Abstract
The proximity of mates can influence mating opportunities and the quantity and quality of offspring, especially in dioecious plant species. Progeny sex ratios modulated by environmental conditions is one of the most radical ways in which offspring quality may be influenced, yet it has rarely been reported in plants. A mechanism proposed to influence progeny sex ratios in dioecious plants involves competition between female- and male-determining microgametophytes (certation) as a result of variation in pollination intensity. However, the role of selective fertilization in dioecious plants is controversial and has not been demonstrated under field conditions. Here we investigate whether natural variation in the spatial arrangement of females and males influences pollination intensity and progeny sex ratios in the wind-pollinated herb Rumex nivalis. Based on previous experimental manipulation of pollination intensity in this species, we predicted that maternal parents in close proximity to males would produce more strongly female-biased progeny sex ratios. We tested this prediction in six alpine populations in Switzerland by measuring the distance between focal females and neighboring males and assessing pollen loads and seed sex ratios of maternal parents. In four of the six populations, females positioned in close proximity to males captured more pollen and exhibited more female-biased sex ratios. Our results demonstrate that demographic aspects of the maternal mating environment can influence progeny sex ratios. The most probable explanation for biased primary sex ratios in Rumex is selective fertilization resulting from pollen tube competition.
Keywords: female-biased sex ratios, pollination intensity, selective fertilization
The spatial context in which reproduction occurs is of critical importance for plants because of their sessile habit. Most plants mate and disperse offspring locally, so that mating success is context-dependent and influenced by plant density and the phenotypic composition of neighborhoods (1–3). Dioecious species are especially sensitive to spatial structure and composition because of the restricted number of mating groups within populations. Female reproductive success can be influenced by male flowering density, depending on the extent of pollen dispersal (4–6). Patch density and the local sex ratio may also affect parental fitness through their influence on pollination intensity. The amount of pollen captured by stigmas could potentially affect both the quality of offspring and progeny sex ratios through gametophytic competition (certation) and selective fertilization (7, 8). However, the relative roles of genetic and environmental factors in governing primary sex ratios in dioecious populations are still poorly understood, and evidence for environmentally induced variation in primary sex ratios is limited despite considerable heterogeneity in seed sex ratios (9).
Sex determination induced by the environment is one of the most direct ways in which progeny sex ratios can vary. Environmental sex determination is expected to be adaptive if the environment experienced during development is variable and exerts a sex-dependent influence on fitness (10, 11). There are numerous examples in the animal kingdom in which environmental sex determination results in biased progeny sex ratios, with environmental triggers such as temperature or parental condition commonly involved (12–14). However, because plant species are generally more plastic than animals in gender expression, they typically show environmentally influenced sex-allocation plasticity or sex inconstancy during flowering (15–17). Evidence for environmental sex determination early in development is relatively rare in seed plants (e.g., Spinacia oleracea: ref. 18), and how frequent environmental influences interact with genetic sex determination mechanisms to influence progeny sex ratios is unclear.
In the dioecious herb Rumex nivalis (Polygonaceae), sex determination is governed by heteromorphic sex chromosomes with females homogametic XX and males heterogametic XY1Y2 (19, 20). Similar to several other dioecious species of Rumex with sex chromosomes (7, 21–24), there is evidence that progeny sex ratios are also influenced by nongenetic factors, specifically the amount of pollen deposited on stigmas. By experimentally manipulating the distance between male pollen donors and female recipients of R. nivalis in a common garden, Stehlik and Barrett (25) demonstrated that seed sex ratios were dependent on the specific maternal pollination environment. Females at closer distances to males had higher stigmatic pollen loads and produced more strongly female-biased seed sex ratios compared with more distant females. However, attempts to demonstrate relations between male proximity and progeny sex ratios have been inconclusive in other Rumex species (23, 24), and the role of maternal pollination environment in affecting progeny sex ratios in natural populations has not been investigated.
Here we investigate whether the composition of the local mating neighborhood in natural populations of dioecious R. nivalis influences progeny sex ratios. Under the certation hypothesis, progeny sex ratios of maternal parents of R. nivalis located in close proximity to males should be more female-biased as a result of higher pollen loads, leading to increased competition between female- versus male-determining pollen tubes. We tested elements of this prediction in six natural populations of R. nivalis in the Swiss Alps. We mapped the location of plants in each population and measured pollen loads of females. Sex-specific molecular markers and censuses of flowering sex ratios of maternal families were then used to determine the sex of offspring. Our results provide evidence for an environmental influence on primary sex ratios in a dioecious plant.
Results
Variation in Population Size, Density, and Flowering Sex Ratios.
The six populations varied in flowering population size (mean number of individuals = 1,145.8, SE = 163.1, range = 614–1,589) and flowering male density (mean males per square meter = 0.64, SE = 0.08, range = 0.12–1.26; Table 1). All populations were strongly female-biased (mean sex ratio = 0.75, SE = 0.03, range = 0.61–0.85; Table 1), a feature typical of R. nivalis populations (26). As a result of variation in male density, there was a wide range of distances between focal females and surrounding males. In the case of the distance between a focal female and its fourth-nearest male (the independent variable in statistical analyses), the mean distance was 1.25 m (SE = 0.07, range = 0.24–6.7).
Table 1.
Summary of population parameters for six natural populations of dioecious R. nivalis in Switzerland used in the study of progeny sex ratio bias
| Population | Geographic coordinates | Altitude, m above sea level | Population size | Male density, no. per m2 | Sex ratio | No. of focal females |
|---|---|---|---|---|---|---|
| Arosa1 | 9°37′7.2″/46°46′6.1″ | 2,500 | 1,333 | 0.98 | 0.61 | 25 |
| Arosa2 | 9°37′7.3″/46°46′9.3″ | 2,250 | 614 | 0.12 | 0.85 | 21 |
| Davos1 | 9°49′20.0″/46°41′32.3″ | 2,500 | 816 | 0.40 | 0.72 | 30 |
| Davos2 | 9°48′33.7″/46°41′49.4″ | 2,330 | 1,542 | 1.26 | 0.77 | 29 |
| Flims | 9°16′21.8″/46°52′40.5″ | 2,500 | 981 | 0.53 | 0.78 | 26 |
| Saentis | 9°21′65.9″/47°14′30.1″ | 2,120 | 1,589 | 0.54 | 0.78 | 28 |
Pollen Load, Seed Set, and Seed Sex Ratios.
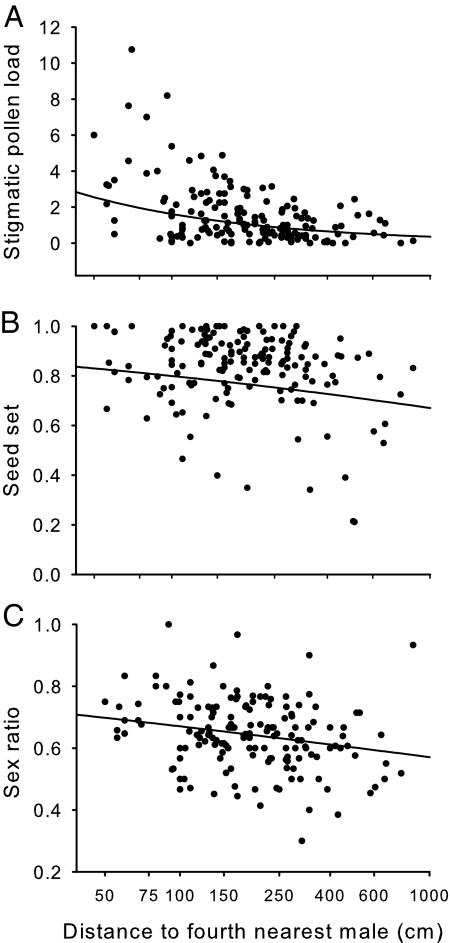
The distance between a focal female and the fourth-nearest male had a significant effect on pollen capture in populations of R. nivalis (Fig. 1A and Table 2). Stigmatic pollen loads were largest in females with males in close proximity, whereas pollen loads decreased in females with male neighbors at further distances (Fig. 1A and Table 2). There was significant variation in pollen capture among the six populations (Table 2). This effect was partially due to significantly lower female pollen loads in Flims compared to other populations (partial regression coefficient, b = −0.269, SE = 0.208, χ2 = 6.76, P < 0.05), and Arosa2, in which pollen capture did not decrease with increasing distance to males (partial regression coefficient, b = 0.512, SE = 0.681, χ2 = 0.57, P = 0.45). We detected no pollen on 44% of sampled stigmas. The mean number of pollen grains per stigma for all females in the six populations was 1.65 (SE = 0.5). If we exclude stigmas that captured no pollen, the mean pollen load was 2.95 (SE = 0.08).
Fig. 1.
The relation between stigmatic pollen loads (A), seed set (B), and seed sex ratios (C) of focal females and their distance to the fourth-nearest male in six populations of R. nivalis in Switzerland. See the text and Table 2 for statistical details. The predicted relations based on the generalized linear models are depicted. The equations for these relationships are as follows: y = e3.51−0.66x (A); y = e2.38−0.14x/(1 + e2.38−0.14x) (B); and y = e1.56−0.18x/(1 + e1.56−0.18x) (C).
Table 2.
Summary of statistical analyses for the influences on stigmatic pollen load, seed set, and seed sex ratio for six natural populations of R. nivalis
| Model | Sources of variation | Test results |
|---|---|---|
| Stigmatic pollen load | Population | χ25 = 13.10* |
| Distance to fourth-nearest male | χ21 = 15.76*** | |
| Population × distance to fourth-nearest male | χ25 = 11.38* | |
| Seed set | Population | χ25 = 110.78*** |
| Distance to fourth-nearest male | χ21 = 7.46** | |
| Population × distance to fourth-nearest male | χ25 = 117.12*** | |
| Sex ratio | Population | χ25 = 12.72* |
| Distance to fourth-nearest male | χ21 = 8.83** | |
| Population × distance to fourth-nearest male | χ25 = 12.57* |
*, P < 0.05;
**, P < 0.01;
***, P < 0.001.
The distance between a focal female and its fourth-nearest male had a significant influence on seed set in R. nivalis (Fig. 1B and Table 2) despite high average seed set in all populations (mean = 0.83, SE = 0.01). Females in closer proximity to males set more seed than females farther away from males (Fig. 1B and Table 2). Additionally, there was a significant population effect and a significant interaction between population and distance to the fourth-nearest male (Table 2). This pattern was largely driven by Arosa1, in which seed set increased with increasing distance to males (partial regression coefficient, b = 0.906, SE = 0.254, χ2 = 13.63, P < 0.001).
The distance between focal females and their male neighbors had a significant influence on the degree of female bias in the progeny sex ratios of seeds (Fig. 1C and Table 2). Females with males in close proximity had the strongest female bias with decreasing bias with increasing distance between female and male plants (Fig. 1C and Table 2). There was a significant population effect and a significant interaction between population and distance to the fourth-nearest male (Table 2). These effects were largely due to the absence of association between seed sex ratios and distance to males for Davos1 (partial regression coefficient, b = 0.014, SE = 0.122, χ2 = 0.01, P = 0.91) and Arosa2 (partial regression coefficient, b = 0.121, SE = 0.126, χ2 = 0.92, P = 0.34). Fifty-seven of the 159 females produced significantly female-biased seed sex ratios, and no female produced a significant male bias. Overall, the mean progeny sex ratio was significantly biased toward daughters with a ratio of 0.65 (SE = 0.01; χ2 = 9.76, P < 0.0001).
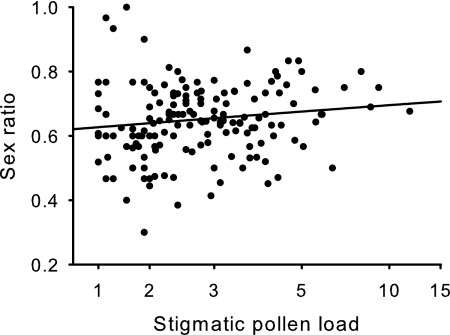
There was a positive relation between seed sex ratio and pollen load (χ21 = 3.88, P < 0.05; Fig. 2), as well as a significant effect of population (χ25 = 13.99, P < 0.05). Females with higher pollen loads produced more female-biased seed sex ratios than females with lower pollen loads, although there was considerable variation in this relation (partial regression coefficient, b = 0.133, SE = 0.068, χ2 = 3.86, P < 0.05; Fig. 2).
Fig. 2.
The relation between the seed sex ratio and stigmatic pollen loads of females in six populations of R. nivalis in Switzerland. For statistical details refer to the text. The predicted relation based on the generalized linear model is depicted. The equation for the relationship is y = e0.52+0.13x/(1 + e0.52+0.13x).
Discussion
Our study demonstrates that the local pollination environment can influence progeny sex ratios in populations of a dioecious plant. Females of R. nivalis positioned in close proximity to males captured the most pollen (Fig. 1A), produced the highest seed set (Fig. 1B), and exhibited the most female-biased sex ratios (Fig. 1C). The most probable explanation for the association between higher stigmatic pollen loads and female-biased primary sex ratios (Fig. 2) is selective fertilization resulting from differential pollen-tube growth of female- versus male-determining pollen (certation). However, the role of certation in affecting sex-ratio variation in dioecious species is controversial (27–29), and previous attempts to demonstrate relations between pollination intensity and female bias under field conditions have failed (23, 24).
Pollen Dispersal and Female Bias in Seed Sex Ratios.
We detected a significant bias toward female offspring in open-pollinated seed families of R. nivalis sampled from six populations in the Swiss Alps compared to the 1:1 ratio expected based on the sex determination system. No family exhibited an excess of males. The average frequency of females (0.65) in seed families was somewhat higher than we obtained in a survey of seed sex ratios in 18 Swiss populations (mean = 0.59; ref. 26) but within the range (0.74–0.63) that we recorded in a common garden experiment conducted at Toronto, Ontario, involving females positioned at different distances from males (25). Female-biased seed sex ratios have also been reported in other Rumex species. By manipulating the amount of pollen applied to stigmas of Rumex acetosa and Rumex hastatulus, Rychlewski and Zarzycki (23) and Conn and Blum (24), respectively, increased female bias with heavier pollen loads. These results indicate that variation in pollination intensity can influence the degree of female bias in progeny sex ratios, and this appears to be a feature of the reproductive system of several Rumex species.
Wind dispersal of pollen typically follows a leptokurtic distribution with most pollen deposited near the source and a long flat tail characterized by low deposition (30, 31). Our previous experiment with small experimental arrays of R. nivalis indicated a steep decrease toward the flat end of the pollen dispersal curve (25). The range of distances used (5–150 cm) was shorter than we investigated in the field, where the mean distance between a female and her closest male was 92.7 cm (SE = 7.6). Pollen dispersal under natural conditions did not show as steep a decrease, probably because of the larger number of males available for pollen donation. Higher wind speeds in the Swiss Alps, in comparison with the earlier artificial array experiment, may have also played a role. In both studies no pollen was detected on a large proportion of stigmas that were sampled (44% in this study and 68%, in ref. 25). However, this was not reflected in a concomitant decrease in seed set in natural populations, which was generally high (mean = 0.83). Our stigma samples probably underestimated the total pollen loads that were captured because many stigmas were harvested before the duration of maximum longevity and pollen may have been washed off of stigmas during preservation in ethanol (see ref. 25).
The signal of a leptokurtic decrease in pollen capture with distance and its effect on female bias was evident in population comparisons. Arosa1, the population with the highest density of males (Table 1), showed the strongest decrease in female bias with increasing distance to males (mean distance between focal females and their closest males = 52.6 cm, SE = 1.3; partial regression coefficient in the generalized linear model, b = −0.532, SE = 0.216, χ2 = 6.04, P < 0.05), whereas Arosa2 and Davos1 had the lowest male densities and showed the weakest responses (mean distances for Arosa2 and Davos1 = 126.7 cm, SE = 7.5, and 110.7 cm, SE = 4.6, respectively; partial regression coefficients, b = 0.121, SE = 0.126, χ2 = 0.92, P > 0.05; b = 0.014, SE = 0.122, χ2 = 0.01, P > 0.05).
Effective wind pollination relies on efficient pollen removal and capture. This is best achieved in habitats unobstructed by vegetation and abiotic barriers, thus allowing the wind to move through the landscape in a laminar way (32, 33). R. nivalis was the dominant vegetation with no physical obstructions in most populations we sampled, including Arosa1. In contrast, nearby Arosa2 had the highest level of intermixing of R. nivalis with other alpine meadow plants, and the terrain was interspersed with large boulders. These differences in composition likely contributed to populations varying in their response of seed sex ratio to male distance, illustrating the potential role of ecological context in affecting progeny sex ratios.
Mechanisms Governing Biased Primary Sex Ratios.
In species with genetic sex determination, Mendelian inheritance should produce primary sex ratios of 1:1. However, both genetic and environmental factors can potentially modify the sex ratio of seeds, although the mechanisms involved are poorly understood (9). In theory, genes modifying primary sex ratios could alter the quantity or quality of female- versus male-determining pollen, or females could selectively abort seeds based on their gender. As yet, there is little evidence to support either mechanism (but see ref. 29), and in Rumex gametophytic selection involving competition between female- and male-determining pollen tubes remains the most viable hypothesis based on the available evidence (7, 21–25). Evolutionary stable strategy models can predict either male- or female-biased primary sex ratios depending on the relative distance of seed and pollen dispersal (34), but the limited empirical data available (see table 10.2 in ref. 9) are not consistent with sex allocation theory.
Investigations of sequential life cycle stages in glasshouse and field populations of R. nivalis have revealed increasing amounts of female bias (figure 6 in ref. 35). On average, male plants produce a small (0.52) excess of female-determining pollen with family means ranging from 0.46 to 0.59. A similar pattern of female bias has also recently been reported in the pollen of R. acetosa (36). However, the degree of bias in R. nivalis is too small to account for the observed bias in seed sex ratios. Rather, the small bias appears to be amplified by postpollination processes leading to the selective fertilization of uniovulate flowers. Abortion of developing seeds can largely be ruled out in R. nivalis because female bias occurs commonly in plants with near maximum seed set, and the uniovulate flowers provide limited scope for ovular competition.
Several lines of evidence indicate that environmental factors influencing the progamic phase of reproduction cause female-biased primary sex ratios in R. nivalis. First, Stehlik and Barrett (26) reported an association between the degree of female bias in seed sex ratios and the flowering sex ratio of natural populations. Females in populations with strongly female-biased flowering sex ratios (proportionally fewer males) produced less female-biased offspring, whereas populations with higher frequencies of flowering males produced more female-biased progeny. This association is consistent with the certation hypothesis because larger pollen loads would be expected in populations with higher male frequencies. Second, direct evidence for the relation between pollination intensity and female-biased progeny sex ratios was obtained by manipulating pollen loads experimentally (25). The results of the present study now corroborate this finding under field conditions. Finally, supporting experimental evidence from pollination experiments on two other Rumex species (7, 22–24) points to the role of gametophytic selection as the principle mechanism governing female-biased sex ratios. However, it is important to emphasize that we have not provided definitive evidence for the certation hypothesis because pollen-tube competition was only inferred and not measured directly in our study. Obtaining evidence for certation remains a daunting challenge because of the technical difficulties of distinguishing female- from male-determining pollen tubes in styles of dioecious species.
The sex ratios of flowering and nonflowering (vegetative) plants in natural populations of R. nivalis are strongly female-biased (flowering mean = 0.87, range = 0.72–0.99; vegetative mean = 0.78, range = 0.36–0.94; n = 18 populations; ref. 26). This indicates that gender-based mortality also plays a role in amplifying biases established during the gametophytic and progamic phases of the life cycle. Extending ideas initially suggested by Smith (22) and Lloyd (37), Stehlik and Barrett (26) proposed that the poor performance of male microgametophytes and sporophytes in R. nivalis may be a consequence of the accumulation of deleterious mutations on Y sex chromosomes (38, 39). According to this hypothesis, deleterious genes expressed during both the gametophytic and sporophytic phases result in poor male performance and an amplification of female bias during the sporophytic life cycle.
Environmental influences on the sex ratios of gender dimorphic plants are commonly reported as a result of variation in site quality or local resources (40, 41). Inbreeding is also known to bias sex ratios in several animal groups (42), although such effects have not been demonstrated in dioecious plants. Mulcahy (43) reported that female frequencies in seed progenies of Silene latifolia were inversely proportional to the male-to-female flower ratio of the parental population. He proposed a group-selection hypothesis based on the “reproductive economy of the sexes” to explain this pattern (and see refs. 44 and 45). Our study is the first to demonstrate an influence of the proximity of males on progeny sex ratios.
Natural populations of R. nivalis are characterized by the nonrandom distribution of sexes across snow-melt gradients (26). Less female-biased seed sex ratios produced in male-scarce environments could offset local male paucity leading to temporal oscillations in sex ratio. However, further work is required to determine whether environmental adjustment of primary sex ratios, mediated by pollination intensity, is an adaptive response for optimizing the fitness of parental plants in local environments characterized by heterogeneous flowering sex ratios. An alternative hypothesis is that female-biased primary sex ratios are simply nonadaptive consequences of the sex chromosome system of R. nivalis.
Methods
Study Species.
R. nivalis (Polygonaceae) is a wind-pollinated perennial herb restricted to the European Alps and mountains of Bosnia-Herzegovina (20). Populations occur almost exclusively in snowbeds interspersed among alpine meadows above tree line. Plants have a basal rosette and one to several inflorescences. Clonal expansion via basal branching is limited, and genets are easily identified. Females have uniovulate flowers, and mature adults produce up to 200 seeds per season. As in other Rumex species of section Acetosa, females of R. nivalis possess one pair of X chromosomes (homogametic sex; 2n = 14), whereas males are characterized by one X and two Y chromosomes (heterogametic sex; 2n = 15; ref. 20).
Selection of Populations, Measurements, and Sex Determination.
In summer 2004, we selected six natural populations of R. nivalis in Switzerland that varied in sex ratios and densities of flowering males and females (Table 1). To assess local sex ratios, we subdivided each population into 4 m × 4 m grids and counted all flowering females and males per quadrant. Within each population, we haphazardly chose 30 focal females from neighborhoods with a range of male densities. To assess the pollination environment of each focal female, we measured the distance to the 50 nearest male inflorescences. For each focal female, we measured stigmatic pollen loads, seed set, and seed sex ratios.
At peak flowering (end of June to beginning of August 2004) we collected 16 flowers per focal female throughout the inflorescence (top to bottom) and preserved them individually in 70% ethanol in microcentrifuge tubes for measurements of stigmatic pollen loads. Stigmas varied in their duration of exposure to pollen. We stained stigmas in 1% fuchsin and counted pollen grains on stigmas under a compound microscope with a ×100 magnification. In September 2004 we collected entire inflorescences of focal females to assess seed set and sex ratios. We counted the number of seeds produced and calculated the total seed set of each female by dividing the number of fully developed seeds by the total number of flowers. Because of animal grazing we recovered only 88% of focal females.
Seed sex ratios were calculated for each of the focal females in all six populations as the number of female offspring divided by the total number of offspring assessed. We determined the sex ratio of progenies following ref. 25 and 26 by either (i) growing maternal half-sib progenies to flowering in the glasshouse or (ii) determining gender using a male-specific SCAR marker (ref. 46; see below). An analysis using a generalized linear model to assess whether the method of sex determination (glasshouse vs. SCAR marker), treated as a fixed factor, affected progeny sex ratios yielded no significant difference between the two techniques (SAS PROC GENMOD 9.1; SAS Institute; χ21 = 0.91, P = 0.34). Because of the loss of some maternal plants in the field, we sexed offspring from a total of 159 females in the six populations (mean number of females per population = 26.5, SE = 1.34, range = 21–30; Table 1) for a total of 4,903 offspring.
Using method i, we grew all available seeds per maternal parent to flowering for Davos1, Davos2 and Saentis. Seeds were fully ripe at harvest in these populations, and the mean number of offspring per female was 38.31 seeds (SE = 2.13). Germination and growth conditions closely resembled those used in refs. 25 and 26, and, as in these studies, germination rates were high (>90%). At the time of final census for sex determination, 98.5% of plants had flowered, yielding a mean sample size of 32.9 (SE = 2.11) offspring per female, for a total of 2,862 individuals. Our previous work on R. nivalis (26) established that the sex ratio of germinated vs. ungerminated seeds was not significantly different. We are therefore confident that flowering sex ratios are equivalent to seed sex ratios. Indeed, this was reflected in the nonsignificant influence of the mode of sex determination in the ANOVA described above.
We assessed the seed sex ratios of Arosa1, Arosa2, and Flims using method ii involving the SCAR marker. DNA extractions, PCR conditions, and sex scoring were identical to those used in ref. 26. Although seeds were fully developed at harvest in these populations, they were still green, which can reduce germination levels. We determined the sex of 30 seeds per female, unless they produced fewer seeds, and we then used all available offspring. This resulted in a mean of 28.35 (SE = 0.50) seeds per female and a total of 2,041 seeds assessed.
Statistical Analyses.
To investigate the relationships among stigmatic pollen load, seed set, and seed sex ratio with each focal female's specific pollination environment, we investigated the fit of the three response variables to measures of male proximity. As occurs in many wind-pollinated plants, pollen dispersal in R. nivalis follows a leptokurtic distribution with females directly adjacent to males receiving the highest pollen loads and a steep decrease occurring over relatively short distances (25). Pollen loads should therefore depend on the proximity of males in the local neighborhood. We initially ran statistical analyses with the distance to the nearest male per focal female as the independent variable. We then re-ran the analyses using distance to the second-nearest male, the third-nearest male, etc., up to the inclusion of all 50 males to determine the model with the best fit to the data, as judged by the model with the lowest log-likelihood. For pollen load and sex ratio, the most relevant response variables for the certation hypothesis, the model with the lowest log-likelihood was the distance to the fourth-nearest male as the independent variable. For seed set, the goodness-of-fit increased slightly with increasing distance past the fourth male but then dropped. We also investigated the relations between male density and the three response variables. We assessed the number of males within 0.25-m incremental distance classes from focal females (i.e., 0–0.25 m, 0–0.5 m, 0–0.75 m, etc.). We used the same technique as above and found that the inclusion of males at a distance of up to 1.75 m yielded the best fit; however, the fit using the distance to the fourth-nearest male still provided a better fit. Because the difference between these two measures of maternal pollination environment resulted in only small qualitative differences, we present results based only on analyses using the distance to the fourth-nearest male as the independent variable.
We investigated stigmatic pollen load with a generalized linear model (SAS PROC GENMOD 9.1; SAS Institute; ref. 47) with a negative binomial distribution and log-link function. We used this distribution to accommodate the zero-inflated data structure and heterogeneous variance. Seed set and seed sex ratios were analyzed with generalized linear models (SAS PROC GENMOD 9.1; SAS Institute; ref. 48) with logit transformations to accommodate the binomial distribution of data. In all of our analyses, we treated population as a block and female pollination environment (distance to the fourth-nearest male) as a fixed factor. We used likelihood-ratio tests to determine the significance of each effect in the model (48). We also examined the relation between sex ratio and stigmatic pollen load irrespective of female-specific pollination environment using generalized linear models (SAS PROC GENMOD). For all analyses, we initially included all factors and all of their interactions. We excluded nonsignificant terms by stepwise backward elimination (α = 0.05) if they did not explain a significant proportion of the variation.
Acknowledgments.
We thank D. Lang for collecting seed and A. Speratti for counting stigmatic pollen loads. I.S. was supported by a postdoctoral fellowship from the Swiss National Science Foundation, J.F. was supported by a Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada, and the research was supported by a Natural Sciences and Engineering Research Council Discovery Grant and funds from the Canada Research Chair's Program (to S.C.H.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Antonovics J, Levin DA. Ecological and genetic consequences of density-dependent regulation in plants. Annu Rev Ecol Syst. 1981;11:411–452. [Google Scholar]
- 2.Davis HG, Taylor CM, Civille JC, Strong DR. An Allee effect at the front of a plant invasion: Spartina in a Pacific estuary. Ecology. 2004;92:321–327. [Google Scholar]
- 3.Stehlik I, Caspersen JP, Barrett SCH. Spatial ecology of mating success in a sexually polymorphic plant. Proc R Soc London Ser B. 2006;273:387–394. doi: 10.1098/rspb.2005.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett SCH, Thomson JD. Spatial pattern, floral sex ratios, and fecundity in dioecious Aralia nudicaulis. Can J Bot. 1982;60:1662–1670. [Google Scholar]
- 5.Heilbuth JK, Ilves KL, Otto SP. The consequences of dioecy for seed dispersal: Modeling the seed-shadow handicap. Evolution. 2001;55:880–888. doi: 10.1554/0014-3820(2001)055[0880:tcodfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Wilson WG, Harder LD. Reproductive uncertainty and the relative competitiveness of simultaneous hermaphroditism versus dioecy. Am Nat. 2003;162:220–241. doi: 10.1086/376584. [DOI] [PubMed] [Google Scholar]
- 7.Correns C. Determination, Inheritance and Distribution of Gender in Higher Plants. Berlin: Bornträger; 1928. (Translated from German) [Google Scholar]
- 8.Jones DA. Selective Fertilization. Chicago: Univ of Chicago Press; 1928. [Google Scholar]
- 9.de Jong TJ, Klinkhamer PGL. Evolutionary Ecology of Plant Reproductive Strategies. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 10.Charnov EL, Bull JJ. When is sex environmentally determined? Nature. 1977;266:828–830. doi: 10.1038/266828a0. [DOI] [PubMed] [Google Scholar]
- 11.Bull JJ. Evolution of Sex Determining Mechanisms. Menlo Park, CA: Benjamin/Cummings; 1983. [Google Scholar]
- 12.Clutton-Brock TH, Albon SD, Guinness FE. Parental investment in male and female offspring in polygynous mammals. Nature. 1981;289:487–489. [Google Scholar]
- 13.Lagomarsino IV, Conover DO. Variation in environmental and genotypic sex-determining mechanisms across a latitudinal gradient in the fish, Menidia menidia. Evolution. 1993;47:487–494. doi: 10.1111/j.1558-5646.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 14.Janzen FJ, Phillips PC. Exploring the evolution of environmental sex determination, especially in reptiles. J Evol Biol. 2006;19:1775–1784. doi: 10.1111/j.1420-9101.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd DG, Bawa KS. Modification of the gender of seed plants in varying conditions. Evol Biol. 1984;17:255–338. [Google Scholar]
- 16.Korpelainen H. Labile sex expression in plants. Biol Rev. 1998;73:157–180. [Google Scholar]
- 17.Delph LF, Wolfe DE. Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytol. 2005;166:119–128. doi: 10.1111/j.1469-8137.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- 18.Freeman DC, Wachocki BA, Stender MJ, Goldschlag DE, Michaels HJ. Seed size and sex ratio in spinach: Application of the Trivers-Willard hypothesis to plants. Ecoscience. 1994;1:54–63. [Google Scholar]
- 19.Żuk J. An investigation on polyploidy and sex determination within the genus Rumex. Acta Soc Bot Pol. 1963;32:5–67. [Google Scholar]
- 20.Wagenitz G. Gustav Hegi, Illustrated Flora of Central Europe. 1. III. Berlin: Parey; 1981. (Translated from German) [Google Scholar]
- 21.Correns C. Sex determination and numerical proportion of genders in Common Sorrel (Rumex acetosa) (Translated from German) Biol Zentralbl. 1922;42:465–480. [Google Scholar]
- 22.Smith BW. The mechanism of sex determination in Rumex hastatulus. Genetics. 1963;48:1265–1288. doi: 10.1093/genetics/48.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rychlewski J, Zarzycki K. Sex ratio in seeds of Rumex acetosa L. as a result of sparse or abundant pollination. Acta Biol Cracov Bot. 1975;18:101–114. [Google Scholar]
- 24.Conn JS, Blum U. Sex ratio of Rumex hastatulus: The effect of environmental factors and certation. Evolution. 1981;35:1108–1116. doi: 10.1111/j.1558-5646.1981.tb04980.x. [DOI] [PubMed] [Google Scholar]
- 25.Stehlik I, Barrett SCH. Pollination intensity influences sex ratios in dioecious Rumex nivalis: A wind-pollinated plant. Evolution. 2006;60:1207–1214. [PubMed] [Google Scholar]
- 26.Stehlik I, Barrett SCH. Mechanisms governing sex-ratio variation in dioecious Rumex nivalis. Evolution. 2005;59:814–825. [PubMed] [Google Scholar]
- 27.Carroll SB, Mulcahy DL. Progeny sex ratios in dioecious Silene latifolia. Am J Bot. 1990;80:551–556. doi: 10.1002/j.1537-2197.1993.tb13839.x. [DOI] [PubMed] [Google Scholar]
- 28.Purrington CB. Parental effects on progeny sex ratio, emergence, and flowering in Silene latifolia (Caryophyllaceae) J Ecol. 1993;81:807–811. [Google Scholar]
- 29.Taylor DR, Saur MJ, Adams E. Variation in pollen performance and its consequences for sex ratio evolution in a dioecious plant. Evolution. 1999;53:1028–1036. doi: 10.1111/j.1558-5646.1999.tb04518.x. [DOI] [PubMed] [Google Scholar]
- 30.Tonsor SJ. Leptokurtic pollen-flow, non-leptokurtic gene-flow in a wind-pollinated herb, Plantago lanceolata L. Oecologia. 1985;67:442–446. doi: 10.1007/BF00384953. [DOI] [PubMed] [Google Scholar]
- 31.Honig MA, Linder HP, Bond WJ. Efficacy of wind pollination: Pollen load size and natural microgametophyte populations in wind-pollinated Staberoha banksii (Restionaceae) Am J Bot. 1992;79:443–448. [Google Scholar]
- 32.Niklas KJ. The aerodynamics of wind pollination. Bot Rev. 1985;51:328–386. [Google Scholar]
- 33.Dowding P. Wind pollination mechanisms and aerobiology. Int Rev Cytol. 1987;107:421–437. [Google Scholar]
- 34.de Jong TJ, van Batenburg FHD, van Dijk J. Seed sex ratio in dioecious plants depends on relative dispersal of pollen and seeds: An example using a chessboard simulation model. J Evol Biol. 2002;15:373–379. [Google Scholar]
- 35.Stehlik I, Kron P, Barrett SCH, Husband BC. Sexing pollen reveals female bias in a dioecious plant. New Phytol. 2007;175:185–194. doi: 10.1111/j.1469-8137.2007.02093.x. [DOI] [PubMed] [Google Scholar]
- 36.Blocka-Wandas M, Sliwinska E, Grabowska-Joachimiak A, Musial K, Joachimiak JA. Male gametophyte development and two different DNA classes of pollen grains in Rumex acetosa L., a plant with an XX/XY1Y2 sex chromosome system and a female-biased sex ratio. Sex Plant Reprod. 2007;20:171–180. [Google Scholar]
- 37.Lloyd DG. Female predominant sex ratios in angiosperms. Heredity. 1974;32:34–44. [Google Scholar]
- 38.Charlesworth D. Plant sex determination and sex chromosomes. Heredity. 2002;88:94–101. doi: 10.1038/sj.hdy.6800016. [DOI] [PubMed] [Google Scholar]
- 39.Vyskot B, Hobza R. Gender in plants: Sex chromosomes are emerging from the fog. Trends Genet. 2004;20:432–438. doi: 10.1016/j.tig.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Case AL, Barrett SCH. Environmental stress and the evolution of dioecy: Wurmbea dioica (Colchicaceaea) in Western Australia. Evol Ecol. 2004;18:145–164. [Google Scholar]
- 41.Ashman T-L. In: Ecology and Evolution of Flowers. Harder LD, Barrett SCH, editors. Oxford: Oxford Univ Press; 2006. pp. 204–222. [Google Scholar]
- 42.Hamilton WD. Extraordinary sex ratios. Science. 1967;15:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 43.Mulcahy DL. Optimal sex ratio in Silene alba. Heredity. 1967;22:411–423. [Google Scholar]
- 44.Lewis D. The evolution of sex in flowering plants. Biol Rev. 1942;17:46–67. [Google Scholar]
- 45.Kaplan SM. Seed production and sex ratio in anemophilous plants. Heredity. 1972;28:281–285. [Google Scholar]
- 46.Stehlik I, Blattner FR. Sex-specific SCAR markers in the dioecious plant Rumex nivalis (Polygonaceae) and implications for the evolution of sex chromosomes. Theor Appl Genet. 2004;108:238–242. doi: 10.1007/s00122-003-1425-7. [DOI] [PubMed] [Google Scholar]
- 47.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. Chicago: Irwin; 1996. [Google Scholar]
- 48.Allison PD. Logistic Regression Using SAS System: Theory and Application. Cary, NC: SAS Institute; 1999. [Google Scholar]