Abstract
Introduction. Pancreas cancer is the fourth commonest cause of cancer-related mortality across the world, with incidence equalling mortality. A recent study has suggested that both the incidence and the mortality of pancreatic cancer are falling in the UK. We investigated whether this trend was being seen all over the world. Methods. Age-standardized mortality (world) rates [ASR(W)] for pancreatic cancer were extracted separately for males and females from a database maintained by the International Agency for Research on Cancer for 51 countries across the world (Europe, 33 countries; Americas, 8 countries; and Asia, 10 countries) for the period 1992–2002; log-linear regression analysis was performed to analyse trends in the past decade. Results. In the period 1992–2002, the ASR(W) remained static across most countries for both sexes. The highest mortality rates (for both sexes) were seen in Central Europe [range: men (8–12), women (4.5–7)] with trends towards increasing mortality in Romania (p<0.001), along with Albania, Spain and Croatia (p<0.01). Korea in the Far East, too, demonstrated increasing mortality trends for both sexes (men p<0.001, women p<0.01). Increasing mortality trends were also observed among women in France (p<0.001). In Canada, there was a decline in mortality [men (7.5–6.4), women (5.9–5); p<0.01], while for men there was a downward trend in Ireland, the UK, Switzerland, Austria, and Poland [p<0.05]. Conclusion. The changes perhaps reflect standardization and consolidation of diagnostic tests for pancreatic cancer in the Western world and further in-depth analysis would be required.
Keywords: Age standardized mortality, pancreatic cancer, trends
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common epithelial, exocrine pancreatic malignancy, i.e. accounting for more than 80% of the malignant neoplasms of the pancreas 1. It remains the fourth most common cause of cancer-related death in the Western world 2. The incidence of pancreatic cancer correlates with increasing age with a peak incidence of the disease occurring in the 65–75 year age group 3. Untreated metastatic pancreatic cancer has a median survival of 3–5 months and 6–10 months for locally advanced disease 4. The majority of cases are diagnosed in the advanced stages, making curative therapy impossible and leading to poor prognosis and incidence equalling mortality 5. In the year 2000, there were 217,000 new cases of pancreatic cancer with 213,000 deaths worldwide, while in Europe there were 60,139 new patients with 64,801 deaths 6. In the UK, 7,152 new cases were seen with 7,250 deaths as a result of PDAC 7. PDAC affects more individuals inhabiting the Western/industrialized parts of the world; the highest incidence has been reported among Maoris in New Zealand, native Hawaiians and Black American populations, while people living in India and Nigeria have the lowest reported incidence 8,9. Being Jewish increases the risk of developing PDAC as compared to other religious faiths 10. Northern and central Europe have a higher incidence of pancreatic cancers compared to southern Europe, while in the United States immigrant populations (Scandinavian, eastern European, and Japanese) living in urban areas have higher incidence rates compared with native populations 8,11. A recent study from England and Wales suggests that the incidence, as well as mortality, for pancreatic cancer perhaps fell over the period 1951–2000. This was particularly pertinent for the male population towards the end of the last century (1996–2000). We investigated whether similar trends could be seen across the world.
Methods
The database maintained by the International Agency for Research on Cancer (IARC) – CANCER Mondial Statistical Information System (http://www-dep.iarc.fr/) was accessed and age-standardized mortality rate (world) [ASR(W)] due to pancreatic cancer was extracted separately for males and females for the period 1992–2002 for 51 countries across the world. Data were available from 33 countries in Europe (Western Europe: Belgium, France, Germany, Ireland, Luxemburg, The Netherlands, Switzerland and the United Kingdom; Eastern Europe: Bulgaria, Estonia, Latvia, Lithuania, Moldova, Poland and Romania. Northern Europe: Denmark, Finland, Iceland, Norway and Sweden; Southern Europe: Albania, Croatia, Greece, Italy, Macedonia, Malta, Portugal, Slovenia and Spain; Central Europe: Austria, Czechoslovakia, Hungary and Slovakia), 10 countries in Asia (South East Asia: Hong Kong, Philippines and Singapore; Far East: Japan and Korea; Northern Asia: Georgia, Kyrgyzstan and Russia; Middle East: Israel and Kuwait) and 8 countries across the Americas (North America: Canada and the USA; South America: Argentina, Chile, Costa Rica, Mexico, Uruguay and Venezuela). Log-linear regression analysis on ASR(W) was used to analyse trends for the period 1992–2002. Correlation coefficients were calculated; a positive value indicated an increasing trend, while a negative value was indicative of a falling trend; p<0.05 was considered significant.
Results
The age-standardized mortality rate due to pancreatic cancer in the period 1992–2002 remained static for both males and females across most of the 51 countries of the world included in our study. The trends are summarized in Table I.
Table I. Significant trends in the mortaility of pancreatic cancer, 1992–2002.
| Males |
Females |
||||||
|---|---|---|---|---|---|---|---|
| Country | coefficient | 95% CI | p-value | coefficient | 95% CI | p-value | |
| Eastern Europe | Bulgaria | NS | 0.021 | (0.006, 0.036) | 0.01 | ||
| Estonia | NS | NS | |||||
| Latvia | NS | NS | |||||
| Lithuania | NS | NS | |||||
| Moldova | NS | NS | |||||
| Poland | −0.006 | (−0.012, −0.001) | 0.02 | NS | |||
| Romania | 0.02 | (0.014, 0.026) | <0.001 | 0.023 | (0.016, 0.030) | <0.001 | |
| Southern Europe | Albania | 0.077 | (0.033, 0.121) | 0.003 | 0.104 | (0.044, 0.163) | 0.003 |
| Croatia | 0.033 | (0.016, 0.050) | 0.002 | 0.027 | (0.004, 0.051) | 0.03 | |
| Greece | 0.009 | (−0.002, 0.020) | 0.09 | 0.014 | (0.004, 0.023) | 0.01 | |
| Italy | NS | 0.006 | (−0.0003, 0.012) | 0.06 | |||
| Macedonia | NS | NS | |||||
| Malta | NS | 0.06 | (0.007, 0.114) | 0.03 | |||
| Portugal | NS | NS | |||||
| Slovenia | NS | NS | |||||
| Spain | 0.011 | (0.006, 0.016) | 0.001 | 0.012 | (0.003, 0.021) | 0.02 | |
| Western Europe | Belgium | NS | NS | ||||
| France | NS | 0.014 | (0.008, 0.020) | <0.001 | |||
| Germany | NS | 0.004 | (0.0003, 0.008) | 0.04 | |||
| Ireland | −0.015 | (−0.30, −0.001) | 0.04 | NS | |||
| Luxembourg | NS | NS | |||||
| Netherlands | NS | NS | |||||
| Switzerland | −0.013 | (−0.025, −0.002) | 0.03 | NS | |||
| UK | −0.008 | (−0.013, −0.002) | 0.01 | NS | |||
| Central Europe | Austria | −0.012 | (−0.020, −0.004) | 0.01 | NS | ||
| Czechoslovakia | NS | NS | |||||
| Hungary | NS | NS | |||||
| Slovakia | NS | NS | |||||
| Northern Asia | Georgia | NS | NS | ||||
| Russia | 0.043 | (−0.011, 0.096) | 0.08 | 0.053 | (−0.019, 0.125) | 0.09 | |
| Kyrgyzstan | NS | NS | |||||
| North East Asia | Japan | NS | 0.004 | (0.001, 0.007) | 0.02 | ||
| Korea | 0.028 | (0.017, 0.039) | <0.001 | 0.26 | (0.014, 0.039) | 0.001 | |
| South East Asia | China | NS | NS | ||||
| Philippines | NS | NS | |||||
| Singapore | NS | NS | |||||
| Thailand | NS | NS | |||||
| North America | Canada | −0.013 | (−0.018, −0.007) | 0.001 | −0.008 | (−0.012, −0.003) | 0.006 |
| USA | −0.003 | (−0.006, 0.0002) | 0.07 | NS | |||
| South America | Colombia | NP | NP | ||||
| Costa Rica | NP | NP | |||||
| Ecuador | NP | NP | |||||
| Mexico | NP | NP | |||||
| Uruguay | NP | NP | |||||
| Venezuela | NP | NP | |||||
NS = not significant; NP = not performed; CI = confidence interval.
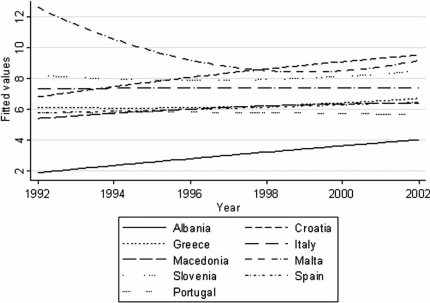
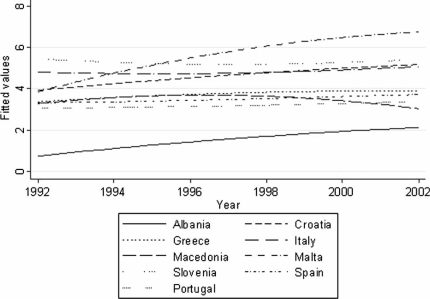
Mortality (ASR(W)) rates were highest in Central Europe for both sexes [range: men (8–12), women (4.5–7)] with highly significant trends towards increasing mortality in Romania [men (6.2–8.2), women (3.2–4.2); p<0.001] along with Albania [men (2–4), women (0.8–2.2); p<0.01]. Increasing mortality trends that attained significance in both sexes were also seen in Spain and Croatia (men p<0.001, women p<0.05; see Figures 1 and 2). In the Far East, Korea demonstrated highly significant increasing mortality trends for both sexes [men (4.8–7.8), p<0.001; women (2.5–4), p<0.01), while women in Japan showed an increasing trend that was significant (p<0.05). In France, a trend towards increasing mortality was observed among women (p<0.001). An upward mortality trend in women achieving significance was also seen in Malta, Bulgaria, Greece, and Germany (p<0.05). A decline in mortality was seen in both sexes only in Canada [men (7.5–6.4), women (5.9–5); p<0.01], while for men there was a downward trend noticeable in Ireland, Switzerland, Austria, the UK, and Poland [p<0.05].
Figure 1. .
Mortality (ASR(W)) due to pancreatic cancer in males in Southern Europe during the period 1992–2002, smoothed using quadratic regression.
Figure 2. .
Mortality (ASR(W)) due to pancreatic cancer in females in Southern Europe during the period 1992–2002, smoothed using quadratic regression.
In the Middle East, Israel showed a dramatic drop in mortality in both sexes from around 8 in 2001 to 0 in 2002 in men, and 6 in 2001 to 0 in 2002 in women, but the overall changes in trends for the period 1992–2002 were insignificant for both men and women. The data from South America were poor and hence analysis of trends from this region could not be performed.
Discussion
Our analysis suggests that in the Western world the mortality due to pancreatic cancer fell in the last decade of the second millennium. This fits well with the data published recently for England and Wales 12. However, this is in stark contrast to an increase seen in Southern Europe and North East Asia. The reasons for the changes observed could be multiple. The first possibility is the variation in quality of data available in international registries, along with an absence of incidence data, population size, and demographics 11,13. We have, thus, assumed the effects in all countries to be equivalent regardless of population size. However, the fact that we used ASR(W), a standard comparative tool, can account for some of the variation. Secondly, the observed variations could reflect a consolidation of use of diagnostic modalities and may thus reflect artificial changes in incidence and mortality rates for pancreatic cancer. For example, the use of CT-guided or EUS-guided biopsy is now a norm in the West, but is not well established in the Southern European countries, though there is an increasing use of cross-sectional imaging. Thus, the initial increase seen in the West during the 1980s (due to increasing use of cross-sectional imaging) may be mirrored in the Southern European countries in the 1990s. However, without biopsy, the differential diagnosis of pancreatic cancers such as chronic pancreatic and other peri-ampullary tumors remains and thus there may be an artificial increase in recording of pancreatic cancer with the increasing diagnosis of peri-ampullary masses.
Lastly, we have to consider whether the changes we have observed are real. However, further in-depth analysis is required for definitive trends to be ascertained. Hence, larger datasets would be required over longer periods (more than 20 years) along with incidence data and demographics. These data are currently unavailable for the majority of countries.
There are multiple risk factors identified for pancreatic cancer. Pancreatic cancer is known to affect older individuals, as only 10% of patients develop this condition below the age of 50; data from the United States show a dramatic increase in rates of pancreas cancer from 9.8/100,000 (age group 50–54) to 57/100,000 (age group 70–74) 3. Smoking is the strongest environmental risk factor. A meta-analysis of cohort and case-control studies shows a significant correlation between cigarette smoking and pancreas cancer, the risk increasing with the number of cigarettes smoked 14. The prevalence of cigarette smoking among Korean men is regarded as the highest in the world (65.4% in 2001) 15,16,17, which may account for the higher mortality due to pancreatic cancer in this region. Similarly, the data for prevalence of smoking among Central Eastern and some Southern European countries, though sparse, suggests that smoking is not declining, but in some countries may be increasing across both sexes 18. In contrast, there are reports of decreasing trends in the incidence of smoking-related cancers across Western Europe 19.
We are thus unable to completely explain the rise and fall of pancreatic cancer-related mortality in different parts of the world, though some insights may be gained from this preliminary study indicating that smoking may effect the changes seen. Additionally, the change in use of various diagnostic modalities may explain the differences seen in our study. The epidemiologies of pancreatic and related cancers deserve a more in-depth study in the near future when more data become available.
Acknowledgements
We thank Dr Jane Warwick, Senior Statistician at the Cancer Research UK Centre for Epidemiology, Mathematics and Statistics, Wolfson Institute of Preventive Medicine, London for helpful criticisms on analysis.
References
- 1.Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91:1410–27. doi: 10.1002/bjs.4794. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan A, Kocher HM. Pancreatic cancer. Br Med J Clin Evid. 2007;11:409–37. [PubMed] [Google Scholar]
- 5.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 7.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134–52. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle P, Hsieh CC, Maisonneuve P, La Vecchia C, Macfarlane GJ, Walker AM, et al. Epidemiology of pancreas cancer 1988. Int J Pancreatol. 1989;5:327–46. doi: 10.1007/BF02924298. [DOI] [PubMed] [Google Scholar]
- 9.Mack TM, Peters JM, Yu MC, Hanisch R, Wright WE, Henderson BE. Pancreas cancer is unrelated to the workplace in Los Angeles. Am J Ind Med. 1985;7:253–66. doi: 10.1002/ajim.4700070307. [DOI] [PubMed] [Google Scholar]
- 10.Phillips RL, Garfinkel L, Kuzma JW, Beeson WL, Lotz T, Brin B. Mortality among California Seventh-Day Adventists for selected cancer sites. J Natl Cancer Inst. 1980;65:1097–107. [PubMed] [Google Scholar]
- 11.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27:87–93. doi: 10.1016/s0361-090x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimmons D, Osmond C, George S, Johnson CD. Trends in stomach and pancreatic cancer incidence and mortality in England and Wales, 1951–2000. Br J Surg. 2007;94:1162–71. doi: 10.1002/bjs.5751. [DOI] [PubMed] [Google Scholar]
- 13.Newnham A, Quinn MJ, Babb P, Kang JY, Majeed A. Trends in the subsite and morphology of oesophageal and gastric cancer in England and Wales 1971–1998. Aliment Pharmacol Ther. 2003;17:665–76. doi: 10.1046/j.1365-2036.2003.01521.x. [DOI] [PubMed] [Google Scholar]
- 14.Boyle P, Maisonneuve P, Bueno de Mesquita B, Ghadirian P, Howe GR, Zatonski W, et al. Cigarette smoking and pancreas cancer: a case control study of the search programme of the IARC. Int J Cancer. 1996;67:63–71. doi: 10.1002/(SICI)1097-0215(19960703)67:1<63::AID-IJC12>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organisation. Tobacco or health: a global status report. Geneva: World Health Organisation; 1997. [Google Scholar]
- 16.Kang HY, Kim HJ, Park TK, Jee SH, Nam CM, Park HW. Economic burden of smoking in Korea. Tob Control. 2003;12:37–44. doi: 10.1136/tc.12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korean Ministry of Health and Welfare. 2001 National Health and Nutrition Survey. Seoul: Ministry of Health and Welfare; 2002. [Google Scholar]
- 18.British Heart Foundation statistics website [home page on the Internet]. Trends in smoking prevalence in Europe [updated 13 September 2005; accessed 9 December 2007]. Available at: http://www.heartstats.org/datapage.asp?id=4669 [Google Scholar]
- 19.Levi F, Lucchini F, Negri E, La Vecchia C. Pancreatic cancer mortality in Europe: the leveling of an epidemic. Pancreas. 2003;27:139–42. doi: 10.1097/00006676-200308000-00006. [DOI] [PubMed] [Google Scholar]