Abstract
Endocannabinoids are involved in synaptic signaling and neuronal protection; however, our understanding of the mechanisms by which endocannabinoids protect neurons from harmful insults remains elusive. 2-Arachidonoylglycerol (2-AG), the most abundant endogenous cannabinoid and a full agonist for cannabinoid receptors (CB1 and CB2), is a substrate for cyclooxygenase-2 (COX-2) and can be metabolized by COX-2. Here we show, however, that 2-AG is also capable of suppressing elevation of hippocampal COX-2 expression in response to proinflammatory and excitotoxic stimuli. 2-AG prevents neurodegeneration from toxic assaults that elevate COX-2 expression and inhibits the COX-2 elevation-enhanced excitatory glutamatergic synaptic transmission. The action of 2-AG on suppression of COX-2 appeared to be mediated via the pertussis toxin-sensitive G protein-coupled CB1 receptor and MAPK/NF-κB signaling pathways. Our results reveal that 2-AG functions as an endogenous COX-2 inhibitor protecting neurons from harmful insults by preventing excessive expression of COX-2, which provides a mechanistic basis for opening up new therapeutic approaches for protecting neurons from inflammation- and excitotoxicity-induced neurodegeneration.
Endocannabinoids (eCBs)2 are endogenous lipid mediators capable of binding to and functionally activating cannabinoid receptors (CB1 and CB2) to modulate synaptic function and produce neuroprotection (1–10). Although the role of eCBs in synaptic signaling has been investigated extensively, the mechanisms underlying the neuroprotection of eCBs are largely unknown (2, 3). Arachidonoylethanolamide (AEA or anandamide) and 2-arachidonoylglycerol (2-AG), the two most studied eCBs, have been demonstrated to be involved in a variety of physiological and pathological processes (1, 4–6, 11). Despite their similar chemical structure, however, the production and degradation of 2-AG and AEA are through different pathways (1, 4–6, 11, 12). 2-AG is produced mainly from diacylglycerol by diacylglycerol lipase and hydrolyzed to arachidonic acid (AA) by monoacylglycerol lipase (MGL), whereas AEA is largely synthesized from N-arachidonoylphosphatidylethanolamine by phospholipase D and degraded to AA by fatty-acid amide hydrolase (FAAH). Moreover, stimulation of glutamate release from Schaffer collaterals in rat hippocampal slices elevates levels of 2-AG but not AEA (13). Both 2-AG and AEA are substrates for cyclooxygenase-2 (COX-2), an inducible enzyme converting AA to classic prostaglandins, and are oxygenated by COX-2 to form new types of prostaglandins: prostaglandin glycerol esters and prostaglandin ethanolamides (14–18). However, there are differences in COX-2 oxidative metabolism of 2-AG and AEA. For instance, COX-2 efficiently metabolizes 2-AG to prostaglandin glycerol esters as it converts AA to classic prostaglandins, whereas the reaction of COX-2 metabolism of AEA to produce prostaglandin ethanolamides is relatively slow (14, 16). Importantly, AEA is a partial CB1 and a weak CB2 agonist, as well as an agonist for the vanilloid receptor (19–23), whereas 2-AG has been demonstrated to be the most abundantly endogenous ligand and a full agonist for both CB1 and CB2 receptors (1, 11, 23, 24). Increasing evidence shows that 2-AG, but not AEA, is likely a signaling molecule in mediating CB1-dependent depolarization-induced suppression of inhibition or depolarization-induced suppression of excitation (4, 23, 25–30). Also, enzymes that synthesize 2-AG are present in postsynaptic dendritic spines, providing direct evidence that 2-AG is synthesized in postsynaptic sites and acts on presynaptic CB1 receptors (31, 32).
Accumulated information suggests that anti-inflammatory effects of eCBs are important properties for eCB-mediated neuroprotection (2–4, 8, 33). Recent evidence indicates that 2-AG protects neurons via a CB1 receptor-dependent inhibition of proinflammatory cytokines and NF-κB (34, 35). This means that inflammatory signaling pathways may be involved in the 2-AG-produced neuroprotection, but direct evidence is still lacking. COX-2 is a key player in neuroinflammation, which has been implicated in the pathogeneses of neurodegenerative diseases (e.g. multiple sclerosis, Parkinson and Alzheimer diseases), and the contribution to the traumatic brain injury- and ischemia-induced neuronal damage (36–42). We hypothesized that the neuroprotective effect of 2-AG may be associated with its suppression of the COX-2 expression in neuroinflammation. We have demonstrated here that 2-AG is capable of suppressing elevation of COX-2 expression in response to proinflammatory and excitotoxic stimuli. The action of 2-AG on COX-2 suppression is mediated via the pertussis toxin (PTX)-sensitive G protein-coupled CB1 receptor and MAPK/NF-κB signaling pathways. Our findings provide a mechanistic basis for opening up new therapeutic approaches of treating, ameliorating, or preventing neurodegenerative diseases resulting from excessive activation of COX-2.
EXPERIMENTAL PROCEDURES
Animals—C57BL6 mice (Charles River), cnr1+/+ and cnr1-/- (NIMH transgenic core) weighing 20–25 g were used according to the guidelines approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center. Mice were intraperitoneally injected with vehicle, lipopolysaccharide (LPS), kainic acid (KA), 2-AG, URB597, SR141716 (SR-1), and SR144528 (SR-2).
Cell Culture—Primary hippocampal neurons were cultured as described previously (43, 44). To test whether astroglial cells are involved in 2-AG-mediated suppression of COX-2, we also made different cultures in which the extent of neurons and astroglial cells, controlled by different treatments, was estimated by using immunostaining with NeuN, a neuronal marker, glial fibrillary acidic protein (GFAP), an astrocytic marker, and OX-42, a microglial marker, in conjunction with staining with 4′,6-diamidino-2-phenylindole (43).
Primary hippocampal neurons from rat embryos (E18) were cultured as described previously (43, 44). After suffocation with carbon dioxide, unconscious animals were decapitated. Embryos were taken out after 75% ethanol was sprayed on the abdominal region of the dam. The hippocampus was dissected out under microscope and triturated in serum-free culture medium after meninges were removed. Tissue was incubated in oxygenated trypsin for 10 min at 37 °C and then mechanically triturated. Cells were spun down and resuspended in neurobasal/B27 medium (Invitrogen) supplemented with 0.5 mm l-glutamine, penicillin/streptomycin, and 25 μm glutamate. Cells (1 × 106) were loaded into poly-d-lysine-coated 35-mm culture dishes for electrophysiological recordings and into 6-well plates for real-time PCR analysis. Cells (4 × 104) were plated on poly-d-lysine-coated glass coverslips for immunocytochemistry. Medium was replaced every 3 days with the same medium without glutamate until use. The extent of astroglial cells in the culture was ∼2–5% at 10 days in vitro (DIV). For electrophysiological recordings, hippocampal neurons were cultured from mouse P0 as described previously (43). To obtain relatively pure hippocampal neurons for terminal transferase dUTP nick end labeling (TUNEL) staining, the proliferation of astroglial cells was inhibited by treatment of cultures with 5–10 μm cytosine arabinoside. Treatment with AraC led to a reduction of astroglial cells in culture to ∼1% (43). Cultures were used between 10 and 21 DIV.
Mixed hippocampal neurons and astroglial cells were cultured from rat P1 pups. The procedure for culturing mixed hippocampal neurons and astroglial cells was similar to that described above for primary hippocampal neurons except that minimum essential medium (MEM) were used during dissection of hippocampal tissue, and the MEM containing 5% fetal-bovine serum was used in cell resuspension and plated for the first 3 days. Then the MEM containing 5% fetal bovine serum was replaced with serum-free neurobasal medium/B27 until the culture was used. The extent of astroglial cells in the culture was estimated to be between 10 and 15% at 10 DIV.
Astroglial cell-enriched primary cultures were prepared using cells from rat P4-P5 pups. Pups were rapidly decapitated, and cerebral hemispheres were immediately dissected out and cleaned of meninges. The tissue were incubated in oxygenated trypsin for 10 min at 37 °C and then mechanically triturated in MEM containing 10% fetal bovine serum. The medium was modified with extra substances to produce a final composition of 7.5 mm glucose, double concentrations of amino acids, quadruple concentrations of vitamins, double concentrations of NaHCO3, 2 mm l-glutamine, penicillin (250,000 IU/liter), and 0.5% streptomycin. Cells were grown on 35-mm plastic Petri dishes. The medium were changed after 3 days of culturing and thereafter three times a week. The cultures were grown at 37 °C in a humidified atmosphere of 5% CO2 until use. The extent of astroglial cells in the culture was estimated at >95%.
Microglial cell-enriched cultures were prepared by shaking primary astroglial culture wells. The weakly adherent microglial cells on top of the astrocytic monolayer were detached. These cells were then transferred to new Petri dishes where they were cultured in MEM. The microglial cultures were used 24 h after reseeding.
Immunocytochemistry—Immunostaining was performed in primary cultures of hippocampal neurons and astroglial cells. The cells were rinsed with PBS after removal of the culture medium and fixed with prewarmed (37 °C) 4% paraformaldehyde, 4% sucrose in 0.1 m phosphate buffer (pH 7.2) at room temperature. Then the cells were washed four times with ice-cold PBS and incubated with blocking buffer (1% BSA and 10% normal goat serum) in PBS at room temperature for 1 h. Primary antibodies at different dilutions (in PBS containing 1% BSA) were applied overnight at 4 °C. Rabbit anti-COX-2 and CB1 (1:200) was purchased from Cayman Chemical (Ann Arbor, MI). NeuN, GFAP, and OX-42 were purchased from Chemicon (Temecula, CA). After four 10-min washes with PBS containing 1% BSA, species-specific and highly cross-adsorbed secondary antibodies coupled to Alexa 488 and CY3 (Molecular Probes), diluted 1/1000 in PBS containing 1% BSA, were applied for 1 h at room temperature. Cells were washed four times, 10 min each, with PBS containing 1% BSA and rinsed with PBS. Images were taken by a deconvolution microscope using Slidebook 4.0 software.
RNA Isolation and DNase Treatment—Total RNA was prepared from harvested tissue or cells with the RNeasy mini kit (Qiagen) and treated with RNase-free DNase (Qiagen) according to the manufacturer's instructions. The RNA concentration was measured by spectrophotometer (DU 640; Beckman). RNA integrity was verified by electrophoresis in a 1% agarose gel.
Reverse Transcription and Real-time Reverse Transcription-PCR—The iScript cDNA synthesis kit (Bio-Rad) was used for the reverse transcription reaction. We used 1 μg of total RNA with 4 μl of 5× iScript reaction mix and 1 μl of iScript reverse transcriptase. The total volume was 20 μl. Samples were incubated for 5 min at 25 °C. All samples were then heated to 42 °C for 30 min, and reactions were stopped by heating to 85 °C for 5 min. Specific primers for COX-1, COX-2, CB1, and glyceraldehyde-3-phosphate dehydrogenase were selected using Beacon Designer software (Bio-Rad) and synthesized by Integrated DNA Technologies (Coralville, IA). The primers are (listed by name, forward primer, reverse primer (amplicon size), and GenBank™ number): COX-1, 5′-AGAAGGAGATGGCTGCTGAG-3′, 5′-CACACGGAAGGAAACATAGGG-3′ (296 bp), BC005573; COX-2, 5-AAGCGAGGACCTGGGTTCAC-3, 5-ACACCTCTCCACCAATGACCTG-3 (142 bp), BC052900; CB1, 5′-TGCTGGTGCTATGTGTCATCC-3′, 5′-GCTGTGAAGGAGGCGGTAAC-3′ (202 bp), BC079564; GAPDH, 5-ACCACAGTCCATGCCATCAC-3, 5-ACCTTGCCCACAGCCTTG-3 (134 bp), M32599. The PCR amplification of each product was further assessed using 10-fold dilutions of mouse brain cDNA library as a template and found to be linear over 5 orders of magnitude and at more than 95% efficiency. All the PCR products were verified by sequencing, and detailed procedures and calculation were described previously (43, 44).
Immunoblot—Hippocampal tissues or hippocampal neurons and astroglial cells in cultures were extracted and immediately homogenized in a one-to-one volume of modified radioimmune precipitation assay lysis buffer consisting of a number of protease inhibitors. Supernatants were fractionated on 10% SDS-polyacrylamide gels (Bio-Rad) and transferred onto polyvinylidene difluoride membranes (Bio-Rad). The membrane was incubated with rabbit caspase-3 and cleaved caspase-3, p38 and phospho-p38 MAPK, p44/42 and phospho-p44/42 MAPK, NF-κB65 and phospho-NF-κB, Akt and phosphor-Akt antibodies (1:1,000, Cell Signaling, Danvers, MA), COX-1 and COX-2 polyclonal antibodies (dilution of 1:1000, Cayman, Ann Arbor, MI), and CB1 antibody (1:1000, Calbiochem) at 4 °C overnight. The blot was washed and incubated with a secondary antibody (goat anti-rabbit 1:10000, Vector Laboratories, Burlingame, CA) at room temperature for 1 h. Proteins were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences). The densities of specific bands were quantified by densitometry using FUJIFILM MultiGauge software (version 3.0). Band densities were normalized to the total amount of protein loaded in each well as determined by mouse anti-β-actin (1:4000, Sigma).
Cytotoxicity Assays—Neurotoxicity assays were performed as described previously (44). Briefly, glutamate or IL-1β was applied as the cytotoxic treatment. Control cultures were incubated with Locke's solution alone for the specified time. For Hoechst staining, cells were fixed with cold methanol (stored at -20 °C) at room temperature for 10 min. After three washes with PBS, Hoechst 33258 in PBS was added to fixed cells for 30 min at room temperature. Stained condensed chromatin appeared small and bright blue, whereas stained normal nuclei were large and light blue. The proportion of nuclei with condensed chromatin was calculated to reflect the level of injury. For TUNEL analysis, we used the DeadEnd™ fluorometric TUNEL system kit (Promega, Madison, WI). The TUNEL-stained cultures also stained with 4′,6-diamidino-2-phenylindole were imaged using a Zeiss inverted deconvolution microscope with Slidebook 4.1 software. The activation of caspase-3 was assayed using the immunoblot technique to detect the cleaved caspase-3.
PGE2 Assay—Mixed hippocampal neurons and astroglial cells in culture were treated with LPS (1 μg/ml) for 24 h in the absence and presence of 2-AG or 2-AG plus SR-1. The levels of PGE2 were measured using a PGE2 enzyme immunoassay kit (Cayman Chemical) according to the procedure described by the manufacturer.
Data Analysis—Data are presented as means ± S.E. Unless stated otherwise, Student's t test and ANOVA with Fisher's PLSD test were used for statistical comparison when appropriate. Differences were considered significant when p was <0.05.
RESULTS
2-AG Prevents Excessive COX-2 Expression in Response to Proinflammatory Stimulus—To determine whether 2-AG is capable of suppressing elevation of COX-2 expression in response to a proinflammatory stimulus, we first determined the expression of COX-2 in hippocampal tissue from mice injected with LPS, a commonly used COX-2 inducer. As shown in Fig. 1a, hippocampal COX-2, but not COX-1, expression was robustly elevated at the level of protein in animals that received LPS for 4 h using the immunoblot analysis. Although 2-AG did not produce a detectable change in basal expression of COX-2 protein in the hippocampus (supplemental Fig. 1), it significantly induced a dose-dependent attenuation of the LPS-induced elevation of COX-2 (Fig. 1a, p < 0.001, n = 6, one-way ANOVA, Fisher's PLSD). The suppression of 2-AG on COX-2 expression was further confirmed at the level of mRNA using quantitative real-time PCR analysis in a mixed culture of hippocampal neurons and astroglial cells (the extent of astroglial cells in the culture was estimated to be between 10 and 15% at 10 DIV; Fig. 1b). 2-AG inhibited LPS-induced elevation of COX-2 mRNA (p < 0.001, n = 3, one-way ANOVA). These data indicate that 2-AG acts as a COX-2 inhibitor preventing excessive elevation of COX-2 expression in response to the proinflammatory stimulus.
FIGURE 1.
2-AG suppresses LPS-induced elevation of COX-2 expression. a1, immunoblot analysis of COX-1 and COX-2 expression in mouse hippocampus. 2-AG was injected intraperitoneally 30 min before intraperitoneal injection of LPS, and COX proteins were analyzed 4 h after LPS injection. a2, quantifications of COX-1 and COX-2 expression. 2-AG induces a dose-dependent reduction of COX-2 elevation in response to LPS injection (n = 6). b, real-time PCR analysis of COX-2 expression in mixed culture of hippocampal neurons and astroglial cells (n = 3). The culture was treated with LPS, and mRNA was assayed 12 h after LPS treatment. Results are from three independent cultures with duplicate wells. *, p < 0.05, and **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with LPS.
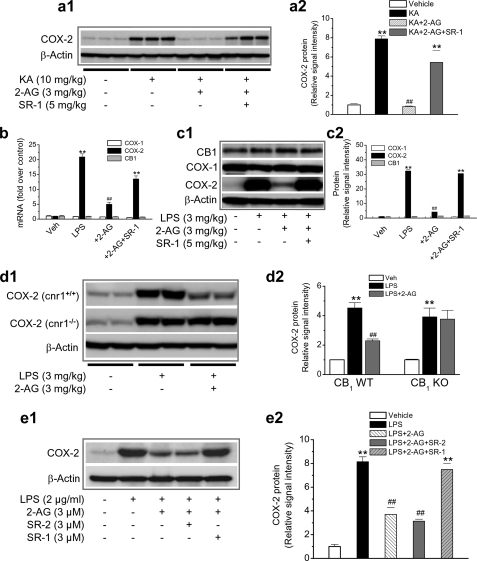
2-AG Limits Excessive Expression of COX-2 in Response to Excitotoxic Stimulus—Excitotoxicity-induced neuronal injury/death has been shown to be closely associated with increased COX-2 expression and activity (45–47). To determine whether 2-AG is also capable of suppressing COX-2 expression in response to an excitotoxic assault, mice were injected intraperitoneally with KA (10 mg/kg) without or with 2-AG (3 mg/kg). As shown in Fig. 2a, KA significantly elevated hippocampal COX-2 expression. This elevation was suppressed in the presence of 2-AG, suggesting that 2-AG is capable of preventing COX-2 elevation from excitotoxicity.
FIGURE 2.
CB1 mediates 2-AG suppression of COX-2 elevation in response to inflammatory and excitotoxic stimuli. a1, immunoblot analysis of COX-2 expression in hippocampus from mice treated with vehicle, KA, KA + 2-AG, and KA + 2-AG + SR-1. COX-2 protein was detected 4 h after injections. a2, quantifications of COX-2 expressions under different treatments (n = 3). b, quantitative real-time PCR analysis of COX-1, COX-2, and CB1 expression in hippocampal tissue from mice treated with vehicle, LPS, LPS + 2-AG, and LPS + 2-AG + SR-1 (n = 4). c1, immunoblot analysis of COX-1, COX-2, and CB1 in the hippocampus from mice treated with vehicle, LPS, LPS + 2-AG, and LPS + 2-AG + SR-1. c2, quantifications of protein expressions under different treatments (n = 4). d1, 2-AG failed to suppress COX-2 elevation in response to LPS injection in CB1 knock-out (cnr1-/-) mice. Immunoblot analysis is shown of COX-2 expression in the hippocampus from animals that received vehicle, LPS, or LPS + 2-AG. d2, quantifications of COX-2 protein expressions in CB1 knock-out (KO) and wild type (WT) animals (n = 4). e1, CB1, but not CB2, mediates 2-AG suppression of COX-2. Immunoblot analysis is shown of COX-2 expression in a mixed culture of neurons and astroglial cells. e2, quantifications of COX-2 under different conditions (n = 3). **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with LPS or KA.
2-AG-induced Suppression of COX-2 Is Mediated via the CB1 Receptor—Several lines of evidence show that 2-AG is a full agonist for CB1 and CB2 receptors (1, 4–6, 11). We therefore decided to determine whether the 2-AG suppression of COX-2 elevation is mediated via CB1, which is expressed mainly in the brain (1, 4–6). As shown in Fig. 2, a–c, SR-1, a selective CB1 antagonist (provided by NIMH Chemical Synthesis and Drug Supply Program), blocked the 2-AG-produced suppression of COX-2 at the levels of protein and mRNA in response to an injection of KA or LPS (p < 0.001; one-way ANOVA), suggesting that the CB1 receptor mediates 2-AG suppression of COX-2. Injection of SR-1 alone did not alter basal COX-2 expression (supplemental Fig. 2). We noticed that the CB1 expression levels at protein and mRNA were not significantly changed in animals treated with LPS or 2-AG. However, 2-AG still suppressed the LPS-elevated COX-2 expression. This is probably because the CB1 receptor is expressed abundantly in the brain (the whole-brain CB1 density is similar to whole-brain densities of receptors for glutamate and γ-aminobutyric acid 11) and because the capacity of the expressed CB1 receptors is likely sufficient to exert their signaling function even if there are no significant changes in their expression levels. To further confirm that the CB1 receptor mediates the 2-AG suppression of COX-2, we examined the effect of 2-AG on COX-2 expression in CB1 knock-out mice (provided by NIMH Transgenic Core) that received LPS (3 mg/kg). As shown in Fig. 2d, the LPS-induced elevation of COX-2 in the hippocampus was attenuated by administration of 2-AG in CB1 wild type animals (cnr1+/+) but not in CB1 knock-out mice (cnr1-/-). This information suggests that the 2-AG suppression of COX-2 elevation is mediated via the CB1 receptor.
It was thought that the CB2 receptor is present mainly in the immune system. Recent evidence shows that the CB2 receptor is also present in the brain (astroglial cells and brain stem neurons) (48–52). To determine whether the CB2 receptor is involved in 2-AG-produced suppression of COX-2, we used SR-2, a selective CB2 antagonist (provided by NIMH Chemical Synthesis and Drug Supply Program). As seen in Fig. 2e, 2-AG significantly suppressed COX-2 elevation induced by LPS in mixed culture of hippocampal neurons and astroglial cells. This suppression was prevented by SR-1 but not by SR-2. These results provide evidence that 2-AG suppression of COX-2 is mediated mainly via the CB1 receptor. This was further confirmed in hippocampal tissue from the animals that received LPS, 2-AG, and SR-2. Inhibition of the CB2 receptor failed to block the 2-AG-produced suppression of COX-2 (Fig. 3c).
FIGURE 3.
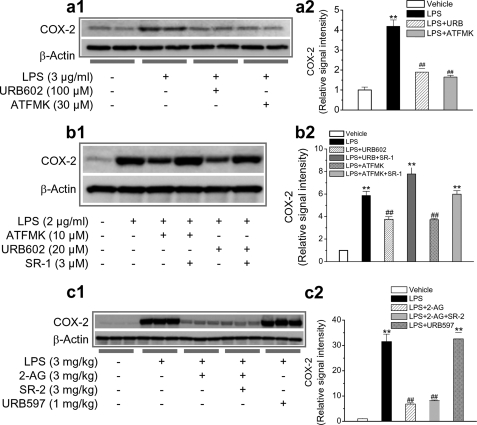
Endogenous 2-AG suppresses COX-2 elevation. a1, MGL inhibitors URB602 and ATFMK prevent LPS-induced elevation of COX-2 in hippocampal slices. Hippocampal slices were treated with LPS in the absence and presence of URB602 or ATFMK. COX-2 expression was detected 6 h after treatments. a2, quantifications of COX-2 in slices treated with LPS in the absence and presence of MGL inhibitors (n = 4). b1, MGL inhibitors reduce COX-2 expression in response to LPS in mixed culture of hippocampal neurons and astroglial cells. COX-2 protein was analyzed 24 h after treatments. b2, quantifications of COX-2 in the culture treated with LPS in the absence and presence of MGL inhibitors or SR-1 (n = 3). c1, FAAH inhibitor or CB2R antagonist does not prevent 2-AG-produced suppression of COX-2 elevation in hippocampal tissue. Mice were injected intraperitoneally with LPS, LPS + 2-AG, LPS + 2-AG + SR144528 (SR-2), and LPS + URB597. COX-2 was determined 4 h after injections. c2, quantifications of COX-2 in the hippocampal tissue from animals that received different treatments (n = 3). **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with LPS.
CB1 receptor -mediated action of 2-AG in suppressing COX-2 elevation in neuroinflammation was further assessed by detecting PGE2, a major COX-2 reaction product. Application of LPS elevated the PGE2 level in a mixed culture of hippocampal neurons and astroglial cells. LPS-induced elevation PGE2 was significantly reduced in the presence of 2-AG; this reduction was reversed by SR-1 (supplemental Fig. 3, p < 0.001; one-way ANOVA). These data provide clear evidence that 2-AG is capable of suppressing COX-2 elevation in neuroinflammation, which is mediated via the CB1 receptor.
Endogenous 2-AG Is Capable of Preventing COX-2 Elevation—As 2-AG was exogenously administrated in the experiments described above, we then sought to determine whether endogenous 2-AG was also able to suppress the LPS-induced elevation of COX-2. Monoacylglycerol lipase is the enzyme responsible for hydrolysis of 2-AG, and thus an inhibition of MGL would raise the levels of 2-AG. To this end, we used a relatively selective MGL inhibitor, URB602, and a nonselective MGL inhibitor, arachidonyl trifluoromethyl ketone (ATFMK), which have been shown to inhibit 2-AG hydrolysis, increase 2-AG levels, and enhance retrograde signaling (26, 30, 53–56). As seen in Fig. 3a, administration of URB602 or ATFMK significantly reduced the LPS-induced elevation of COX-2 in hippocampal slices (p < 0.001, n = 3, one-way ANOVA). The MGL inhibitor-produced suppression of COX-2 elevation was also determined in a mixed culture of neurons and astroglial cells. As shown in Fig. 3b, URB602 and ATFMK significantly decreased the LPS-induced elevation of COX-2; this decrease was blocked by the CB1 antagonist SR-1. This means that an increase in endogenous 2-AG level, either by inhibiting hydrolysis of 2-AG or by exogenously supplying 2-AG, is able to prevent COX-2 elevation in neuroinflammation. As ATFMK has been shown to inhibit both MGL and FAAH (53), we decided to use URB597, a selective FAAH inhibitor, to see whether inhibiting FAAH would prevent the LPS elevation of COX-2. It appeared that administration of URB597 failed to suppress LPS-elevated hippocampal COX-2 expression (Fig. 3c), suggesting that the ATFMK-induced reduction of COX-2 expression was a result of its inhibition of MGL activity in turn resulting in an elevated level of 2-AG.
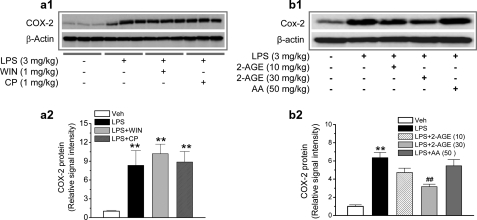
Exogenous Cannabinoids Fail to Prevent LPS Elevation of COX-2—The results presented above clearly show that 2-AG as an endogenous cannabinoid is capable of preventing COX-2 elevation in response to proinflammatory and excitotoxic stimuli. To determine whether exogenous cannabinoids are also capable of inhibiting COX-2 in neuroinflammation, we detected hippocampal COX-2 expression in mice that received LPS and WIN55,212-2 (WIN) or CP55,940 (CP), synthetic cannabinoid receptor agonists. As shown in Fig. 4a, the cannabinoids WIN or CP failed to suppress LPS-induced elevation of COX-2. To confirm the capability of 2-AG in suppressing COX-2, we used 2-arachidonyl glycerol ether, a less potent analogue of 2-AG, on LPS-elevated COX-2 expression. We found that 2-arachidonyl glycerol ether significantly reduced the LPS-elevated hippocampal COX-2 but that a higher dosage was required for the suppression (Fig. 4b). Because 2-AG is easily hydrolyzed by MGL to AA, it is possible that the 2-AG-produced suppression of COX-2 may result from the action of AA. To test this possibility, we treated animals with AA (50 mg/kg, intraperitoneally). It appears that AA did not significantly inhibit LPS-induced COX-2 elevation (Fig. 4b). To explore possible mechanisms underlying differences in suppression of COX-2 between exogenous and endogenous cannabinoids, we detected mRNA of the CB1 in a mixed culture of astroglial cells and hippocampal neurons in the presence of 2-AG, WIN, or CP. As shown in supplemental Fig. 4, WIN and CP induced a down-regulation of the CB1 mRNA, whereas 2-AG did not induce a detectable change in the CB1 mRNA. It is likely that WIN- and CP-induced down-regulation of the CB1 may be an important mechanism contributing, at least in part, to their failure of suppressing COX-2. This information indicates that the molecular mechanisms of 2-AG in suppressing COX-2 elevation in response to LPS insult are different from those of exogenous cannabinoids.
FIGURE 4.
Cannabinoids WIN55,212-2 and CP55,940 fail to prevent LPS-induced elevation of hippocampal COX-2. a1, immunoblot analysis of COX-2 expression in hippocampi from mice treated with vehicle, LPS, LPS + WIN, or LPS + CP. WIN and CP were injected 30 min before LPS injection. COX-2 protein was detected 4 h after the injections. a2, quantifications of COX-2 expressions under different treatments (n = 3). **, p < 0.01 compared with vehicle controls. b1, immunoblot analysis of COX-2 expression in hippocampi from mice treated with vehicle, LPS, LPS + 2-arachidonyl glycerol ether (2-AGE), or LPS + AA. 2-Arachidonyl glycerol ether and AA were injected 30 min before LPS injection. COX-2 protein was detected 4 h after injections. b2, quantifications of COX-2 expressions under different treatments (n = 3). **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with LPS.
Neurons and Astroglial Cells Are Involved in the 2-AG Suppression of COX-2—Because COX-2 is expressed in both neurons and astroglial cells (Fig. 5, a1–a3), we decided to determine the involvement of neurons and astroglial cells in the 2-AG-mediated suppression of COX-2 induction stimulated by proinflammatory factors. We measured the expression levels of COX-2 in primary cultures with relatively pure hippocampal neurons, a mixed culture of hippocampal neurons and astroglial cells, and an astroglial cell-enriched culture. The extent of astroglial cells in the culture was estimated by staining with NeuN, a neuronal marker, GFAP, an astrocytic marker, and OX-42, a microglial marker, in conjunction with 4′,6-diamidino-2-phenylindole staining as described previously (43, 44). We found that 2-AG reduced IL-1β- or LPS-induced increase in COX-2 mRNA in different cultures (Fig. 5, c–e). Similarly, the 2-AG suppression of COX-2 was blocked by SR-1, indicating that the CB1 receptor mediates the 2-AG suppression of COX-2 in both neurons and astroglial cells. We detected that the CB1 receptor is expressed in neurons and astroglial cells (Fig. 5, b1–b3), which is consistent with reports by others (8, 12, 33, 57, 58). These results suggest that both hippocampal neurons and astroglial cells are involved in the 2-AG suppression of COX-2 elevation in response to proinflammatory stimuli and that the CB1 receptor mediates this suppression process.
FIGURE 5.
Neurons and astroglial cells are involved in the 2-AG-produced suppression of COX-2. a1–a3, COX-2 is expressed in neurons and astroglial cells. Shown is COX-2 immunostaining with NeuN (neuronal marker), GFAP (astrocytic marker), and OX-42 (microglial marker) in different cultures. Images were taken with a Zeiss deconvolution microscope using Slidebook 4.0 software with magnification ×40. Scales bars = 10 μm. b1–b3, CB1 receptors are expressed in neurons and astroglial cells. Shown is CB1 immunostaining with NeuN, GFAP, and OX-42 in different cultures. c, 2-AG induces a CB1-dependent suppression of COX-2 elevation in response to IL-1β stimulus in relatively pure neuronal culture (astroglial cells ∼1%). Neurons in culture were treated with IL-1β (50 ng/ml), IL-1β + 2-AG (3 μm), and IL-1β + 2-AG + SR-1 (3 μm). COX-2 mRNA was analyzed using real-time PCR 24 h after treatments. d, 2-AG suppresses COX-2 expression in response to LPS (1 μg/ml) stimulus in mixed culture of hippocampal neurons and astroglial cells (astroglial cells 10∼15%). The culture was treated with LPS, LPS + 2-AG, and LPS + 2-AG + SR-1. e, 2-AG prevents excessive expression of COX-2 in astroglial cell-enriched culture (astroglial cells >95%). Astroglial cells in culture were treated with LPS (0.5 μg/ml), LPS + 2-AG (3 μm), and LPS + 2-AG +SR-1 (3 μm). **, p < 0.01 compared with controls; ##, p < 0.01 compared with IL-1β or LPS.
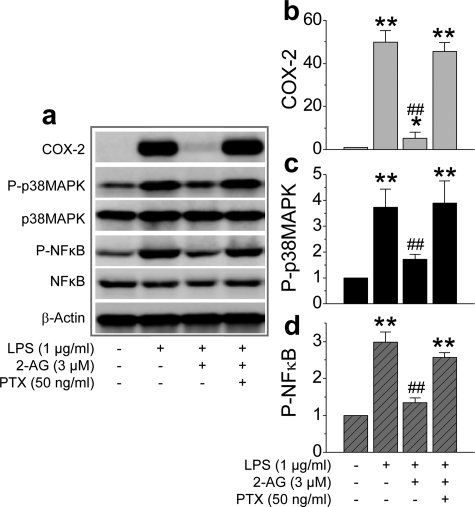
Signaling Pathways Involved in the 2-AG Suppression of COX-2—To examine possible signal transduction mechanisms underlying the 2-AG suppression of COX-2 elevation in response to a proinflammatory and excitotoxic stimuli, we probed the phosphorylation status of p38 MAPK and NF-κB in hippocampal tissue from mice that had received LPS, 2-AG, and SR-1. As indicated in Fig. 6, a and b, 2-AG attenuated the LPS-induced phosphorylation of p38 MAPK and NF-κB. 2-AG-Induced attenuation of the phosphorylation of p38 MAPK and NF-κB was blocked by SR-1. This information indicates that the 2-AG-induced suppression of COX-2 elevation in neuroinflammation is likely mediated via the CB1 receptor and p38 MAPK/NF-κB signaling pathways. However, p38 MAPK and NF-κB were not significantly phosphorylated in the hippocampus from the animals injected with KA (data not shown). To further explore other possible signaling pathways that might be involved in COX-2 expression in inflamed tissue, we detected phosphoinositide 3-kinases and extracellular signal-regulated kinases (ERK) in hippocampi from animals that had received LPS, KA, 2-AG, or SR-1. Fig. 6c shows that LPS significantly induced ERK phosphorylation, but not Akt, and that 2-AG inhibits the LPS-induced phosphorylation of ERK. Interestingly, KA induced phosphorylation of both ERK and Akt, and 2-AG prevented KA-induced ERK and Akt phosphorylation (Fig. 6d). These data suggest that the downstream signaling pathways that mediate LPS- and KA-elevated COX-2 expression are different. However, 2-AG still is able to suppress the COX-2 elevation, suggesting that the CB1 receptor is linked to multiple signaling pathways.
FIGURE 6.
ERK, MAPK, phosphoinositide 3-kinase, and NF-κB signaling pathways are involved in the 2-AG-induced suppression of COX-2 elevation. a1, Western immunoblot analysis of phosphorylation of p38 MAPK in hippocampal tissue from mice that received vehicle, LPS, LPS + 2-AG, and LPS + 2-AG + SR-1. a2, quantifications of p38 MAPK phosphorylation. b1, immunoblot analysis of NF-κB phosphorylation in the mouse hippocampus. b2, quantifications of NF-κB phosphorylation (n = 3). **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with LPS. c1, Western blot analysis of ERK1/2 and Akt phosphorylation in hippocampal tissue from mice injected with vehicle, LPS, and LPS + 2-AG. c2 and c3, quantifications of ERK1/2 and Akt phosphorylation. **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with LPS (n = 3). d1, Western blot analysis of ERK1/2 and Akt phosphorylation in hippocampal tissue from mice injected with vehicle, KA, KA + 2-AG, and KA + 2-AG + SR-1. c2 and c3, quantifications of ERK1/2 and Akt phosphorylation. **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with KA (n = 3).
It has been well documented that the CB1 receptor is coupled to a PTX-sensitive Gi/o protein. To ascertain whether the 2-AG suppression of COX-2 elevation is mediated via the Gi/o, we treated mixed culture of hippocampal neurons and astroglial cells with PTX (50 ng/ml) in the presence of LPS or LPS plus 2-AG. Fig. 7 shows that pretreatment with PTX blocked 2-AG suppression of LPS-elevated COX-2 and phosphorylation of p38MAP and NF-κB. These results demonstrate that the CB1 receptor-coupled PTX-sensitive G protein, Gi/o, mediates 2-AG-induced suppression of COX-2.
FIGURE 7.
Gi/o protein mediates 2-AG suppression of COX-2. a, immunoblot analysis of COX-2 expression and ERK1/2 and NF-κB phosphorylation in mixed culture of hippocampal neurons and astroglial cells treated with LPS, 2-AG, and PTX. Neurons in culture were treated with PTX (50 mg/ml) for 30 min before application of 2-AG and 60 min before application of LPS. Proteins were detected 6 h after treatments. b–d, quantifications of COX-2 expression, p38 MAPK, and NF-κB phosphorylation. *, p < 0.05, and **, p < 0.01 compared with vehicle controls; ##, p < 0.01 compared with LPS (n = 3).
2-AG Protects Neurons from Proinflammatory and Excitotoxic Insults—To determine the functional consequence of 2-AG-meidated suppression of COX-2 elevation in response to proinflammatory and excitotoxic insults, we treated hippocampal neurons in culture with IL-1β or glutamate (Glu) to induce neurotoxicity using Hoechst and TUNEL staining and analysis of capase-3 activation, an apoptotic marker. As seen in Fig. 8, a and b, 2-AG significantly attenuated the numbers of TUNEL-positive neurons in culture treated with IL-1β or Glu. The blockade of the CB1 receptor with SR-1 reversed the 2-AG-produced reduction of injured/dead neurons. Similarly, 2-AG reduced LPS-induced neuronal death (supplemental Fig. 5). Hoechst staining also showed that 2-AG or NS398 (a selective COX-2 inhibitor) reduced Glu-induced apoptotic neurons, and SR-1 blocked the effect of 2-AG (Fig. 8c). In addition, 2-AG decreased Glu-induced cleavage of caspase-3, an effect that was also blocked by SR-1 (Fig. 8d). To determine whether the elevation of COX-2 contributed to the Glu-induced excitotoxicity, we measured COX-2 expression in hippocampal neurons in cultures treated with Glu in the absence and presence of 2-AG and SR-1. Fig. 8e shows that glutamate elevated COX-2 expression and that the elevation was reduced by 2-AG. The ability of 2-AG to protect neurons from toxic insults was also evaluated by URB602, which inhibits hydrolysis of MGL and raises endogenous 2-AG. The inhibition of MGL also significantly decreases Glu-induced neuronal injury/death (supplemental Fig. 6). These data clearly demonstrated that 2-AG produced a neuroprotective effect against inflammatory or excitotoxic insults, and the neuroprotective effect is likely via 2-AG suppression of COX-2 elevation.
FIGURE 8.
2-AG protects hippocampal neurons from proinflammatory and excitotoxic insults. a1–a5, TUNEL images of hippocampal neurons in control (a1), IL-1β (50 ng/ml (a2)), IL-1β + 2-AG (1 μm (a3)), IL-1β + 2-AG (5 μm (a4)), and IL-1β + 2-AG (5 μm) + SR-1 (5 μm (a5)) for 24 h. a6, percentages of injured neurons under different treatments (n = 10–12). b1–b5, TUNEL images of hippocampal neurons in control (b1), Glu (50 μm (b2)), Glu + NS398 (20 μm (b3)), Glu + 2-AG (5 μm (b4)), and Glu + 2-AG (5 μm) + SR-1 (5 μm (b5)) for 24 h. b6, percentages of injured neurons under different treatments (n = 10). c1, Hoechst staining of hippocampal neurons in control, Glu (50 μm), Glu + NS398 (20 μm), Glu + 2-AG (3 μm), and Glu + 2-AG + SR-1 (5 μm) for 24 h. c2, percentages of apoptotic neurons under different treatments (n = 5). d, Western blot analysis of cleaved caspase-3 in control, Glu (50 μm), 2-AG (1 and 5 μm), and SR-1 (1 μm). e, glutamate elevates COX-2 expression, and 2-AG attenuates Glu-induced elevation of COX-2. Hippocampal neurons in culture were treated with Glu for 24 h in the absence and presence of 2-AG and 2-AG + SR-1. **, p < 0.01, compared with control; #, p < 0.05, and ##, p < 0.01 compared with IL-1β or glutamate.
2-AG Inhibits COX-2 Elevation-enhanced Excitatory Synaptic Transmission—We demonstrated previously that an elevation of COX-2 expression by LPS or IL-1β in hippocampal neurons in culture enhances excitatory glutamatergic synaptic release, and the enhancement is inhibited by a selective COX-2 inhibitor (43). If 2-AG is capable of preventing excessive expression of COX-2, then 2-AG should inhibit COX-2 elevation-induced enhancement of glutamatergic synaptic transmission. To test this idea, we treated hippocampal neurons in culture with IL-1β or LPS in the absence and presence of 2-AG or SR-1. As shown in Fig. 9, IL-1β or LPS enhanced the frequency of miniature excitatory postsynaptic currents (mEPSCs). 2-AG reduced IL-1β- or LPS-induced enhancement of mEPSCs, and this reduction was reversed by SR-1 (p < 0.001; one-way ANOVA). To confirm that the LPS-induced increase in mEPSCs was attributable to the increased expression of COX-2, LPS was added to the culture of hippocampal neurons from COX-2 knock-out mice. It appeared that LPS failed to enhance synaptic transmission in cultured neurons from COX-2 null mice while significantly increasing the frequency of mEPSCs in neurons from wild type animals (supplemental Fig. 7), suggesting that the elevation of COX-2 augments excitatory synaptic transmission. These results provided convincing information that 2-AG is able to suppress COX-2 elevation in neuroinflammation and to reduce the COX-2 elevation-induced increase in excitatory synaptic transmission. There were no significant changes in the frequency or amplitude of mEPSCs in neurons treated with 2-AG or SR-1 alone for 12 h (supplemental Fig. 8).
FIGURE 9.
2-AG inhibits COX-2 elevation-induced enhancement of hippocampal excitatory synaptic transmission. a1, representative sweeps of mEPSCs recorded in control, IL-1β (10 ng/ml)-, IL-1β + 2-AG (1 μm)-, and IL-1β + 2-AG + SR-1 (1 μm)-treated neurons. The culture was treated with IL-β for 16 h. 2-AG or 2-AG + SR-1 was added 30 min before IL-1β application. a2, cumulative probability of mEPSC frequency recorded in neurons with different treatments. a3, mean percentage of change in the frequency of mEPSCs in neurons with different treatments (n = 10 to 16). a4, cumulative probability of mEPSC amplitude. a5, mean percentage of change in the amplitude of mEPSCs. Scale bar:20 pA/2 s. b1, representative sweeps of mEPSCs recorded in control, LPS (1 μg/ml)-, LPS + 2-AG (1 μm)-, and LPS + 2-AG + SR-1 (1 μm)-treated neurons. The culture was treated with LPS for 24 h. 2-AG or 2-AG + SR-1 was added 30 min before application of LPS. b2, cumulative probability of mEPSC frequency recorded in neurons with different treatments. b3, mean percentage of change in the frequency of mEPSCs (n = 7–12). b4, cumulative probability of mEPSC amplitude. b5, mean percentage of change in the amplitude of mEPSCs. Scale bar: 20 pA/2 s. **, p < 0.01 compared with the control; ##, p < 0.01 compared with IL-1β or LPS.
DISCUSSION
Several lines of evidence show that eCBs play a variety of physiological and pharmacological roles (1, 4–6). In particular, eCBs are likely an endogenous system protecting neurons from harmful insults (2, 3, 7–10, 59, 60). However, the mechanisms by which eCBs protect neurons from harmful insults are still not clear. Our findings provide the first mechanistic evidence showing that the neuroprotective effect of endocannabinoid 2-AG against inflammatory and excitotoxic assaults is associated with its capability of limiting COX-2 elevation.
Recent evidence shows that the neuroprotective effect of 2-AG against inflammation and traumatic brain injury is associated with its reduction of cytokines, reactive oxygen species, and PGE2 (10, 34, 35, 59, 61), meaning that the action of 2-AG may be related to the inhibition of COX-2. In the present study, we have provided convincing evidence that 2-AG is capable of suppressing COX-2 elevation in response to inflammatory and excitotoxic insults. Importantly, COX-2 elevation could be suppressed by the application of MGL inhibitors, which prevent the hydrolyzing of endogenous 2-AG, suggesting that 2-AG may function as an endogenous COX-2 inhibitor. 2-AG suppression of COX-2 appears to be mediated via the CB1 receptor, which is expressed predominantly in the central nervous system. This is evident by the facts that the inhibitory effect of 2-AG on COX-2 is blocked or attenuated by SR141716, a selective CB1 antagonist, but not by SR 144528, a selective CB2 antagonist. This is further confirmed by experiments in which 2-AG failed to suppress COX-2 elevation in mice deficient in the CB1 receptor. There is a report showing that Δ9-tetrahydrocannabinol, a major psychoactive component in marijuana but not 2-AG, induces CB2 receptor-mediated suppression of LPS-induced COX-2 induction and PGE2 production in mouse macrophage cell lines (62). However, it has also been shown that marijuana smoking or Δ9-tetrahydrocannabinol elevates PGE2; this elevation could be antagonized by nonsteroidal anti-inflammatory drugs such as indomethacin (63–65). The synthetic cannabinoid WIN55,212-2 also has been demonstrated to elevate COX-2 expression in murine brain-derived endothelial cells (66). In the present study, we observed that WIN55,212-2 and CP55,940 failed to suppress COX-2. 2-Arachidonyl glycerol ether, an endogenous analogue of 2-AG, is also capable of suppressing COX-2 elevation in response to the LPS stimulus. Therefore, there are differences in the mediation of COX-2 induction or suppression between 2-AG and synthetic cannabinoids. The mechanisms responsible for the differences are still not clear. We observed that 2-AG did not affect basal mRNA expression of the CB1 receptor, whereas WIN and CP down-regulated it. The difference in down-regulation of the CB1 mRNA between the two agonists and 2-AG may not fully account for the difference in suppressing COX-2, but it may, in part, explain why WIN55212-2 and CP55940 failed to attenuate COX-2 elevation in response to a proinflammatory stimulus. It is possible that regulation of the CB1 expression levels and different downstream signaling pathways may contribute to cannabinoid receptor-mediated effects (67, 68).
In the present study, we have demonstrated that the signaling pathways mediating LPS- and KA-elevated COX-2 expression are different. ERK, p38 MAPK, and NF-κB signal transduction pathways are involved in the LPS-elevated COX-2 expression, whereas ERK and phosphoinositide 3-kinase contribute to KA-elevated COX-2. Despite differences in signaling pathways triggered by LPS and KA, the phosphorylation of these signaling pathways is attenuated by 2-AG. 2-AG suppression of COX-2 is mediated mainly via the CB1 receptor, which indicates that the CB1 receptor is connected to multiple intracellular signaling transduction pathways. Moreover, the action of 2-AG in suppressing COX-2 appears to be mediated via a PTX-sensitive Gi/o protein, because the inhibitory effects of 2-AG on COX-2 expression and phosphorylation of p38 MAPK and NF-κB are prevented by pretreatment with PTX.
Astroglial cells play an important role in neurotoxicity. Similar to neurons, COX-2 in astroglial cells is rapidly up-regulated in response to inflammatory and excitotoxic stimuli. We observed that the magnitude of COX-2 elevation in response to the stimuli in mixed cultures of neurons and astroglial cells and astroglial cell-enriched culture was much greater than that in relatively pure neuronal cultures. This means that the elevated COX-2 expression in response to LPS or KA stimulus is derived largely from astroglial cells. In this study, we confirmed that the CB1 receptor is expressed in both hippocampal neurons and astroglial cells, which is consistent with reports that the CB1 receptor is present in neurons and astroglial cells (8, 12, 33, 57, 58). Thus, 2-AG suppression of COX-2 may involve both neurons and astroglial cells.
2-AG has been shown to protect neurons from brain ischemia, traumatic brain injury, and proinflammatory stimuli (7, 10, 33–35, 59, 60). We have demonstrated here that 2-AG protects neurons from inflammatory and excitotoxic insults and that this protective effect is through the CB1 receptor-dependent suppression of COX-2 elevation. Previously, we showed that COX-2 elevation enhances excitatory synaptic transmission (43). Interestingly, COX-2 elevation-enhanced excitatory synaptic transmission is prevented by 2-AG in a CB1 receptor-dependent manner. These results provide functional evidence that 2-AG protects neurons by limiting excessive COX-2 expression. 2-AG has been shown to reduce mEPSCs/EPSCs and synaptic release of excitatory neurotransmitter glutamate (27, 29, 44). It is likely that the immediate effect of 2-AG is to inhibit excitatory synaptic transmission, whereas the delayed effect of 2-AG is to limit excessive expression of COX-2 in neuroinflammation (supplemental Fig. 9).
Recent studies reveal that eCBs are substrates for COX-2 and can be degraded by COX-2 to form novel prostaglandins (14–18). In particular, 2-AG is a natural substrate for COX-2, and its oxygenation is as effective as that of AA (16). Because 2-AG is capable of suppressing COX-2 expression, as revealed by the present study, the capacity of 2-AG to inhibit COX-2 induction will therefore be greatly weakened when COX-2 expression is abnormally elevated in neuroinflammation. This may be an important mechanism underlying COX-2-meidated neurodegeneration. Our results suggest that an elevation of endogenous 2-AG levels by facilitating its synthesis, inhibiting its hydrolysis, or directly supplying 2-AG may result in new therapeutic approaches to protecting neurons from harmful insults by preventing excessive expression of COX-2. In addition, the inhibitory effect of 2-AG on COX-2 expression can also be inferred as interpreting the analgesic and anticancer effects of 2-AG because of the involvement of COX-2 in pain and certain carcinogenic developments.
Supplementary Material
Acknowledgments
We thank Dr. Nan Sang for help in the recording of mEPSCs.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 NS054886. It was also supported by Alzheimer's Association Grant IIRG-05-13580. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–9.
Footnotes
The abbreviations used are: eCBs, endocannabinoids; COX-2, cyclooxygenase-2; 2-AG, 2-arachidonoylglycerol; AEA, arachidonoylethanolamide; PGE2, prostaglandin E2; TUNEL, terminal transferase dUTP nick end labeling; mEPSC, miniature excitatory postsynaptic current; CB, cannabinoid; PTX, pertussis toxin; MAPK, mitogen-activated protein kinases; NF-κB, nuclear factor-κB; ERK, extracellular signal-regulated kinase; AA, arachidonic acid; MGL, monoacylglycerol lipase; FAAH, fatty acid amide hydrolase; LPS, lipopolysaccharide; KA, kainic acid; SR-1, SR141716; SR-2, SR144528; GFAP, glial fibrillary acidic protein; DIV, days in vitro; ATFMK, arachidonyl trifluoromethyl ketone; WIN, WIN55,212-2; CP, CP55,940; MEM, minimum essential medium; PBS, phosphate-buffered saline; BSA, bovine serum albumin; IL, interleukin; ANOVA, analysis of variance.
References
- 1.Piomelli, D. (2003) Nat. Rev. Neurosci. 4 873-884 [DOI] [PubMed] [Google Scholar]
- 2.Van der Stelt, M., and Di Marzo, V. (2005) Neuromol. Med. 7 37-50 [DOI] [PubMed] [Google Scholar]
- 3.Sarne, Y., and Mechoulam, R. (2005) Curr. Drug Targets CNS Neurol. Disord. 4 677-684 [DOI] [PubMed] [Google Scholar]
- 4.Mackie, K. (2006) Annu. Rev. Pharmacol. Toxicol. 46 101-122 [DOI] [PubMed] [Google Scholar]
- 5.Chevaleyre, V., Takahashi, K. A., and Castillo, P. E. (2006) Annu. Rev. Neurosci. 29 37-76 [DOI] [PubMed] [Google Scholar]
- 6.Freund, T. F., Katona, I., and Piomelli, D. (2003) Physiol. Rev. 83 1017-1066 [DOI] [PubMed] [Google Scholar]
- 7.Marsicano, G. Goodenough, S., Monory, K., Hermann, H., Eder, M., Cannich, A., Azad, S. C., Cascio, M. G., Gutiérrez, S. O., van der Stelt, M., López-Rodríguez, M. L., Casanova, E., Schütz, G., Zieglgänsberger, W., Di Marzo, V., Behl, C., and Lutz, B. (2003) Science 302 84-88 [DOI] [PubMed] [Google Scholar]
- 8.Eljaschewitsch, E., Witting, A., Mawrin, C., Lee, T., Schmidt, P. M., Wolf, S., Hoertnagl, H., Raine, C. S., Schneider-Stock, R., Nitsch, R., and Ullrich, O. (2006) Neuron 49 67-79 [DOI] [PubMed] [Google Scholar]
- 9.Monory, K., Massa, F., Egertová, M., Eder, M., Blaudzun, H., Westenbroek, R., Kelsch, W., Jacob, W., Marsch, R., Ekker, M., Long, J., Rubenstein, J. L., Goebbels, S., Nave, K. A., During, M., Klugmann, M., Wölfel, B., Dodt, H. U., Zieglgänsberger, W., Wotjak, C. T., Mackie, K., Elphick, M. R., Marsicano, G., and Lutz, B. (2006) Neuron 51 455-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panikashvili, D., Simeonidou, C., Ben-Shabat, S., Hanus, L., Breuer, A., Mechoulam, R., and Shohami, E. (2001) Nature 413 427-531 [DOI] [PubMed] [Google Scholar]
- 11.Sugiura, T., Kishimoto, S., Oka, S., and Gokoh, M. (2006) Prog. Lipid Res. 45 405-446 [DOI] [PubMed] [Google Scholar]
- 12.Stella, N. (2004) Glia 48 267-277 [DOI] [PubMed] [Google Scholar]
- 13.Stella, N., Schweitzer, P., and Piomelli, D. (1997) Nature 388 773-778 [DOI] [PubMed] [Google Scholar]
- 14.Kozak, K. R., Rowlinson, S. W., and Marnett, L. J. (2000) J. Biol. Chem. 275 33744-33749 [DOI] [PubMed] [Google Scholar]
- 15.Kozak, K. R., Crews, B. C., Morrow, J. D., Wang, L. H., Ma, Y. H., Weinander, R., Jakobsson, P. J., and Marnett, L. J. (2002) J. Biol. Chem. 277 44877-44885 [DOI] [PubMed] [Google Scholar]
- 16.Kozak, K. R., Prusakiewicz, J. L., and Marnett, L. J. (2004) Curr. Pharm. Des. 10 659-667 [DOI] [PubMed] [Google Scholar]
- 17.Sang, N., and Chen, C. (2006) Neuroscientist 12 425-434 [DOI] [PubMed] [Google Scholar]
- 18.Yu, M., Ives, D., and Ramesha, C. S. (1997) J. Biol. Chem. 272 21181-21186 [DOI] [PubMed] [Google Scholar]
- 19.Zygmunt, P. M., Petersson, J., Andersson, D. A., Chuang, H., Sorgard, M., Di Marzo, V., Julius, D., and Hogestatt, E. D. (1999) Nature 400 452-457 [DOI] [PubMed] [Google Scholar]
- 20.Van der Stelt, M., and Di Marzo, V. (2004) Eur. J. Biochem. 271 1827-1834 [DOI] [PubMed] [Google Scholar]
- 21.De Petrocellis, L., and Di Marzo, V. (2005) Life Sci. 77 1651-1666 [DOI] [PubMed] [Google Scholar]
- 22.Ross, R. A. (2003) Br. J. Pharmacol. 140 790-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alger, B. E. (2005) Sci. STKE 2005, pe51, 1-4 [DOI] [PubMed]
- 24.Bisogno, T., Ligresti, A., and Di Marzo, V. (2005) Pharmacol. Biochem. Behav. 81 224-238 [DOI] [PubMed] [Google Scholar]
- 25.Kim, J., and Alger, B. E. (2004) Nat. Neurosci. 7 697-698 [DOI] [PubMed] [Google Scholar]
- 26.Makara, J. K., Mor, M., Fegley, D., Szabo, S. I., Kathuria, S., Astarita, G., Duranti, A., Tontini, A., Tarzia, G., Rivara, S., Freund, T. F., and Piomelli, D. (2005) Nat. Neurosci. 8 1139-1141 [DOI] [PubMed] [Google Scholar]
- 27.Melis, M., Perra, S., Muntoni, A. L., Pillolla, G., Lutz, B., Marsicano, G., Di Marzo, V., Gessa, G. L., and Pistis, M. (2004) J. Neurosci. 24 10707-10715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safo, P. K., and Regehr, W. G. (2005) Neuron 48 647-659 [DOI] [PubMed] [Google Scholar]
- 29.Straiker, A., and Mackie, K. (2005) J. Physiol. (Lond.) 569 501-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo, B., Urbanski, M. J., Bisogno, T., Di Marzo, V., Mendiguren, A., Baer, W. U., and Freiman, I. (2006) J. Physiol. (Lond.) 577 263-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katona, I., Urban, G. M., Wallace, M., Ledent, C., Jung, K. M., Piomelli, D., Mackie, K., and Freund, T. F. (2006) J. Neurosci. 26 5628-5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida, T., Fukaya, M., Uchigashima, M., Miura, E., Kamiya, H., Kano, M., and Watanabe, M. (2006) J. Neurosci. 26 4740-4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter, L., and Stella, N. (2004) Br. J. Pharmacol. 141 775-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panikashvili, D., Mechoulam, R., Beni, S. M., Alexandrovich, A., and Shohami, E. (2005) J. Cereb. Blood Flow Metab. 25 477-484 [DOI] [PubMed] [Google Scholar]
- 35.Panikashvili, D., Shein, N. A., Mechoulam, R., Trembovler, V., Kohen, R., Alexandrovich, A., and Shohami, E. (2006) Neurobiol. Dis. 22 257-264 [DOI] [PubMed] [Google Scholar]
- 36.Chen, C., and Bazan, N. G. (2005) Prostaglandins Other Lipid Mediat. 77 65-76 [DOI] [PubMed] [Google Scholar]
- 37.Dubois, R. N., Abramson, S. B., Crofford, L., Gupta, R. A., Simon, L. S., Van De Putte, L. B., and Lipsky, P. E. (1998) FASEB J. 12 1063-1073 [PubMed] [Google Scholar]
- 38.Ho, L., Pieroni, C., Winger, D., Purohit, D. P., Aisen, P. S., and Pasinetti, G. M. (1999) J. Neurosci. Res. 57 295-303 [DOI] [PubMed] [Google Scholar]
- 39.Patrignani, P., Tacconelli, S., Sciulli, M. G., and Capone, M. L. (2005) Brain Res. Rev. 48 352-359 [DOI] [PubMed] [Google Scholar]
- 40.Hurley, S. D., Olschowka, J. A., and O'Banion, M. K. (2002) J. Neurotrauma 19 1-15 [DOI] [PubMed] [Google Scholar]
- 41.Iadecola, C., Niwa, K., Nogawa, S., Zhao, X., Nagayama, M., Araki, E., Morham, S., and Ross, M. E. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 1294-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakayama, M., Uchimura, K., Zhu, R. L., Nagayama, T., Rose, M. E., Stetler, R. A., Isakson, P. C., Chen, J., and Graham, S. H. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 10954-10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sang, N., Zhang, J., Marcheselli, V., Bazan, N. G., and Chen, C. (2005) J. Neurosci. 25 9858-9870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sang, N., Zhang, J., and Chen, C. (2007) J. Neurochem. 102 1966-1977 [DOI] [PubMed] [Google Scholar]
- 45.Domoki, F., Thrikawala, N., Robins, G. S., Bari, F., and Busija, D. W. Neuroreport 11 3435-3438 [DOI] [PubMed]
- 46.Kim, E. J., Lee, J. E., Kwon, K. J., Lee, S. H., Moon, C. H., and Baik, E. J. (2001) Brain Res. 908 1-9 [DOI] [PubMed] [Google Scholar]
- 47.Kunz, T., and Oliw, E. H. (2001) Eur. J. Neurosci. 13 569-575 [DOI] [PubMed] [Google Scholar]
- 48.Cabral, G. A., and Marciano-Cabral, F. (2005) J. Leukocyte Biol. 78 1192-1197 [DOI] [PubMed] [Google Scholar]
- 49.Carrier, E. J., Kearn, C. S., Barkmeier, A. J., Breese, N. M., Yang, W., Nithipatikom, K., Pfister, S. L., Campbell, W. B., and Hillard, C. J. (2004) Mol. Pharmacol. 65 999-1007 [DOI] [PubMed] [Google Scholar]
- 50.Van Sickle, M. D., Duncan, M., Kingsley, P. J., Mouihate, A., Urbani, P., Mackie, K., Stella, N., Makriyannis, A., Piomelli, D., Davison, J. S., Marnett, L. J., Di Marzo, V., Pittman, Q. J., Patel, K. D., and Sharkey, K. A. (2005) Science 310 329-332 [DOI] [PubMed] [Google Scholar]
- 51.Gong, J. P., Onaivi, E. S., Ishiguro, H., Liu, Q. R., Tagliaferro, P. A., Brusco, A., and Uhl, G. R. (2006) Brain Res. 1071 10-23 [DOI] [PubMed] [Google Scholar]
- 52.Pertwee, R. G. (2001) Prog. Neurobiol. (Oxf.) 63 569-611 [DOI] [PubMed] [Google Scholar]
- 53.Dinh, T. P., Carpenter, D., Leslie, F. M., Freund, T. F., Katona, I., Sensi, S. L., Kathuria, S., and Piomelli, D. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 10819-10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hohmann, A. G., Suplita, R. L., Bolton, N. M., Neely, M. H., Fegley, D., Mangieri, R., Krey, J. F., Walker, J. M., Holmes, P. V., Crystal, J. D., Duranti, A., Tontini, A., Mor, M., Tarzia, G., and Piomelli, D. (2005) Nature 435 1108-1112 [DOI] [PubMed] [Google Scholar]
- 55.Suplita, R. L., Gutierrez, T., Fegley, D., Piomelli, D., and Hohmann, A. G. (2006) Neuropharmachology 50 372-379 [DOI] [PubMed] [Google Scholar]
- 56.Walter, L., Dinh, T., and Stella, N. (2004) J. Neurosci. 24 8068-8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinha, D., Bonner, T. I., Bhat, N. R., and Matsuda, L. A. (1998) J. Neuroimmunol. 82 13-21 [DOI] [PubMed] [Google Scholar]
- 58.Waksman, Y., Olson, J. M., Carlisle, S. J., and Cabral, G. A. (1999) J. Pharmacol. Exp. Ther. 288 1357-1366 [PubMed] [Google Scholar]
- 59.Gopez, J. J., Yue, H., Vasudevan, R., Malik, A. S., Fogelsanger, L. N., Lewis, S., Panikashvili, D., Shohami, E., Jansen, S. A., Narayan, R. K., and Strauss, K. I. (2005) Neurosurgery 56 590-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melis, M., Pillolla, G., Bisogno, T., Minassi, A., Petrosino, S., Perra, S., Muntoni, A. L., Lutz, B., Gessa, G. L., Marsicano, G., Di Marzo, V., and Pistis, M. (2006) Neurobiol. Dis. 24 15-27 [DOI] [PubMed] [Google Scholar]
- 61.Howlett, A. C., Mukhopadhyay, S., and Norford, D. C. (2006) J. Neuroimmune Pharmacol. 1 305-316 [DOI] [PubMed] [Google Scholar]
- 62.Chang, Y.-H., Lee, S. T., and Lin, W.-W. (2001) J. Cell. Biochem. 81 715-723 [DOI] [PubMed] [Google Scholar]
- 63.Burstein, S. H., Hull, K., Hunter, S. A., and Latham, V. (1988) FASEB J. 2 3022-3026 [DOI] [PubMed] [Google Scholar]
- 64.Burstein, S. H., Hull, K., Hunter, S. A., and Shilstone, J. (1989) Mol. Pharmacol. 35 6-9 [PubMed] [Google Scholar]
- 65.Perez-Reyes, M., Burstein, S. H., White, W. R., McDonald, S. A. and Hicks, R. E. (1991) Life Sci. 48 507-515 [DOI] [PubMed] [Google Scholar]
- 66.Mestre, L., Correa, F., Docagne, F., Clemente, D., and Guaza, C. (2006) Biochem. Pharmacol. 72 869-880 [DOI] [PubMed] [Google Scholar]
- 67.Mukhopadhyay, S., Shim, J.-Y., Assi, A.-A., Norford, D., and Howlett, A. C. (2002) Chem. Phys. Lipids 121 91-109 [DOI] [PubMed] [Google Scholar]
- 68.Calandra, B., Portier, M., Kernéis, A., Delpech, M., Carillon, C., Fur, G. L., Ferrara, P., and Shire, D. (1999) Eur. J. Pharmacol. 374 445-455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.