Abstract
Background and aim. The aim of this study was to report our 44-year experience (1963–2006) in the management of primarily infected hydatid cyst of the liver. This is a retrospective review of demographic data, clinical presentation, diagnostic work-up, surgical management, and long-term outcome of patients treated at our center. Material and methods. There were 77 patients with operated infected liver cysts. In the same period, a total of 460 cases with liver hydatidosis were treated surgically. Of those with suppurated cysts, 27 were men and 50 were women, with a mean age 54.5 years. Results. Clinical manifestations of an abscess were identified in 75% of the patients. In the earlier cases of the study, the diagnosis was made from the clinical picture, laboratory studies, in combination with plain X-ray, hepatic scintigraphy, and in the later cases with US (ultrasonography), CT (computed tomography) or MRI (magnetic resonance imaging), and ERCP (endoscopic cholangiopangreatography). Abdominal and, rarely, thoracic and abdominal or thoracoabdominal incisions were used. Total cystopericystectomy in 8 patients and partial pericystectomy and proper drainage with one or two drainage tubes of the cystic cavity in the other 69 patients were carried out. Hospital stay was between 13 and 146 days with 5 re-operations. Two patients with grossly suppurated cysts and coexistent medical problems died. The disease recurred in five patients. Conclusions. We conclude that, under good perioperative antibiotic and metabolic coverage, the infected hydatid cysts have to be completely evacuated and properly drained. The application of “conservative” surgical procedures should be preferred. Further studies are needed to solve the clinical and therapeutic problems of this serious complication.
Keywords: Hepatic echinococcosis, infected hepatic hydatid cyst, liver echinococcosis, liver hydatidosis, primarily suppurated parasitic cysts
Introduction
Echinococcal cysts (ECs) of the liver is still a problem in Greece 1, with echinococcosis remaining an important public health problem in communities in which it occurs. It has a considerable economic impact on the endemic areas owing to multiple complications leading to disability and even death. Besides the known complications of rupture, calcification, and multiplicity, suppuration is another complicated form of EC and presents like a pyogenic abscess 1,2,3,4. Primarily infected hydatidosis is relatively common, with a frequency, varying widely, between 5% and 40% 5,6,7,8. Well-planned surgical techniques and appropriate procedures based on correct evaluation of disease complications and performed by an experienced team with adequate postoperative support constitute the mainstay of therapy.
The purpose of this retrospective study was to evaluate clinical presentation, surgical management, and long-term outcome of patients treated operatively for primarily infected hydatidosis at our center. The study aims to emphasize modifications of the surgical treatment for which this serious and sometimes life-threatening complication calls.
Patients and methods
Patients
We carried out a retrospective review of all patients older than 14 years who underwent an operation for primary suppuration of liver hydatid cysts between 1 January 1963 and 31 December 2006 at a Level I, university-affiliated tertiary hospital in Northern Greece.
Data collection
Institutional review board approval for the study was obtained before initiation of data collection, which was performed in two stages. First, an electronic search of the hospital database was performed using the specific key words of the study. Second, using a standardized form, data were collected for the eligible patients from their medical records; all patients without primary suppuration of ECs were excluded. The term primary suppuration of EC is used for apparently intact hydatid cysts which are invaded by bacteria either through small-caliber peripheral cystobiliary communications or rarely through the hematogenous route of spread. Secondary bacterial infection of hydatid cysts applies in the case of maltreated or incompletely evacuated cysts which have an obvious source of infection, such as communication through a sinus tract with the biliary tree, the peritoneal cavity, bronchi, alimentary tract, or skin.
The distinction between primary and secondary suppuration in earlier decades of the study was not reliable based on the indolent, without heavy anaphylaxis, febrile clinical picture, the atypical radiological signs, such as air-fluid or haziness around the elevated hemidiaphragm, the vague isotopic liver defects and the preoperative existence of the suppurated cyst with absence of gross cystobiliary rupture. In later decades, besides the history, with the characteristic findings of the modern diagnostic technology, a higher preoperative diagnostic distinction rate was achieved.
The high incidence of suppurated hydatid cysts reported in some series is attributed to the absence of the above distinction, summing all cases, primary and secondary, together 7. Demographic data, clinical presentation, as well as the diagnostic work-up and management technique were extracted from the medical record.
Method
In this study, the diagnostic work-up consisted of blood tests, i.e. including complete blood count, liver function tests, the Casoni skin test (in the earlier three decades) and hydatid serology with the ELISA hemagglutination test and the Weinberg reaction test (in the last decade). Radiologic work-up comprised plain abdominal/chest X-ray and hepatic scintigraphy in the earlier decades of the study (before 1980) as well as ultrasonography (US), computed tomography (CT) magnetic tomography (MRI) and endoscopic cholangiopancreatography (ERCP) in more recent decades (after 1980).
Our methodology in the management of cases was the same in all periods of the study. Preoperative treatment was based on examination results and according to individual needs. Generally, prophylaxis of hepato-renal failure was undertaken, i.e. management of purulent-septic complications and hemobilia. For prophylaxis of purulent complications, more recently the broad-spectrum antibiotic of choice has been ceftriaxon.
Operative strategy, similar in all the years of the study, was directed at drainage of the abscess cavity and removal of the parasitic elements without spillage. The choice of operative access was based on number, size, and location of cysts, nature of complications, and previous surgery. Drapes soaked in hypertonic sodium chloride solution (20%) were placed around the cysts before their emptying to produce scolisidal effect and avoid peritoneal contamination. The liver abscess was aspirated via a special transparent canulla with bevelled tip for culture and initial decompression at its most superficial point, as close to the liver capsule as possible. The cyst was then punctured with a trocar and the cyst fluid was rapidly aspirated under continuous suction. Digital destruction of septations and loculations led to one large cavity. Inactivation/sterilization of the cyst was performed routinely in this series.
Antiparasitic treatment with albendazole (Eskazol, Zentel 400 mg tablets) was given in a few patients. The total oral daily dosage of the drug varied between 600 and 800 mg in two divided doses, preoperatively for 7 days and postoperatively in 2–3 monthly cycles. The anthelminthic drugs, with the exception of their routine use in the last 6 years of the study, were previously used only in selected cases in multiple or in difficult position cysts.
The initial diagnosis was researched in all cases postoperatively by histological and anatomo-pathological examinations.
The following data were analysed for each admission: Age; gender; clinical, laboratory, and imaging/instrumental evaluation; number, size, and location of cysts; choice of operative access; surgical procedures performed; histological and anatomo-pathological examinations; pus culture results; previous surgery for liver hydatidosis; length of stay; postoperative morbidity; mortality; and recurrence rate.
Follow-up was organized by our team or by the primary care physician. The follow-up work-up included blood tests and abdominal ultrasonography at regular intervals, plus CT in some cases. Patients and/or their physicians were sent a questionnaire asking about any recurrence.
Results
Four-hundred-and-sixty patients with hydatid disease of the liver were managed surgically between 1963 and 2006 in our clinic. Patients with primary suppuration of ECs comprised 16.7% (n=77) of the total number of patients who underwent surgery for liver echinococcosis during this period. There were 27 females and 50 males aged between 23 and 78 years (median 54.5 years).
Clinical presentation was variable and included abdominal pain (n=37, 48%), dyspepsia (n=10, 13%), fever (n=58, 75.4%), jaundice (n=17, 22%), weight loss (n=8, 10.4%), and anaphylaxis (n=4, 5.2%). Twenty-seven patients (35%) presented with symptoms persisting for over 15 days. Thirteen patients with recurrent hydatid disease had undergone a previous operation, five of them at another center. Hepatomegaly was noticed in 38 patients (49.35%) and an abdominal mass was detected in 25 (32.5%). Decreased breathing sounds at the base of the right lung were found in 13 patients (16.9%) (Table I).
Table I. Clinical picture of 77 patients with primary suppuration of hydatid liver cysts.
| Cases | |
|---|---|
| Upper right abdominal pain | 37 (48%) |
| High fever | 40 (52%) |
| Low fever | 18 (23.4%) |
| Upper abdominal discomfort or dyspepsia | 10 (13%) |
| Jaundice | 17 (22%) |
| Weight loss | 8 (10.4%) |
| Allergic manifestation | 4 (5.2%) |
| Palpable liver | 38 (49.35%) |
| Abdominal mass | 25 (32.5%) |
| Diminished respiration | 13 (16.9%) |
In 19 of our earlier cases the final diagnosis was made during operation and histologic examination.
Leukocytosis with a shift to the left on the blood smear was found in all cases, while eosinophilia was detected in 11 (14.3%) (Table II).
Table II. Laboratory findings of 77 patients with primary suppuration of hydatid liver cysts.
| Cases | Positive | % | |
|---|---|---|---|
| Leucocytosis | 77 | 77 | 100 |
| Eosinophilia | 77 | 11 | 14.3 |
| Casoni | 18 | 12 | 65 |
| Elisa | 14 | 8 | 57 |
| I.H.A. | 20 | 10 | 50 |
| Weinberg | 20 | 8 | 40 |
The Casoni skin test, utilized in the earlier years of the study, was performed in 18 patients (23.4%) and was found to be positive in 12 cases (65%). The ELISA test was used in 14 patients (18.2%) and was positive in 8 (57%). The hemagglutination test was applied in 20 patients (26%) and was positive in half of them. The Weinberg reaction was used in 20 patients (26%) and was positive in 8 (40%).
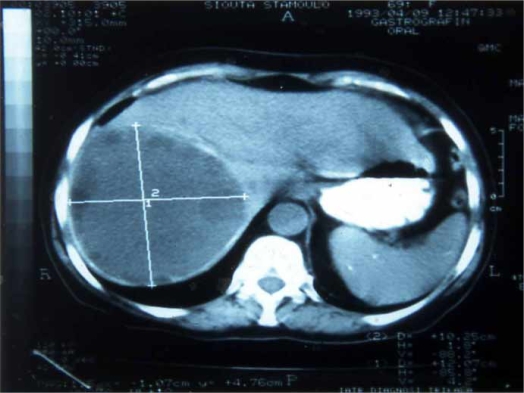
Chest/abdominal X-rays revealed an elevation of the right diaphragm, atelectatic changes at the right lower lobe or obliteration of the costophrenic angle in 38 cases (49.4%). Moreover, air–fluid level and/or calcified cyst wall was discovered in 40 cases (52%). Hepatic scintigraphy was performed in 25 patients (32.5%) and was helpful in 22 (88%). Abdominal US and CT were utilized in 45 (58.45%) and 35 (45.5%) patients, demonstrating the primarily infected hydatid cyst of the liver in 92% and 95% of cases, respectively (Figure 1). These imaging techniques came into use after 1980 and have since become the main diagnostic tools. MRI and ERCP were utilized successfully in 3 (3.9%) and 20 (26%) patients, respectively. MRI with gadolinium correctly detected all the hydatid cysts on both T1 and T2 weighted images and with ERCP led to discrimination of primary or secondary suppurated cysts from absence or presence of gross cystobiliary communication, respectively (Table III).
Figure 1. .
Appearance of primarily infected hydatid cyst on CT scan.
Table III. Imaging findings of 77 patients with primary suppuration of hydatid liver cysts.
| Cases | Positive | % | |
|---|---|---|---|
| Chest X-ray (air fluid/calcification) | 77 | 40 | 52 |
| Right hypochondrium X-ray (elevation/reaction) | 77 | 35 | 45.5 |
| Isotopic scans | 25 | 22 | 88 |
| US | 45 | 41 | 92 |
| CT | 35 | 33 | 95 |
| MRI | 3 | 3 | 100 |
| ERCP | 20 | 20 | 100 |
In most patients (n=58, 75%) primary suppuration of EC was diagnosed preoperatively, and in only 19 patients (25%) was an unexpected primarily infected hydatid cyst discovered intraoperatively.
In 57.1% of patients (n=44), an abdominal incision was used; right paramedian in the first decade and right subcostal in the later three decades of the study. A left subcostal incision combined with a separate right thoracotomy was performed in 10 patients (13%) and a right thoracoabdominal incision was employed in 5 cases (6.5%). A thoracotomy incision approach was made in 23.4% of patients (n=18).
In 61 cases (79.2%) the infected cyst was single and in 3 cases multiple (3.9%). Coexisting hydatid cysts were found in 13 patients (16.9%), 4 of which were removed at the time of hepatic surgery.
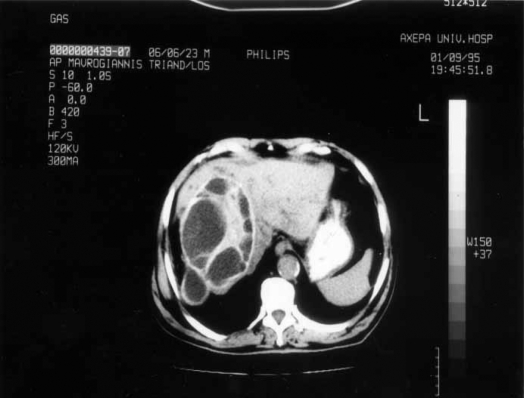
Most primarily infected hydatid cysts of the liver were located in the right lobe (58 patients, 75.32%), but in 11 patients (14.3%) they were found in the left lobe and in 8 cases (10.38%) in both lobes. The mean diameter of the cyst was 13 cm (range 4.8 to 25 cm) (Figure 2).
Figure 2. .
Extended infected hepatic echinococcosis on CT scan.
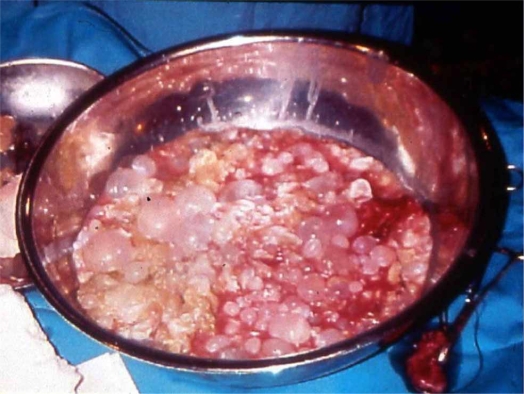
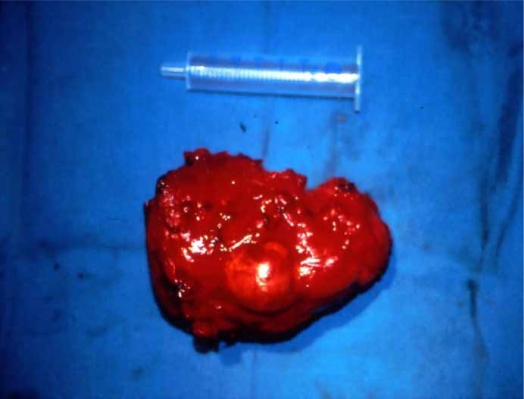
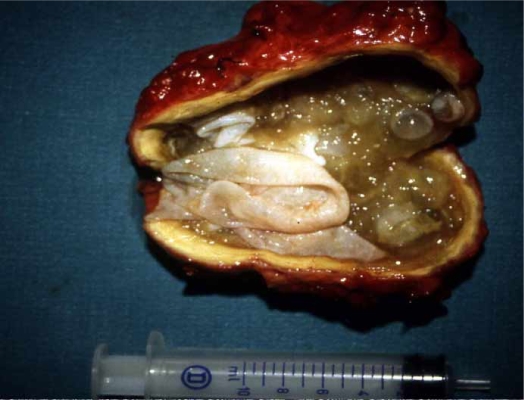
After careful, complete removal of the contents of the cyst in 69 patients (89.6%), partial pericystectomy was performed (Figure 3), with closure of the remaining cavity drained with large bore soft tubes brought out through a separate stab wound. Closed suction drains were favored in most cases. Only in 8 patients (10.4%) were the suppurated cysts managed with cystopericystectomy (Table IV) (Figures 4 and 5). In 12 patients, cholecystectomy was performed because of gallstones and in 9 cases because the hydatid cyst was adjacent to the gallbladder fossa. Bile duct exploration was conducted in 6 patients and sphincteroplasty in 3 cases due to odditis or retrieval of parasitic elements.
Figure 3. .
Complete removal of the contents of primarily infected echinococcal cyst.
Table IV. Surgical management of the suppurated cysts.
| Procedure | No. of cases |
|---|---|
| Cystopericystectomy | 8 |
| Partial pericystectomy, drainage | 69 |
| Total | 77 |
Figure 4. .
Cystopericystectomy of the primarily infected echinococcal cyst.
Figure 5. .
Opened primarily infected echinococcus cyst.
In all cases, the initial diagnosis was confirmed postoperatively by histological and anatomo-pathological examinations.
The results of a pus culture available in 61 patients revealed Enterobacter (14 cases), Pseudomonas (11 cases), E. coli (8 cases), Proteus rettgeri (4 cases), and Klebsiela (3 cases). In 21 cases the culture was negative for microorganisms.
Perioperative anthelminthic therapy with albedazole was given in 13 patients. The morbidity and mortality rates were similar in all decades of the study. Repeat laparotomy was performed on three patients before discharge and on two after discharge owing to incomplete evacuation of infected contents of the cyst. Six patients developed an external fistula, which was closed spontaneously without surgical intervention long after their discharge. Acute respiratory insufficiency as a result of pneumonia and exudative pleurisy was recorded in 5 patients, wound infection in 9 patients, erosive intraperitoneal hemorrhage in 2 patients and all were easily treated conservatively. The postoperative complication rate was 19% and no correlation was found between early postoperative complications and type of surgical procedure. The mean duration of hospital stay was 32 days (range 13–146 days). A fatal outcome was recorded in two (2.6%) jaundiced, aged patients, the causes of death being related to sepsis and coexistent severe general condition. One of them survived 146 days, the other 23 days postoperatively.
Of the 77 patients, follow-up was complete in 54 (70.1%) and partial in 23 (29.9%) with a median of 113 months (12–348) or 9.6 years. Cysts recurred in 5 patients (6.5%) and no correlation was found between recurrence of cysts and albendazole use, type of surgical procedure, or in number and size of the cysts. Two patients died from unrelated reasons during our study period.
Discussion
The clinical presentation of a hydatid cyst is largely asymptomatic until complications occur 9. The expanding cysts and resultant portal vein or bile duct obstruction cause a degree of segmental or lobar liver atrophy in the cyst-bearing lobes 10. The cysts may become infected and present themselves as liver abscesses. Identification of hydatid cyst is considered to be of crucial importance; percutaneous puncture has to be avoided as it may lead to intraperitoneal dissemination of the infection. For the complicated with primary or secondary suppuration hydatid cyst of the liver, surgery is the treatment of choice 11. Specific management and long-term outcome of primarily infected hydatid cyst of the liver have not been evaluated extensively.
This complication has been reported in the literature as occurring in 5% to 40% of patients 5,6,7. In our series of 460 patients with hydatid disease of the liver, who were managed surgically, primary suppuration of ECs occurred in 77 (16.75%) cases.
While history of recent travel or immigration to endemic areas, as well as characteristic eosinophilia, may suggest the diagnosis, unfortunately it is often difficult to establish the correct diagnosis preoperatively and an unexpected primarily infected hydatid cyst is discovered intraoperatively. Symptoms and signs are often not specific and severe disease may follow an indolent course. This relatively benign clinical presentation in some patients could be explained by factors such as partial decompression of the pus through small cystobiliary communications, low virulence of the infectious agent in certain cases, and the protective role of the pericyst, which prevents the infection process extending to the liver parenchyma 8. Fever, combined with right upper quadrant pain, is the most characteristic clinical manifestation. In our study, primary suppuration of EC was diagnosed preoperatively in 58 patients (75%). The high rate observed at our center could be explained by the referral of patients from endemic areas.
A number of abnormal laboratory findings may be found in hydatid liver abscesses 12. The non-specific white blood count elevation is usual and the sensitivity of eosinophilia or specific serological tests is low due to dead parasite, reduced absorption of the antigen, and impaired immune reaction 6,13.
Differential diagnosis relies heavily on current imaging techniques. Chest or/and right upper quadrant X-ray films or isotopic scanning have been valuable diagnostic tools. Plain films reveal hepatic calcification or non-specific findings of right pleural effusion, atelectasis, elevated hemidiaphragm or right upper-quadrant air-fluid level. Although liver scans are useful (sensitivity varying from 80% to 97%), they do not provide information about the etiology of the liver image filling defect 12. More recently, US (sensitivity 85% to 95%), CT (sensitivity 95%) and MRI have been utilized 12,13. Ultrasonography findings vary and range from purely cystic to solid-appearing pseudotumors. Daughter cysts and the water-lily sign are characteristic but not always present. Calcification may also be seen. Hydatid cysts can die with calcification of the wall, but calcification does not always imply that the cyst is dead 14,15. With CT, it appears as a well-defined, hypoattenuating lesion with a distinguishable wall. In our series, coarse wall calcifications were present in 50% of cases and daughter cysts were identified in approximately 75%. MRI, with its superior contrast resolution on both T1 and T2 weighted images, better demonstrates the pericyst, matrix, and daughter cysts and establishes the diagnosis 16. Primarily infected hydatid cysts, however, often present as typical liver abscesses and only the existence of peripheral calcifications can be of help. Moreover, these imaging techniques enabled us to evaluate extrahepatic disease and distinguish detailed hepatic anatomic relationships or rupture of the cyst, while they were useful in the postoperative follow-up. In patients with suspected biliary involvement and to differentiate primary from secondary suppuration, endoscopic retrograde cholangiopancreatography (ERCP) was also necessary in some cases.
For the primarily infected hydatid cyst of the liver, open surgery is the treatment of choice 17,18. At present, efficacy and safety of the PAIR method (puncture of cysts percutaneously, aspiration of fluid, introduction of protoscolicidal agent and re-aspiration) need documentation in large series, especially in complicated echinococcosis 5,18. In general, percutaneous drainage should be avoided, because it may lead to intraperitoneal dissemination of the infection 11, and simple open drainage does not seem to be a satisfactory approach. It is necessary to evacuate the contents of the cyst without spillage, inactivate/sterilize the cavity, and select the appropriate management of the residual cavity. Surgical intervention can be performed using either a transperitoneal or an extraperitoneal approach. In either case, the incision must be lengthy enough to offer maximum exposure of the liver and surrounding structures. The number and location of cysts, and their connection to adjacent structures, determine the surgical approach. It is important to aspirate the cyst at its most superficial point, as close to the liver capsule as possible, not exposing the extensive part of the liver to purulent material. The extraperitoneal approach through the bed of the 12th rib, or the transpleural approach for drainage of a large cyst, located in the dome of the liver, was the classical approach in earlier cases. An anterior subcostal incision with the extraperitoneal approach was employed for an anterior hepatic cyst. Since the introduction of antibiotics/scolicidals, the improvement in surgical techniques and the management of seriously ill patients, the transperitoneal approach has become the gold standard, as it confers the additional advantages of being able to drain all infected hydatid cysts, irrespectively of size and location within the liver, and of allowing a thorough exploration of the abdomen 8,12. Right paramedian incision was used in our earlier, before 1980, cases, while a lengthy right subcostal incision was preferred in most of the later cases. The abdominal approach was helpful for the appropriate exposure of biliary tract, incidental cholecystectomy and common bile duct exploration. For cysts located in the dome of the liver, more “radical” approaches were utilized, in particular combined thoracic and abdominal or thoracoabdominal incisions. Wide exposure is fundamental, since it allows management of “difficult” encapsulated abscess cavities and demonstrates the most direct route for puncture. In most of our cases, where partial pericystectomy and closure of the remaining cavity was performed, inadequate length of the incision had resulted in inadequate evacuation of cysts, spillage, incomplete roof excision, or insufficient treatment of coexistent cysts with biliary-cyst communication, which should be controlled with sutures and drainage, or with more complicated procedures. The management of multiple cysts is sometimes difficult and requires sound operative experience 1. In the event of other uncomplicated cysts, obscure cysts, or when the patient's condition was poor, we left the cysts in place and planned management in a more appropriate condition, unless they could be readily reached through the already evacuated cyst. Cystopericystectomy was indicated in penduculated, peripheral, small cysts. Wide exposure was yet again necessary for complete mobilization of the liver and laborious dissection of surrounding adhesions.
Laparoscopic management of primarily infected hydatid cysts, not used in our series, demands experience in open as well as advanced liver laparoscopic surgery. In our opinion, it should only be applied when adequate experience and technical support are available and then restricted to those cysts that are readily accessible. As with the PAIR method, not used in our study, the foremost concern is the risk of spillage and spreading of the cyst content in the abdominal cavity as well as the risk of anaphylactic shock 17,20. Although these methods eliminate the problems of surgical incision and shorten hospitalization, they do not cope with the disease-related complication 17,18,19,20.
Despite the implementation of n ew technology in the later cases of the study, postoperative morbidity and mortality have not changed over time. The outcomes, as we noted in a previous study, are comparable in infected and non-infected cysts 1. Postoperative complications involved 19% of our patients, which can be considered acceptable since Chautems et al. recently reported a complication rate of 37% in a series of patients treated surgically for complicated liver hydatid cysts 21. We have realized that treatment failures commonly resulted from catheter obstruction by purulent material or from untreated loculations within the abscess, a fact that fits in with the conclusions of Barnes & Lillemoe 12. In our series, repeat laparotomy was performed on 5 patients, approaching a rate of 6.5%. The re-operation rates reported by Milicevic 14 and Gonzalez et al. 22 were 3.2% and 14.1%, respectively. Cysts recurred in 5 of our patients (6.5%). The crucial factor in low rates of recurrence is the cautious operative technique. The effectiveness of a traditional injection of local scolicidal agents (hypertonic normal saline in our study) is unknown, with dose-dependent toxicity 5,8,14. Also the benefit of preoperative or postoperative oral use of bezimidazoles is questionable. These drugs are recommended, as in our series, for cases in which spillage of protoscoleces may have occurred during surgery 5,6. Sepsis, advanced age, and coexistent serious general conditions were implicated as the cause of death in two of our patients, leading to a mortality rate of 2.6%.
Our study is subject to a number of limitations inherent in the use of retrospective administrative data. First, we identified study subjects on the basis of an electronic search of the hospital database using specific key words. If coding was inaccurate, patients may not have been recognized as having primary suppuration of ECs. Similarly, because all data reported in this study were derived from information recorded by the treating institution, the validity of our results is dependent on the accuracy of its record-keeping.
Based on the existing literature and on our own experience, there is a relative diagnostic and treatment algorithm preoperatively and postoperatively. The diagnosis of primarily infected hydatid cyst of the liver, due to mild symptoms and signs of a localized inflammatory process, is not always easy 8. Open surgery is the treatment of choice. In most cases, a “conservative” surgical method through a lengthy incision has to be preferred. To avoid exposure of healthy liver parenchyma to purulent material, it is crucial to evacuate the cyst properly by its most superficial point. External drainage of the cyst is always undertaken. Surgical treatment may be improved with early management, wider use of known and newer chemotherapeutics, and proper perioperative evaluation and support of the patients. In managing such complicated hydatid cysts, we see at the moment little place for unconventional treatment, such as the PAIR method or laparoscopic surgery 17,18. Because in these “promising” procedures the risk of anaphylaxis and peritoneal dissemination may have been overestimated, there is need to continue relative studies 20.
References
- 1.Prousalidis J, Kosmidis Ch, Fahantidis E, Harlaftis N, Aletras O. Surgical treatment of multiple cystic echinococcosis. HPB. 2004;6:110–14. doi: 10.1080/16515320410026068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osmanov AO. Moscow; 1997. Complicated liver echinococcosis. [Dissertation] p. 287. [Google Scholar]
- 3.Chen WQ, Chai FL, Gu SN. Experience in the surgical treatment of hepatic hydatidosis. Chung Hua Wai Ko Tsa Chich. 1994;32:166–8. [PubMed] [Google Scholar]
- 4.Ferrari A. Hepatic echinococcosis. Min Gastroenterol Dietol. 1995;41:311–12. [PubMed] [Google Scholar]
- 5.Amman RW, Eckert J. Cestodes Echinococcus. Gastr Clin N Am. 1996;25:655–89. doi: 10.1016/s0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- 6.Erguney S, Tortum O, Taspinal AH, et al. Complicated hydatid cysts of the liver. Ann Chirurg. 1991;45:584–9. [PubMed] [Google Scholar]
- 7.Vilardell F. Bockus HL. Saunders; London: 1976. Echinococcus (hydatid) cysts of the liver, Gastroenterology. [Google Scholar]
- 8.Saidi F. Saunders; London: 1976. Surgery of hydatid disease; pp. 148–53. [Google Scholar]
- 9.D'Angelica M, Fong Y. Townsend C, Beauchamp R, Evers B, Mattox K. Elsevier Saunders; Philadelphia: 2004. The liver, Sabiston textbook of surgery. The biological basis of modern surgical practice17th edn; pp. 1542–4. [Google Scholar]
- 10.Prousalidis J, Tzardinoglou E, Kosmidis C, Katsohis C, Aletras H. Hepatic regeneration after pericystectomy for hydatid disease of the liver. HPB. 1999;1:153–8. [Google Scholar]
- 11.Contis J, Voros D. Karaliotas C, Broelsch C, Habib N. Springer; Vienna: 2006. Hepatic abscess, Liver and biliary tract surgery. Embryological anatomy to 3D-imaging and transplant innovations; p. 499. [Google Scholar]
- 12.Barnes SA, Lillemoe KD. Appleton and Lange; New York: 1997. Liver abscess and hydatid cyst disease in Maingots abdominal operations10th edn; pp. 1513–45. [Google Scholar]
- 13.Kammerer WS, Schantz PM. Echinococcal disease infections disease. Clin North Am. 1993;7:605–18. [PubMed] [Google Scholar]
- 14.Milicevic MN. Blumgart LH, Fong Y. Saunders; London: 2000. Hydatid disease, Surgery of the liver and biliary tract; pp. 1167–204. [Google Scholar]
- 15.Pedrosa I, Saiz A, Arrazola J, et al. Hydatid disease: Radiologic and pathologic features and complications. Radiographics. 2000;20:795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 16.Kalovidouris A, Gouliamos A, Vlahos L, et al. MRI of abdominal hydatid disease. Abdom Imaging. 1994;19:489–94. doi: 10.1007/BF00198247. [DOI] [PubMed] [Google Scholar]
- 17.Koray A. Controversies in the laparoscopic treatment of hepatic hydatid disease. HPB. 2004;6:213–21. doi: 10.1080/13651820410024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayek I, Onat D. Diagnosis and treatment of uncomplicated hydatid cyst of the liver. World J Surg. 2001;25:21–7. doi: 10.1007/s002680020004. [DOI] [PubMed] [Google Scholar]
- 19.Zaouche A, Haouet K, Jouini M, El Hachaichi A. Management of liver hydatid cysts with a large biliocystic fistula: Multicenter retrospective study. World J Surg. 2001;25:28–39. doi: 10.1007/s002680020005. [DOI] [PubMed] [Google Scholar]
- 20.Filice C, Brunetti E. Use of PAIR in human cystic echinococcosis. Acta Trop. 1977;64:95–107. doi: 10.1016/s0001-706x(96)00642-0. [DOI] [PubMed] [Google Scholar]
- 21.Chautems R, Bühler L, Gold B, Giostra E, Poletti P, Chilcott M, et al. Surgical management and long-term outcome of complicated liver hydatid cysts caused by Echinococcus granulosus. Surgery. 2005;137:312–16. doi: 10.1016/j.surg.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez EM, Selas PR, Martinez B, Garcia G, Capazo FP, Pascual MH. Results of surgical treatment of hepatic hydatidosis: Current therapeutic modification. World J Surg. 1993;15:254–63. doi: 10.1007/BF01659061. [DOI] [PubMed] [Google Scholar]