of mRNAs encoding receptors and voltage-activated channels in the postmortem rat brain. Proc Natl Acad Sci USA. 1991;88:1854–1858. doi: 10.1073/pnas.88.5.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fucile S, Miledi R, Eusebi F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc Natl Acad Sci USA. 2006;103:2943–2947. doi: 10.1073/pnas.0511063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusano K, Miledi R, Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samaco R-C, Hogart A, LaSalle J-M. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fatemi S-H, et al. Reelin signaling is impaired in autism. Biol Psychiatry. 2005;57:777–787. doi: 10.1016/j.biopsych.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y-H, et al. A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am J Med Genet. 2004;131:1–10. doi: 10.1002/ajmg.a.30297. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen Q-T, Miledi R. A Windows software package to record from voltage-clamped Xenopus oocytes. J Neurosci Methods. 1995;61:213–219. doi: 10.1016/0165-0270(95)00047-x. [DOI] [PubMed] [Google Scholar]