Abstract
The autonomic nervous system maintains homeostasis through its sympathetic and parasympathetic divisions. During infection, cells of the immune system release cytokines and other mediators that cause fever, hypotension, and tissue injury. Although the effect of cytokines on the nervous system has been known for decades, only recently has it become evident that the autonomic nervous system, in turn, regulates cytokine production through neural pathways. We have previously shown that efferent vagus nerve signals regulate cytokine production through the nicotinic acetylcholine receptor subunit α7, a mechanism termed “the cholinergic antiinflammatory pathway.” Here, we show that vagus nerve stimulation during endotoxemia specifically attenuates TNF production by spleen macrophages in the red pulp and the marginal zone. Administration of nicotine, a pharmacological agonist of α7, attenuated TNF immunoreactivity in these specific macrophage subpopulations. Synaptophysin-positive nerve endings were observed in close apposition to red pulp macrophages, but they do not express choline acetyltransferase or vesicular acetylcholine transporter. Surgical ablation of the splenic nerve and catecholamine depletion by reserpine indicate that these nerves are catecholaminergic and are required for functional inhibition of TNF production by vagus nerve stimulation. Thus, the cholinergic antiinflammatory pathway regulates TNF production in discrete macrophage populations via two serially connected neurons: one preganglionic, originating in the dorsal motor nucleus of the vagus nerve, and the second postganglionic, originating in the celiac-superior mesenteric plexus, and projecting in the splenic nerve.
Keywords: brain, cytokine, innate, spleen, vagus
The autonomic nervous system is divided into two major branches based on anatomy and function. These divisions, sympathetic and parasympathetic, control organ function through a set of two serially connected neurons, the first of which is located in the CNS and the second in the peripheral ganglia. Typically, parasympathetic neurons originate in the brainstem medulla and sacral portion of the spinal cord, and the ganglia are located close to or within the innervated organ. Sympathetic neurons originate in the thoracic and lumbar portion of the spinal cord, and the ganglia are situated close to the spinal cord. Classically, sympathetic and parasympathetic signals act in opposition to maintain organ function in dynamic equilibrium. This anatomical and functional architecture facilitates control over physiological responses to environmental and internal threat, and it maintains homeostasis.
During infection, homeostasis is significantly perturbed, in part, because cells of the immune system release soluble factors that act on the brain and other tissues to mediate physiological responses. For example, cytokines like TNF, IL-1β, and other soluble factors mediate fever, anorexia, shock, and tissue injury (1). Although the effect of cytokines on the nervous system has been known for decades, only recently has it become evident that the autonomic nervous system, in turn, regulates cytokine production through neural pathways. We have described the “cholinergic antiinflammatory pathway” as a neural mechanism in which the efferent vagus nerve regulates systemic cytokine levels through a nicotinic acetylcholine receptor containing the α7 subunit (2–4). This pathway is controlled by brain muscarinic networks that depend on an intact vagus nerve to attenuate systemic cytokine production (5). Activation of the cholinergic antiinflammatory pathway, either by electrical stimulation of the vagus nerve or through pharmacological approaches, has been shown to significantly ameliorate cytokine-mediated disease models, including endotoxemia (2, 6), sepsis (6, 7), colitis (8), pancreatitis (9), and ischemia reperfusion (10, 11).
In the course of studying the functional and anatomical basis of cholinergic regulation of cytokines in disease models, we discovered that the spleen is required for the cytokine regulatory effect of vagus nerve stimulation (12). This finding was surprising because experimental evidence indicates that efferent fibers from the celiac branch of the vagus nerve terminate in the celiac-superior mesenteric plexus ganglia (13), but not in the spleen (14). Innervation of the spleen is provided by catecholaminergic fibers that originate in the celiac-superior mesenteric plexus ganglia (15–17), which are in turn under the control of preganglionic neurons located in the intermediolateral gray column of the thoracic spinal cord (16). Here, we show that catecholaminergic splenic nerve fibers terminate adjacent to TNF-producing macrophages and that surgical ablation of the splenic nerve or catecholamine depletion by reserpine abrogates the suppressive effect of vagus nerve stimulation on TNF production.
Results
Macrophages Are the Major Source of Spleen TNF During Endotoxemia.
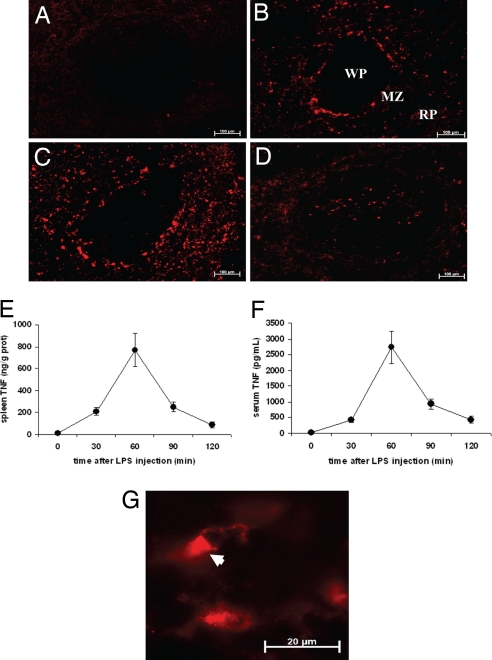
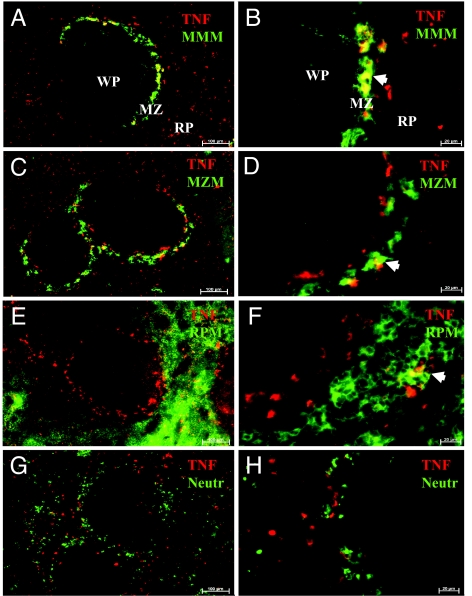
We previously established that the cholinergic antiinflammatory pathway regulates TNF production in the spleen (12), but the cellular source of spleen TNF during endotoxemia was previously unknown. Macrophages, a component of the reticuloendothelial system, are a major source of TNF in endotoxemia (18). TNF was detected by immunofluorescence 30 min after endotoxin administration in the red pulp and the marginal zone (MZ) (Fig. 1B). At 60 min, the number of TNF-positive cells reached a peak (Fig. 1C), decreasing at the 120-min time point (Fig. 1D). The number of spleen TNF-positive cells paralleled TNF protein levels in spleen and serum, which peaked at 60 min (Fig. 1 E and F). Higher magnification of spleen sections showed polarized TNF arranged in vesicle-like structures, a distinct pattern that indicates active TNF secretion by producer cells (Fig. 1G) (19, 20). Spleen macrophages are classified into subpopulations that vary in origin, location, and function (21–23). In the MZ, TNF costained with MOMA-1- and SIGN-R1-positive cells, markers of marginal metallophilic macrophages (Fig. 2 A and B) and MZ macrophages (Fig. 2 C and D), respectively. In the red pulp, TNF costained with F4/80-positive cells, a marker of red pulp macrophages (Fig. 2 E and F). These findings implicate macrophages as a major source of spleen TNF during endotoxemia.
Fig. 1.
Anatomical distribution of spleen TNF in endotoxemia. Mice received 10 mg/kg LPS i.p. and were killed 10, 30, 60, 90, or 120 min later. (A) TNF was not detectable at 10 min. (B) At 30 min, TNF immunoreactivity was observed in the MZ, a thin rim of specialized cells surrounding the white pulp (WP), and in the red pulp (RP). (C) At 60 min, the number of TNF-positive cells increased in the MZ and the RP. (D) At 2 h, TNF was observed in cells of the WP. (Magnification: ×100.) (E and F) TNF protein content in the spleen peaked at 60 min (E) and correlated with serum TNF concentration (F). (G) High-power magnification showing TNF within spleen cells (arrowhead). (Magnification: ×630.) Pictures are representative of at least five mice per group and three to four sections per mouse.
Fig. 2.
Spleen macrophages costain with TNF in endotoxemia. Spleens harvested 60 min after LPS administration were stained for TNF (red) and markers for macrophage subpopulations (green). (A–F) TNF costained with (arrowheads) marginal metallophilic macrophages (MMM) (A and B), MZ macrophages (MZM) (C and D), and RP macrophages (RPM) (E and F). (G and H) Neutrophils, Gr1-positive cells, did not costain with TNF. (Magnification: A, C, E, and G, ×100; B, D, F, and H, ×400.) MZ, marginal zone; WP, white pulp; RP, red pulp.
Vagus Nerve Stimulation and Nicotine Target Spleen Macrophages to Attenuate Spleen TNF in Endotoxemia.
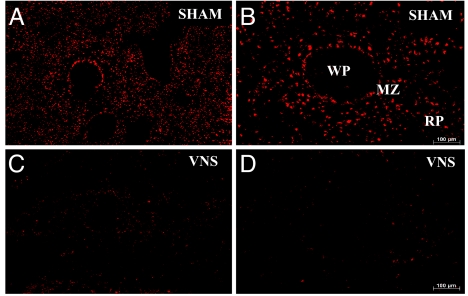
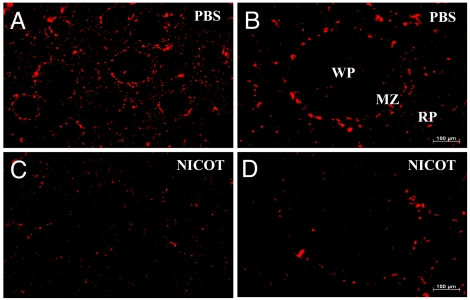
To determine whether TNF production in spleen macrophages is regulated by the cholinergic antiinflammatory pathway, we performed vagus nerve stimulation in endotoxemic rats. In spleen sections from sham-treated animals, we observed homogeneous distribution of TNF immunoreactivity in the MZ and red pulp (Fig. 3 A and B). In contrast, vagus nerve stimulation led to attenuated TNF immunoreactivity in the MZ and the red pulp (Fig. 3 C and D). We have shown previously that suppression of TNF production by vagus nerve stimulation depends on the nicotinic acetylcholine receptor α7 subunit (4). Nicotine attenuates LPS-induced TNF release by human macrophages in an α7-dependent manner in vitro (4). Nicotine administration significantly attenuated TNF immunoreactivity in the spleen compared with the vehicle-treated controls (Fig. 4). The effect of nicotine administration on the expression of TNF in spleen was similar to the effect of vagus nerve stimulation, in that we observed a generalized attenuation of TNF expression in the MZ and red pulp macrophage populations. Together, these findings provide direct evidence that the cholinergic antiinflammatory pathway controls TNF production in the specific subpopulation of spleen macrophages.
Fig. 3.
Vagus nerve stimulation attenuates spleen TNF immunoreactivity in endotoxemia. (A–D) Rats were subjected to vagus nerve stimulation (C and D) or sham surgery (A and B). Spleens were harvested 60 min after LPS. In sham animals, TNF was detected in the MZ and RP. Vagus nerve stimulation significantly decreased TNF immunoreactivity throughout the spleen. (Magnification: A and C, ×25; C and D, ×100.) MZ, marginal zone; WP, white pulp; RP, red pulp.
Fig. 4.
Nicotine attenuates spleen TNF immunoreactivity in endotoxemia. Mice were treated with 2 mg/kg nicotine i.p. 30 min before 10 mg/kg LPS i.p. Spleens were harvested 60 min after LPS administration and stained for TNF. (A–D) Spleens of nicotine-treated mice (C and D) showed attenuated TNF immunoreactivity in the MZ and RP compared with spleens from PBS-treated mice (A and B). (Magnification: A and C, ×25; B and D, ×100.) MZ, marginal zone; WP, white pulp; RP, red pulp.
Nerve Terminals in the Spleen Are Found Adjacent to TNF-Producing Macrophages.
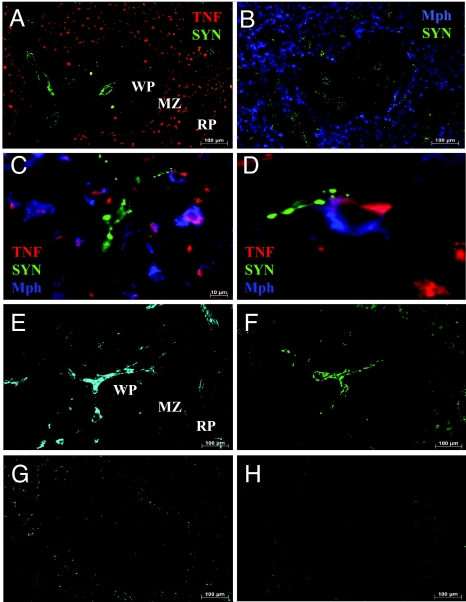
To determine the spatial relationship between nerve endings and TNF-producing macrophages in spleen, we looked for the distribution pattern of the presynaptic vesicle-associated protein synaptophysin, a synaptic vesicle protein (24, 25). Synaptophysin punctae were distributed in networks in the parenchyma of the white pulp and around the central artery. In the red pulp, nerve endings were found predominantly in the trabecullae of the venous sinusoids (Fig. 5 A and B) in close proximity to TNF-producing cells. Higher magnification revealed synaptophysin in close apposition to TNF-producing red pulp macrophages (Fig. 5 C and D). Previous studies indicate that innervation of the spleen is supplied by the splenic nerve, which is comprised principally of catecholaminergic fibers originating in the ganglia of the celiac-superior mesenteric plexus (15–17). Spleen tissue treated with glyoxylic acid, a standard procedure to stain catecholamines (26), revealed an extensive nerve fiber network that followed the same distribution as that of synaptophysin (Fig. 5 E and F). Surgical ablation of the splenic nerve completely eliminated catecholamine and synaptophysin staining (Fig. 5 G and H), indicating that nerve terminals found in juxtaposition to TNF-producing macrophages are catecholaminergic. In agreement with studies that indicate lack of vagus innervation of the spleen (14, 16, 17), staining of spleen sections with antibodies against choline acetyltransferase or vesicular acetylcholine transporter, markers of cholinergic neurons, failed to reveal cholinergic nerve fibers (data not shown). Together, these results indicate that the nerve fibers supplying the TNF-secreting macrophages are catecholaminergic, not cholinergic.
Fig. 5.
Nerve endings terminate adjacent to TNF-producing macrophages in spleen. Rat spleens harvested 60 min after 15 mg/kg LPS i.p. were stained for the synaptic vesicle protein synaptophysin, TNF, and macrophages. Synaptophysin-positive nerve endings were found in close proximity to TNF-producing macrophages especially in the RP. (A) Synaptophysin (green) and TNF (red). (B) Synaptophysin (green) and RP macrophages (blue). (C) Synaptophysin (green), TNF (red), and RP macrophages (blue). (D) Digital magnification of C. (E and F) Sequential slices (10 μm thick) of a normal spleen showing similar spatial distribution of catecholamines (E) and synaptophysin (F). (G and H) Sequential slices of a spleen harvested 7 days after splenic neurectomy stained for catecholamines (G) and synaptophysin (H). (Magnification: A, B, E, F, G, and H, ×100; C, ×630.) Pictures are representative of at least four animals. MZ, marginal zone; WP, white pulp; RP, red pulp.
Surgical Ablation of the Splenic Nerve and Catecholamine Depletion by Reserpine Abrogate the TNF-Suppressive Effect of Vagus Nerve Stimulation.
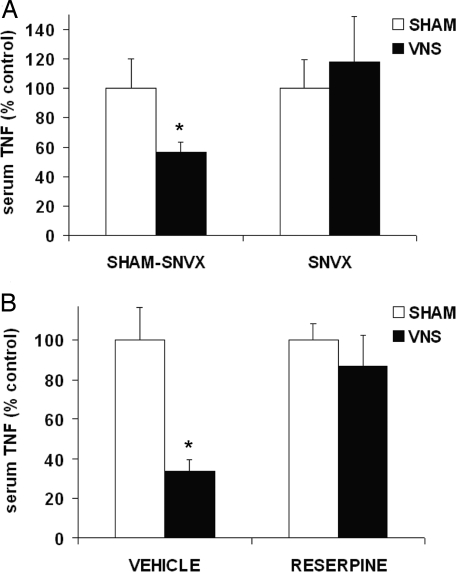
Efferent fibers of the common celiac branch of the vagus terminate in the celiac and superior mesenteric ganglia (27), the site where catecholaminergic splenic nerve fibers originate (15–17). We performed vagus nerve stimulation in endotoxemic rats that had previously undergone surgical ablation of the splenic nerve. Electrical stimulation of the vagus nerve attenuated systemic TNF levels in rats subjected to sham surgery (Fig. 6A). In contrast, vagus nerve stimulation failed to suppress systemic TNF in rats devoid of spleen innervation, indicating that cervical stimulation of the vagus nerve requires an intact splenic nerve to suppress TNF production (Fig. 6A). To further investigate the role of catecholamines in this pathway, we performed vagus nerve stimulation in endotoxemic rats previously depleted of catecholamine stores by treatment with reserpine. Vagus nerve stimulation failed to attenuate systemic TNF levels in reserpine-treated animals (Fig. 6B). Together, these findings indicate that vagus nerve stimulation attenuates TNF production in specific spleen macrophages by functionally signaling through catecholaminergic nerve fibers that travel along the splenic nerve.
Fig. 6.
Surgical ablation of the splenic nerve abrogates the TNF-suppressive effect of vagus nerve stimulation. (A) Rats underwent splenic neurectomy (SNVX) or sham-SNVX surgery. Eight to 11 days later, animals received 5 mg/kg LPS i.v. and were subjected to sham surgery or vagus nerve stimulation. Data are presented as mean ± SEM (n = 10–15 per group; *, P < 0.05). (B) Rats received 5 mg/kg reserpine i.p. or vehicle. Twenty-four hours later, rats received 5 mg/kg LPS i.v. and were subjected to vagus nerve stimulation or sham surgery. Data are presented as mean ± SEM (n = 4–6 per group; *, P < 0.05).
Discussion
The results herein expand our knowledge of the anatomical basis of the cholinergic antiinflammatory pathway (28, 29). Macrophage subpopulations in the spleen produce TNF in endotoxemia and electrical stimulation of the vagus nerve controls TNF release in these cells through a mechanism that depends on an intact, catecholaminergic splenic nerve.
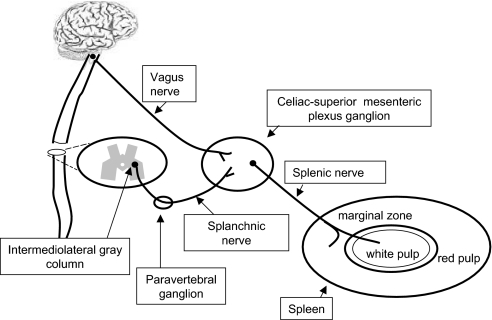
The spleen is innervated by nerve fibers that originate in the celiac-superior mesenteric plexus ganglia (15–17). Although most of its fibers are catecholaminergic (30), several other neurotransmitters are associated with splenic nerve function (31–34). Previous studies have failed to show cholinergic fibers in the spleen, and neuronal tracing studies indicate that primary vagus nerve fibers do not innervate the spleen. In agreement with these studies, we confirmed the absence of cholinergic fibers in the spleen and found that some TNF-producing macrophages in the spleen are in close proximity to catecholaminergic nerve terminals. Previous studies revealed that the vagus nerve, through efferent fibers of its celiac branches, terminates in synapatic-like structures around principal cells of the celiac-superior mesenteric ganglia (13, 27). It is now clear that the vagus nerve controls immune cell function in the spleen through a system of two serially connected neurons: one preganglionic, which originates in the dorsal motor nucleus of the vagus embodied in the vagus nerve, and the other postganglionic, which originates in the ganglia of the celiac-superior mesenteric plexus and travels along the splenic nerve (Fig. 7).
Fig. 7.
Anatomical basis of the cholinergic antiinflammatory pathway. Two-neuron model of vagus nerve modulation of cytokine production via the splenic nerve: The preganglionic neuron originates in the dorsal motor nucleus of the vagus; the postganglionic neuron, located in ganglia of the celiac-superior mesenteric plexus, reaches the spleen through the splenic nerve.
The splenic nerve is supplied by preganglionic neurons that originate in the intermediolateral column of the thoracic spinal cord (16). Our results indicate that the vagus nerve functionally communicates with the splenic nerve. Vagal regulation of postganglionic catecholaminergic fibers has been demonstrated previously. In dogs, electrical stimulation of the cervical vagus nerve decreases pancreatic norepinephrine release induced by electrical stimulation of thoracic (sympathetic) nerves, an effect that is independent of muscarinic receptors (35). Electric stimulation of the splenic nerve induces norepinephrine release from the spleen and attenuates LPS-induced TNF through a β-adrenergic-dependent mechanism in the ex vivo perfused rat spleen (36). Given that electrical stimulation of the vagus nerve suppresses TNF in a splenic nerve and catecholamine-dependent way, it is plausible that vagus nerve stimulation induces release of spleen catecholamines and other neurotransmitters, whose presence has been identified in nerve fibers close to spleen macrophages (37, 38). Stimulation of adrenergic receptors on macrophages can enhance or decrease TNF production depending on whether α or β receptors are activated, respectively (39, 40). Norepinephrine attenuates TNF production by LPS-stimulated rat splenic macrophages, an effect that is partially reversed by propranolol (41). Thus, it is possible that norepinephrine release from postganglionic adrenergic nerve terminals in spleen, acting through β-adrenergic receptors expressed in splenic macrophages, can attenuate spleen TNF. However, this observation does not take into account the requirement of nicotinic acetylcholine receptor α7 subunit signaling in the cholinergic antiinflammatory pathway (3, 28).
We and others have observed that α7 KO mice are insensitive to the TNF-suppressive effect of vagus nerve activity (4). In vitro, acetylcholine and other cholinergic agonists attenuate LPS-induced TNF in human and mouse macrophages and in mouse splenocytes through an α7-dependent mechanism (2, 4). The nicotinic acetylcholine receptor subunit α7 is expressed in autonomic ganglia (42), where it mediates fast synaptic transmission (43). It is possible that acetylcholine released by the vagus nerve acts on α7 expressed in the ganglia of the celiac-superior mesenteric plexus to modulate splenic nerve function. But it is also clear that acetylcholine down-regulates cytokine release by splenic macrophages, and this effect requires the α7 subunit of the nicotinic acetylcholine receptor (12, 44). Furthermore, the spleen contains and secretes acetylcholine, which may be released by stimulation of the splenic nerve (45–47). It is possible that catecholaminergic signals via the splenic nerve enhance acetylcholine levels in the spleen, which attenuate cytokine production by signaling through α7 on TNF-producing cells.
The splenic nerve is an inherent component of a pathway that originates in the brain and terminates in the spleen to control the immune response to invasion. For example, electrical stimulation of the hypothalamus (48) or central administration of angiotensin (49), IL-1β (50), or IFNα (51) modulate spleen immune cell function via the splenic nerve, an effect that has been ascribed solely to the sympathetic nervous system. It is now clear that the cholinergic antiinflammatory pathway, which transmits information via the vagus nerve and requires signaling of the nicotinic acetylcholine receptor subunit α7, utilizes the splenic nerve to suppress cytokine release.
Materials and Methods
Antibodies.
Antibodies used for immunofluorescence were as follows: marginal zone macrophages, biotinylated rat anti-mouse SIGN-R1 (clone ER-TR9; BMA Biomedicals); marginal metallophilic macrophages, biotinylated rat anti-mouse sialoadhesin (clone MOMA-1; BMA Biomedicals); red pulp macrophages: biotinylated rat anti-mouse F4/80 (clone A3–1; Serotec); neutrophils, rat anti-mouse Gr-1 (clone RB6–8C5; R&D Systems); rat red pulp macrophages, mouse anti-rat CD163 (clone ED2; Serotec); goat anti-mouse TNF (R&D Systems) and goat anti-rat TNF (R&D Systems); and rabbit anti-synaptophysin (Abcam) polyclonal antibody. Secondary reagents include: Cy3-conjugated Affinipure donkey anti-goat IgG (Jackson Immunoresearch Laboratories), FITC-conjugated Affinipure donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories), Alexa Fluor 568-conjugated streptavidin (Molecular Probes), and FITC-conjugated avidin (Molecular Probes).
Immunofluorescence.
All samples were fresh-frozen with dry ice, embedded in O.C.T. compound (Tissue-Tek), and kept at −20°C until processing. Spleen slices (10 μm) were cut by using a cryostat, mounted on glass slides, and air-dried for 5 min. The tissue was then incubated with PBS-saponin 0.1% for 30 min. All incubation periods were performed at room temperature in a humidity chamber. Primary antibodies were diluted in PBS-saponin 0.1% at the following concentrations: TNF (1:6 dilution), MOMA-1 (1:1,000 dilution), ER-TR9 (1:100 dilution), and F4/80 (1:50 dilution). After a 2-h incubation period, the slides were washed three times in washing buffer (PBS, tween 20 0.02%) and incubated with avidin-FITC and Cy3-labeled rat anti-goat IgG, which were both diluted 1:250 in PBS-saponin 0.1% for 30 min. Finally, slides were washed, dried, mounted (Vectashield), and observed through a Zeiss Axiovert 20-inverted microscope. Images were analyzed by using the AxioVision V5 software (Carl Zeiss).
Catecholamine Staining.
To visualize catecholamines, we performed the glyoxylic acid method described by de la Torre (26). Briefly, 10-μm spleen sections were dipped three times in a sucrose, monobasic potassium phosphate, and glyoxylic acid solution immediately after cutting and dried for 30 min using cool air from a hair dryer. Next, they were covered with mineral oil and heated in an oven at 95°C for 3 min, after which excess oil was removed and coverslips were added. Catecholamines were visualized with a Zeiss Axiovert 20-inverted microscope by using the Zeiss filter set 05.
Animals.
Adult male BALB/c mice 8 to 12 weeks old (20–25 g; Taconic) and adult male Sprague–Dawley rats 8 to 12 weeks old (250–300 g; Charles River Laboratories) were housed at 25°C on a 12-h light/dark cycle and acclimatized for 1 week before experiments were conducted. Water and regular rodent chow were available ad libitum. Experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Endotoxemia.
Endotoxin (LPS from Escherichia coli, 0111:B4; Sigma–Aldrich) was administered to animals (10 mg/kg, i.p. corresponding to an LD75 dose). Blood and spleens were harvested 10, 30, 60, or 120 min after LPS administration. TNF concentration in serum was determined by ELISA (R&D Systems). Spleens were either snap frozen for further immunofluorescence analysis or homogenized in PBS with protease inhibitor mixture (complete, mini; Roche). TNF content in spleen tissue was determined by ELISA and normalized to protein concentration (protein assay; Bio-Rad). In some experiments, PBS or nicotine (Sigma–Aldrich) was administered 30 min (2 mg/kg, i.p.) before endotoxin administration. Animals were then killed 60 min later, and spleens were harvested for TNF immunofluorescence or TNF quantification in tissue homogenates.
Electrical Stimulation of the Vagus Nerve.
Male Sprague–Dawley rats were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine i.m. Vagus nerve stimulation was performed as described previously (12). Briefly, a bipolar platinum electrode (Plastics One) was placed across the isolated left cervical vagus nerve. Electrical stimulation (5 V, 2 ms, 5 Hz) was generated by a stimulation module (STM100A) under the control of the AcqKnowledge software (Biopac Systems). Rats underwent 5 min of vagus nerve stimulation before and after injection of 5 mg/kg endotoxin i.v. Rats were killed 90 min after endotoxin injection, and serum was obtained for TNF determination. In rats subjected to sham surgery, the vagus nerve was exposed, but not touched or manipulated.
Splenic Neurectomy.
Male Sprague–Dawley rats 10 to 11 weeks old were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. The peritoneal cavity was accessed through a midline abdominal incision. After the main branches of the splenic vessels were identified and isolated, the splenic nerve, which traverses along the splenic artery, was incised at several points with forceps. Special attention was given to avoid manipulation of the celiac ganglion. In sham-operated rats, the splenic vessels were isolated, but the nerve was left intact. Animals recovered for 8–11 days before undergoing endotoxemia and vagus nerve stimulation. To assess the efficacy of splenic neurectomy, spleens were treated with the glyoxylic acid method to visualize the catecholaminergic fibers. Only animals whose spleens showed complete absence of catecholamines were included in the data series.
Catecholamine Depletion.
Reserpine (Sigma–Aldrich) was dissolved in glacial acetic acid (Sigma–Adrich) and diluted with sterile saline to a final glacial acetic acid concentration of 0.5%. The 5 mg/kg reserpine i.p. was administered to rats 24 h before the beginning of experiments. The efficacy of catecholamine depletion was confirmed by subjecting spleens to the glyoxylic acid method. Only animals whose spleens showed complete absence of catecholamines were included in the data series.
Statistical Analysis.
Data in figures are expressed as means ± SEM. A two-tailed t test was used to compare mean values between groups; P values <0.05 were considered significant.
Acknowledgments.
This work was supported in part by National Institute of General Medical Sciences Grants R01 GM57226 and R01 GM62508 (to K.J.T).
Footnotes
Conflict of interest statement: K.J.T. and M.R.-B. are inventors on technology related to the topic.
This article is a PNAS Direct Submission.
References
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 5.Pavlov VA, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlov VA, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 7.van Westerloo DJ, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 8.Pullan RD, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 9.van Westerloo DJ, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Bernik TR, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 11.Altavilla D, et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500–506. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 12.Huston JM, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: Lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 15.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989;3:291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 16.Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 17.Nance DM, Burns J. Innervation of the spleen in the rat: Evidence for absence of afferent innervation. Brain Behav Immun. 1989;3:281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- 18.Grivennikov SI, et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 20.Palmblad K, Andersson U. Identification of rat IL-1beta, IL-2, IFN-gamma and TNF-alpha in activated splenocytes by intracellular immunostaining. Biotech Histochem. 2000;75:101–109. doi: 10.3109/10520290009066487. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 22.van FR, esselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. J Exp Med. 1984;160:1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijffels JF, De RZ, Beelen RH, Kraal G, Van RN. Macrophage subpopulations in the mouse spleen renewed by local proliferation. Immunobiology. 1994;191:52–64. doi: 10.1016/s0171-2985(11)80267-6. [DOI] [PubMed] [Google Scholar]
- 24.Alder J, Xie ZP, Valtorta F, Greengard P, Poo M. Antibodies to synaptophysin interfere with transmitter secretion at neuromuscular synapses. Neuron. 1992;9:759–768. doi: 10.1016/0896-6273(92)90038-f. [DOI] [PubMed] [Google Scholar]
- 25.Walaas SI, Jahn R, Greengard P. Quantitation of nerve terminal populations: Synaptic vesicle-associated proteins as markers for synaptic density in the rat neostriatum. Synapse. 1988;2:516–520. doi: 10.1002/syn.890020507. [DOI] [PubMed] [Google Scholar]
- 26.De la Torre JC. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980;3:1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: Morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35:80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tracey KJ. Fatal Sequence: The Killer Within. Washington, DC: Dana Press; 2005. pp. 128–204. [Google Scholar]
- 30.Klein RL, Wilson SP, Dzielak DJ, Yang WH, Viveros OH. Opioid peptides and noradrenaline co-exist in large dense-cored vesicles from sympathetic nerve. Neuroscience. 1982;7:2255–2261. doi: 10.1016/0306-4522(82)90135-x. [DOI] [PubMed] [Google Scholar]
- 31.Lorton D, Bellinger DL, Felten SY, Felten DL. Substance P innervation of spleen in rats: Nerve fibers associate with lymphocytes and macrophages in specific compartments of the spleen. Brain Behav Immun. 1991;5:29–40. doi: 10.1016/0889-1591(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 32.Jobling P. Electrophysiological events during neuroeffector transmission in the spleen of guinea-pigs and rats. J Physiol. 1994;476:153–165. [PMC free article] [PubMed] [Google Scholar]
- 33.Bellinger DL, et al. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides. 1997;18:1139–1149. doi: 10.1016/s0196-9781(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 34.Romano TA, Felten SY, Felten DL, Olschowka JA. Neuropeptide-Y innervation of the rat spleen: Another potential immunomodulatory neuropeptide. Brain Behav Immun. 1991;5:116–131. doi: 10.1016/0889-1591(91)90011-x. [DOI] [PubMed] [Google Scholar]
- 35.Benthem L, Mundinger TO, Taborsky GJ., Jr Parasympathetic inhibition of sympathetic neural activity to the pancreas. Am J Physiol Endocrinol Metab. 2001;280:E378–E381. doi: 10.1152/ajpendo.2001.280.2.E378. [DOI] [PubMed] [Google Scholar]
- 36.Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol. 2003;145:77–85. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Meltzer JC, Grimm PC, Greenberg AH, Nance DM. Enhanced immunohistochemical detection of autonomic nerve fibers, cytokines and inducible nitric oxide synthase by light and fluorescent microscopy in rat spleen. J Histochem Cytochem. 1997;45:599–610. doi: 10.1177/002215549704500412. [DOI] [PubMed] [Google Scholar]
- 38.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. 118–121. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 39.Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145:1430–1434. [PubMed] [Google Scholar]
- 40.Chou RC, Stinson MW, Noble BK, Spengler RN. Beta-adrenergic receptor regulation of macrophage-derived tumor necrosis factor-alpha production from rats with experimental arthritis. J Neuroimmunol. 1996;67:7–16. doi: 10.1016/0165-5728(96)00023-9. [DOI] [PubMed] [Google Scholar]
- 41.Hu XX, Goldmuntz EA, Brosnan CF. The effect of norepinephrine on endotoxin-mediated macrophage activation. J Neuroimmunol. 1991;31:35–42. doi: 10.1016/0165-5728(91)90084-k. [DOI] [PubMed] [Google Scholar]
- 42.Lips KS, et al. Coexpression and spatial association of nicotinic acetylcholine receptor subunits alpha7 and alpha10 in rat sympathetic neurons. J Mol Neurosci. 2006;30:15–16. doi: 10.1385/JMN:30:1:15. [DOI] [PubMed] [Google Scholar]
- 43.Del Signore A, Gotti C, Rizzo A, Moretti M, Paggi P. Nicotinic acetylcholine receptor subtypes in the rat sympathetic ganglion: Pharmacological characterization, subcellular distribution and effect of pre- and postganglionic nerve crush. J Neuropathol Exp Neurol. 2004;63:138–150. doi: 10.1093/jnen/63.2.138. [DOI] [PubMed] [Google Scholar]
- 44.Toyabe S, et al. Identification of nicotinic acetylcholine receptors on lymphocytes in the periphery as well as thymus in mice. Immunology. 1997;92:201–205. doi: 10.1046/j.1365-2567.1997.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulloch K, Damavandy T, Badamchian M. Characterization of choline O-acetyltransferase (ChAT) in the BALB/C mouse spleen. Int J Neurosci. 1994;76:141–149. doi: 10.3109/00207459408985999. [DOI] [PubMed] [Google Scholar]
- 46.Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J Neuroimmunol. 1998;81:31–37. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
- 47.Brandon KW, Rand MJ. Acetylcholine and the sympathetic innervation of the spleen. J Physiol. 1961;157:18–32. doi: 10.1113/jphysiol.1961.sp006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katafuchi T, Ichijo T, Take S, Hori T. Hypothalamic modulation of splenic natural killer cell activity in rats. J Physiol. 1993;471:209–221. doi: 10.1113/jphysiol.1993.sp019898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganta CK, et al. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–H1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 50.Brown GL, Gillespie JS. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957;138:81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katafuchi T, Take S, Hori T. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J Physiol. 1993;465:343–357. doi: 10.1113/jphysiol.1993.sp019680. [DOI] [PMC free article] [PubMed] [Google Scholar]