Abstract
One of the defining properties of β2-adrenergic receptor (β2AR) signaling is the transient and rapidly reversed accumulation of cAMP. Here we have investigated the contribution of different PDE4 proteins to the generation of this transient response. To this aim, mouse embryonic fibroblasts deficient in PDE4A, PDE4B, or PDE4D were generated, and the regulation of PDE activity, the accumulation of cAMP, and CREB phosphorylation in response to isoproterenol were monitored. Ablation of PDE4D, but not PDE4A or PDE4B, had a major effect on the β-agonist-induced PDE activation, with only a minimal increase in PDE activity being retained in PDE4D knock-out (KO) cells. Accumulation of cAMP was markedly enhanced, and the kinetics of cAMP accumulation were altered in their properties in PDE4DKO but not PDE4BKO cells. Modest effects were observed in PDE4AKO mouse embryonic fibroblasts. The return to basal levels of both cAMP accumulation and CREB phosphorylation was greatly delayed in the PDE4DKO cells, suggesting that PDE4D is critical for dissipation of the β2AR stimulus. This effect of PDE4D ablation was in large part due to inactivation of a negative feedback mechanism consisting of the PKA-mediated activation of PDE4D in response to elevated cAMP levels, as indicated by experiments using the cAMP-dependent protein kinase inhibitors H89 and PKI. Finally, PDE4D ablation affected the kinetics of β2AR desensitization as well as the interaction of the receptor with Gαi. These findings demonstrate that PDE4D plays a major role in shaping the β2AR signal.
By transducing the action of catecholamines into changes in intracellular cAMP levels, β-adrenergic receptors (βARs)2 play a critical role in cellular homeostasis (1, 2). Occupancy of the β2-adrenergic receptor (β2AR), the prototype G protein-coupled receptor, promotes GDP/GTP exchange in Gαs transducer proteins, which then activate adenylyl cyclases to synthesize cAMP. Although the interaction with Gαs is the primary outcome of receptor occupancy, β2AR also stimulates several other signaling cascades through interaction with Gαi and other effectors (3).
Stimulation of cAMP accumulation by β2AR is usually transient because numerous feedback regulations are activated to finely tune the cAMP signal in intensity, time, and its propagation through the intracellular space. Occupancy of the β2AR causes rapid phosphorylation of the receptor by different kinases, with PKA- and GRK-mediated phosphorylation having been the most widely studied in terms of their effects. These phosphorylations directly modulate receptor coupling to G proteins but also constitute a signal for the recruitment of additional proteins involved in receptor signaling and trafficking of the receptor in and out of the plasma membrane (3). At low concentrations of agonist, PKA phosphorylation of the unoccupied or occupied β2AR causes uncoupling from Gαs and heterologous desensitization, as documented by the reduced ability of the phosphorylated receptor to stimulate GDP/GTP exchange in Gαs (4). In addition, it has been reported that β2AR phosphorylation at the PKA site promotes coupling to the inhibitory Gαi protein through several direct and indirect mechanisms (3). Indeed, regions of the third intracellular loop of β2AR have some affinity for Gαi, and the phosphorylated receptor shows increased affinity for Gαi in reconstitution systems (3, 4). At higher concentrations of ligand, GRK-mediated phosphorylation of the occupied receptor increases the affinity of the receptor for the scaffold protein β-arrestin, which precludes the interaction with G proteins and promotes receptor internalization via binding to ADP-ribosylation factors (ARFs), dynamin, and clathrin-coated pits (5). The sequestration and trafficking of the receptor through these endocytic vesicles has several functions including resensitization of the receptor. These mechanisms are essential for cell signaling and homeostasis as documented by the phenotypes of β-arrestin null mice (3, 6, 7).
In addition to the modulation of receptor-G protein interactions and receptor internalization, other mechanisms are likely to be involved in defining the β2AR signal in a cell. An emerging concept is that the properties of β-adrenergic signals are shaped by the presence of a large array of cyclic nucleotide phosphodiesterases (PDEs) (8–10). These enzymes compose a superfamily of proteins that mediate cyclic nucleotide degradation. More than 20 genes are present in the mammalian genome and are subdivided into 11 PDE families on the basis of sequence homology, substrate specificity, and inhibitor sensitivity (10). In addition, most PDE genes are expressed as multiple variants through the use of different promoters or alternative splicing, generating up to 100 individual PDE proteins, which implies a complex array of functions for these enzymes.
PDE4s were initially identified pharmacologically by their sensitivity to the inhibitor rolipram. The four genes composing this family, PDE4A, PDE4B, PDE4C, and PDE4D, are widely expressed and are a major component of the cAMP degradation machinery in most cells (11). Ablation of three of the four PDE4 genes (PDE4A, PDE4B, and PDE4D) results in numerous phenotypes, underscoring the importance of these enzymes in cell homeostasis (12). PDE4s share the property of being rapidly phosphorylated and activated by PKA as part of a negative feedback loop regulating cAMP levels during hormone action (11). The function of this regulation has been addressed indirectly by pharmacological manipulation (13), but no information is available on how a cell responds to β2AR agonists in the absence of this feedback loop. The impact of this regulation has been explored in the present study using a genetic approach.
In line with the idea that PDEs contribute to the compartmentalization of signaling, many PDEs show unique subcellular distributions due to the formation of protein-protein or protein-lipid interactions (11, 14). Most of the PDEs form complexes with components of cAMP signaling distal to the receptor, such as the formation of complexes with PKA through binding to A-kinase-anchoring proteins (AKAPs) (15, 16). However, others become associated with the G protein-coupled receptors themselves (17–19). PDE4s have been shown to regulate several aspects of β2AR and cAMP signaling by being recruited to the receptor in association with β-arrestin. PDE4 recruitment has important effects on the properties of the signal emanating from the receptor as indicated by its effects on PKA activity (18). More recently, it has been proposed that PDE4D recruitment to the receptor in complex with β-arrestin serves to negatively modulate the β2AR “switch” from Gαs signaling to Gαi-mediated responses (17). According to this view, β2AR occupancy causes local activation of PKA, which in turn phosphorylates the receptor. This phosphorylation promotes the switch in β2AR coupling from Gαs to Gαi and then activation of other kinases, such as the tyrosine kinase Src, as well as other pathways such as Raf/Erk. It has been reported that PDE4D down-regulation with small interfering RNAs or overexpression of an inactive PDE4D functioning as a dominant negative causes enhanced phosphorylation of the β2AR as well as increased activation of Erk; both events are consistent with an increased switching from Gαs to Gαi. However, the regulations occurring after receptor occupancy may be even more complex, because it has recently been proposed that some of the events linking Erk and β2AR are independent of PKA activation but dependent on β-arrestin recruitment (20).
Here we have investigated the role of different PDE4 subtypes in β2AR signaling using mouse embryonic fibroblasts (MEFs) deficient in single PDE4 genes. This approach allows us to monitor the effects of PDE4 ablation on the endogenous β2AR without overexpression of receptors or PDEs. Our data demonstrate that PKA-mediated phosphorylation and activation of PDE4D is a major determinant in the control of cAMP accumulation after β2AR stimulation. PDE4D, but not PDE4A or PDE4B, is also involved in defining the time course of β2AR uncoupling and desensitization. Surprisingly, we found that PDE4D ablation disrupts desensitization and β2AR coupling to Gαi rather than enhancing it.
EXPERIMENTAL PROCEDURES
Materials—Dulbecco's modified Eagle's medium (catalog No. 11965), fetal bovine serum, penicillin/streptomycin stock solution, 0.25% trypsin-EDTA solution, and l-glutamine were obtained from Invitrogen. (±)-isoproterenol (1-(3′,4′-dihydroxyphenyl)-2-isopropylaminoethanol hemisulfate salt), ICI118551 ((±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride), CGP20712A ((±)-2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl] amino]ethoxy]-benzamide methanesulfonate salt), cilostamide (N-cyclohexyl-N-methyl-4-(1,2-dihydro-2-oxo-6-quinolyloxy)-butyramide), rolipram (4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone), IBMX (3-isobutyl-1-methylxanthine), milri-none (1,6-dihydro-2-methyl-6-oxo-(3,4′-bipyridine)-5-carbonitrile), and Crotalus atrox snake venom were purchased from Sigma. The PKA inhibitor H89 (N-[2-(p-bromocinnamylamino)-ethyl]-5-isoquinolinesulfonamide dihydrochloride) was from Calbiochem.
Generation of MEF Cell Lines—The generation of mice deficient in PDE4A, PDE4B, and PDE4D has been described previously (21–23). For the present study, the PDE4 null alleles were transferred to a pure C57Bl/6 background by backcrossing heterozygous mice of mixed C57Bl/6-129/Ola background over 13 generations. To derive MEF cells, embryos were retrieved from pregnant females 13.5 days post-coitum. The brain and internal organs of the embryos were removed, and the remaining carcasses were minced and incubated with 0.25% trypsin-EDTA for 10 min at 37 °C. Complete medium (consisting of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 5 mm l-glutamine, 30 μg/ml penicillin, and 100 μg/ml streptomycin) was added to the cell suspensions, and cells were disaggregated by up-and-down pipetting. Large tissue clumps, which settled to the bottom of the tube during a 1-min incubation at room temperature, were removed. The remaining cell suspensions were then transferred onto cell culture dishes. Fibroblasts were grown at 37 °C under a 5% CO2 atmosphere to confluence. Cells were replated, grown to 80–90% confluence, and then harvested and frozen in liquid nitrogen at 4 × 106 cells/ml in complete medium supplemented with 10% DMSO and 15% fetal bovine serum.
Cell Culture and Harvest—For experiments, 1-ml aliquots of MEFs were thawed, and cells were grown on 10-cm cell culture plates to 80–90% confluence. The cells were then replated into 6-well culture dishes at a density of 2 × 10 5 cells/well. After overnight culture, cells were serum-starved in Dulbecco's modified Eagle's medium supplemented with 5 mm l-glutamine, 30 μg/ml penicillin, 100 μg/ml streptomycin, and 25 mm HEPES for 16 h before cell treatment and harvest. Cells were pretreated for 5 min with rolipram (10 μm), IBMX (1 mm) or H89 (10 μm) as indicated and were then stimulated with isoproterenol (ISO, 10 μm) for different times while cell culture plates were resting in a 37 °C water bath. For restimulation experiments (Fig. 11) cells were washed four times with prewarmed phosphate-buffered saline (PBS) between treatments. For measurement of PDE activity, MEFs were lysed in 50 mm Tris/HCl (pH 7.4) containing 150 mm NaCl, 5% glycerol, 1 mm EDTA, 0.2 mm EGTA, 10 mm NaF, 100 mm Na4P2O7, 1 mm Na3VO4, 1.4 mm β-mercaptoethanol, 0.5% Triton X-100, 100 nm okadaic acid, and complete protease inhibitor tablets (Roche Diagnostics). Cell lysates were centrifuged at 14,000 × g, and supernatants were either used directly for PDE assay or first subjected to immunoprecipitation (IP) as described below.
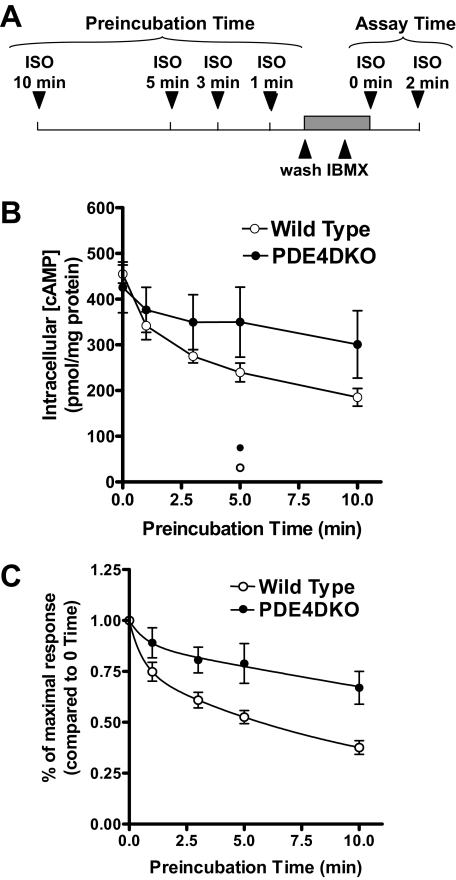
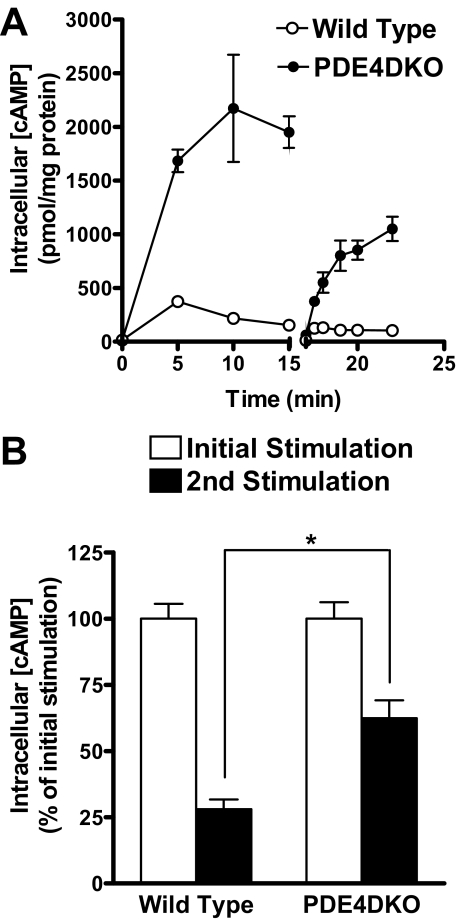
FIGURE 11.
β2AR desensitization is altered in the absence of PDE4D. Wild type and PDE4DKO MEFs were preincubated with 10 μm ISO for various times. After this first exposure, the medium was removed, and cells were washed three times with PBS within a period of 1 min and then incubated with 1 mm IBMX for 1 min. Cells were then restimulated a second time with 10 μm ISO for 2 min. Stimulations were terminated, and intracellular cAMP concentrations were measured by RIA. A, shown is a scheme illustrating the experimental design. B, shown is the cAMP accumulation during the second ISO stimulation as a function of the time of preincubation. Data represent the means ± S.E. of five to six experiments. The open and closed circles at the bottom of the graph represent the cAMP accumulation after 5-min ISO preincubation followed by a 2-min treatment with IBMX. In C, cAMP accumulation for each preincubation time is reported as a fraction of the response of cells not prestimulated with ISO. The differences between wild type and PDE4DKO data were highly significant (p < 0.001) using two-way ANOVA.
Measurement of cAMP Accumulation—After the appropriate treatment, the cell culture medium was aspirated and the reactions terminated by addition of 0.8 ml of 95% ice-cold ethanol containing 0.1% trichloroacetic acid to each well. After a 30-min incubation of the plates on ice, the trichloroacetic acid solution containing cAMP was collected. The cell protein that remained on the cell culture plates was dissolved in 300 μl of 1 n NaOH/well, and this solution was used for determination of protein content as described below. The cAMP-containing trichloroacetic acid solutions were dried in a spin vacuum and reconstituted in 500 μl of PBS, and cAMP concentrations were determined using a radioimmunoassay (RIA) as described previously (24).
PDE Assay—PDE activity was measured according to the method of Thompson and Appleman (25) and as described in detail previously (26). In brief, samples were assayed in a reaction mixture of 200 μl (containing 40 mm Tris-HCl (pH 8.0), 1 mm MgCl2, 1.4 mm β-mercaptoethanol, 1 μm cAMP, 0.75 mg/ml bovine serum albumin, and 0.1 μCi of [3H]cAMP) for 30 min at 33 °C. The reaction was terminated by heat inactivation in a boiling water bath for 1 min. The PDE reaction product 5′-AMP was then hydrolyzed by incubating the assay mixture with 50 μgof C. atrox snake venom for 20 min at 33 °C, and the resulting adenosine was separated by anion exchange chromatography using 1 ml of AG1-X8 resin (Bio-Rad) and quantitated by scintillation counting.
Antibodies and IP—Rabbit anti-PDE4D3, anti-PDE4D5, anti-PDE4D9, PAN-PDE4A (AC55), and PAN-PDE4B (K118) antibodies, as well as the mouse PAN-PDE4D (M3S1) antibody, have been described previously (27). For immunoprecipitations, these antibodies were coupled to 25 μl of protein G-Sepharose and incubated with the respective cell extracts for 2 h at 4 °C, after which pellets were washed three times with cell lysis buffer and the resulting IP pellets subjected to SDS-PAGE and Western blotting or used for measurement of PDE activity as described above.
Design of a PDE4D Adenoviral Vector—Cloning of the open reading frame of the rat PDE4D variants PDE4D2, PDE4D5, and PDE4D9 has been described previously (19, 27). In the present study, these constructs were subcloned into the pAd/CMV/V5-DEST vector to generate an adenovirus encoding a C-terminally Myc-tagged PDE4D5 using the ViraPower adenoviral expression system (Invitrogen).
Protein Assay—Protein concentrations were measured by the method of Lowry et al. (28) using bovine serum albumin as the standard.
Data Analysis—Experimental data of cAMP accumulation were fitted using single or double exponential decay equations using the GraphPad Prism program (GraphPad Inc., San Diego, CA). Statistical significance was determined with Student's t test or ANOVA, as indicated, using the Prism software.
RESULTS
Generation of MEFs Deficient in Different PDE4 Subtypes—MEFs deficient in the PDE4 subtypes, PDE4A, PDE4B, and PDE4D, were generated by mating heterozygous PDE4 mice on a pure C57Bl/6 background. Wild type and homozygous PDE4KO cells were derived from embryos of the same pregnancy; thus, PDE4KO and wild type MEFs are considered congenic except for the targeted locus. Indeed, cells of the same genotype prepared from different embryos produced comparable results. In the same vein, wild type cells from the different PDE4 knock-out lines behaved in a similar fashion. Thus, data from different wild type lines were pooled. In all experiments performed, cells were used within four passages. At these early passages, these MEFs express β-adrenergic receptors at levels sufficient to produce a 20-fold increase in cAMP concentration after a 2-min exposure to 10 μm ISO (see for instance supplemental Fig. 1). The β2AR is the predominant β-adrenergic receptor subtype expressed in MEFs, as cAMP elevation in response to ISO is blocked by a β2AR antagonist (ICI118551) but not by a β1AR antagonist (CGP20712A) (supplemental Fig. 1).
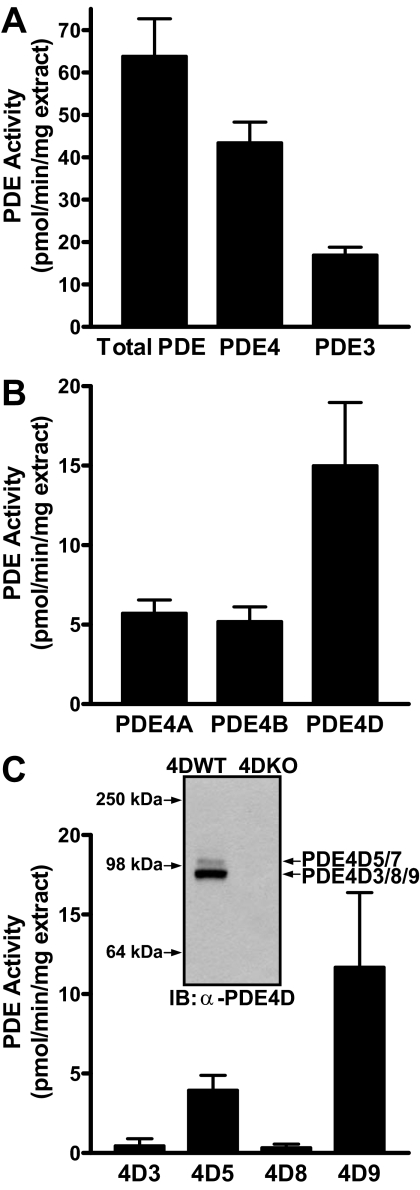
PDE Expression Pattern in MEFs—To define the repertoire of PDEs present in MEFs, PDE activity assays were performed on cell extracts in the presence of specific PDE inhibitors or after IP with PDE4 subtype-selective antibodies. PDE4 and PDE3 are the major PDE families expressed in these cells, contributing ∼60 and 30% of the total PDE activity, respectively (Fig. 1A). Of the four PDE4 genes present in the mouse genome, PDE4D is the major form expressed in these MEFs, accounting for about 25% of the overall PDE activity, whereas PDE4A and PDE4B each account for ∼10–15% of the activity (Fig. 1B). To date, at least nine different PDE4D variants (PDE4D1–PDE4D9) have been described. The PDE activity immunoprecipitated with PDE4D5 and PDE4D9 antibodies (Fig. 1C) accounted for the PDE4D activity expressed in these MEFs (Fig. 1B), indicating that PDE4D5 and -9 are the major, if not the only, PDE4D variants expressed. PDE4D3 and PDE4D8 are expressed at low levels (Fig. 1C). Western blot analysis of MEF extracts using a PAN-PDE4D antibody (Fig. 1C) revealed a predominant band migrating at ∼90 kDa, corresponding to variants PDE4D3, -8, and -9, and a weaker band at ∼100 kDa, corresponding to the migration of PDE4D5 and -7 (27). Thus, this PDE4D immunoreactive pattern is consistent with the predominant expression of PDE4D9 and lower expression level of PDE4D5 as determined by IP in Fig. 1C.
FIGURE 1.
PDE expression pattern in MEF cells. A, PDE activity in detergent extracts prepared from wild type MEFs was measured in the absence or presence of the PDE4-selective inhibitor rolipram and the PDE3-selective inhibitor milrinone to determine the contribution of PDE4 and PDE3 to the overall PDE activity, respectively. B and C, detergent extracts prepared from wild type MEFs were subjected to immunoprecipitation with PDE4 subtype-selective (B) or PDE4D variant-selective antibodies (C). The PDE activity recovered in the IP pellets was corrected for the amount of protein used as IP input. All data represent means ± S.E. of at least three experiments. C, inset, 50 μg of detergent extract prepared from wild type and PDE4DKO MEFs was separated on SDS-PAGE and probed in Western blotting using α-PAN-PDE4D (Icos4D) antibody.
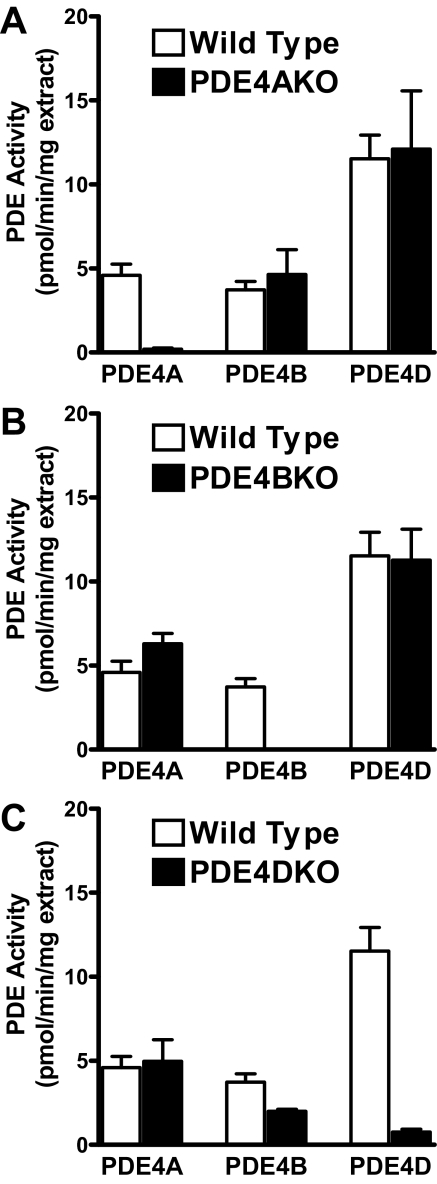
To confirm the identity of the PDEs expressed in MEFs and to determine whether compensation occurs after individual PDE4 gene ablation, the activity of PDE4 subtypes was measured in PDE4KO and matching wild type MEFs. The activity corresponding to the disrupted gene in each of the PDE4KO cell lines could not be detected. Other than the loss of the activity corresponding to the ablated gene, the knock-out MEFs displayed a similar PDE4 activity pattern compared with that of their matched wild type cells, indicating that compensatory up-regulation of the remaining PDE4 genes does not occur (Fig. 2).
FIGURE 2.
Expression of PDE4 subtypes in PDE4KO MEFs. Detergent extracts prepared from MEFs deficient in PDE4A (A), PDE4B (B), and PDE4D (C) and the respective wild type controls were immunoprecipitated using PDE4 subtype-selective antibodies. The PDE activity recovered in IP pellets, corrected for the amount of protein used as IP input, is reported.
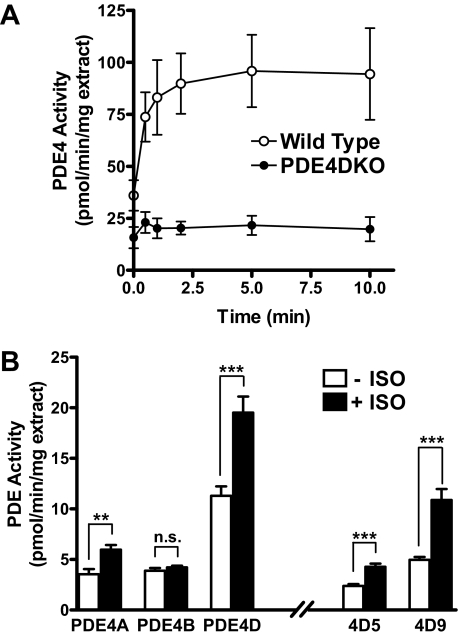
β2AR-dependent Activation of PDE4 in MEFs—Elevated cAMP levels resulting from β2AR stimulation promote the PKA-mediated phosphorylation and activation of PDE4, a ubiquitous negative feedback loop involved in cAMP homeostasis (11, 14, 29). To define which PDE4 subtypes are activated upon β-adrenergic signaling in MEFs, PDE activity was monitored at different times after treatment with ISO (Fig. 3A). Wild type MEFs displayed a rapid, ISO-dependent PDE4 activation, with an approximate 2-fold increase over basal activity within the first 30 s of stimulation, a time course overlapping that reported for β2AR phosphorylation (30, 31). PDE4 activity continued to increase up until 5 min, reaching a plateau at about 2.5 times the basal activity. In contrast, in MEFs deficient in PDE4D only a small response to ISO stimulation could be detected (Fig. 3A). Consistent with this finding, immunoprecipitated PDE4D activity increased significantly in response to a 5-min ISO treatment (Fig. 3B). PDE4A activity was also elevated, whereas PDE4B activity did not change in response to ISO. Both the PDE4D variants PDE4D5 and -9 contributed to the ISO-dependent increase in PDE4D activity in wild type MEFs (Fig. 3B). The activity of PDEs other than PDE4 was not affected by ISO treatment (data not shown). Thus, together with a moderate overall decrease in basal PDE activity, PDE4DKO cells display minimal PDE activation upon ISO treatment.
FIGURE 3.
Isoproterenol-dependent activation of PDE4 in MEFs. A, quiescent wild type and PDE4DKO MEFs were treated with 10 μm ISO for the times indicated on the abscissa. Cells were then lysed, and PDE activity in the resulting detergent extracts was determined in the presence or absence of rolipram. The graph depicts the rolipram-sensitive PDE4 activity. Data represent the means ± S.E. of three experiments. B, detergent extracts prepared from wild type MEFs treated for 5 min with or without 10 μm ISO were subjected to immunoprecipitation using subtype-selective PDE4 or splice form-selective PDE4D antibodies. Shown is the PDE activity recovered in immunoprecipitates corrected for the amount of protein used as IP input. All data represent means ± S.E. of the three experiments performed. The statistical significance of ISO-induced PDE activation was determined using Student's t test. ***, p < 0.001; **, p < 0.01; n.s., not significant.
Global cAMP Accumulation in Cells Deficient in the Different PDE4 Subtypes—To determine the effects of PDE4 subtype ablation on cAMP signaling, cAMP accumulation in response to ISO was followed for up to 10 min (Fig. 4). Exposure to ISO rapidly stimulated cAMP accumulation in wild type MEFs, with a transient maximum reached at 1 min (see Fig. 4B) and cAMP levels rapidly declining thereafter. PDE4B inactivation produced no changes in cAMP accumulation, whereas a small but significant increase in cAMP accumulation was observed in PDE4AKO MEFs (Fig. 4A). Conversely, PDE4D ablation had dramatic effects. The time course of β2AR-dependent cAMP accumulation in PDE4DKO MEFs was altered in three distinct properties. The maximal cAMP accumulated was increased (cAMPmax WT = 255 ± 56; cAMPmax PDE4DKO = 900 ± 130 pmol/mg protein), and the half-time required to reach the maximum (tmax½) was delayed (30–45 s in WT to 90–100 s in the PDE4DKO). Finally, the rate of return to basal levels was decreased (tdecay½WT = 2.8 min; tdecay½PDE4DKO = 4.3 min). Indeed, even after a 20-min incubation with the agonist, cAMP levels in PDE4DKO cells were still higher than those in wild type cells at any time point (Fig. 7A). Thus, PDE4D, but not PDE4B, ablation has a major impact on the shape of the β2AR-induced cAMP transients. PDE4A had a minor effect. It should be noted that PDE4B was not activated by PKA in response to ISO treatment, whereas PDE4D and PDE4A were activated (Fig. 3). Thus, the impact of the different PDE4 subtypes on cAMP accumulation is proportional to the degree of their activation by PKA. Inhibition of PDE3 activity in MEFs using the PDE3-selective inhibitor cilostamide had no significant effect on cAMP accumulation elicited by ISO (Fig. 4C). Although contributing to overall cAMP hydrolysis under basal conditions to the same extent as PDE4D (∼30%; see Fig. 1A), the PDE3 family of enzymes does not affect β2-AR signaling in this model.
FIGURE 4.
Effect of PDE inactivation on cAMP accumulation in MEFs. A, MEFs deficient in PDE4A, PDE4B, or PDE4D as well as matched wild type controls were incubated for the indicated times with 10 μm ISO. The cell culture medium was then aspirated and incubations terminated by the addition of 0.1% trichloroacetic acid in 95% ethanol. Cyclic AMP concentration in the resulting extracts was measured by RIA. A representative experiment of the three performed is reported. Inactivation of PDE4A and PDE4D resulted in significant changes in cAMP accumulation (p < 0.001; two-way ANOVA), whereas ablation of PDE4B had no effect (p > 0.05). B, the graph reports the mean ± S.E. of five separate experiments measuring cAMP accumulation in wild type and PDE4DKO MEFs. C, wild type MEFs were pretreated for 5 min with or without the PDE3-selective inhibitor cilostamide (10 μm). Cells were then stimulated with 10 μm ISO for the times indicated, after which the incubations were terminated and cAMP concentrations were determined by RIA. The graph reports the mean ± S.E. of three separate experiments.
FIGURE 7.
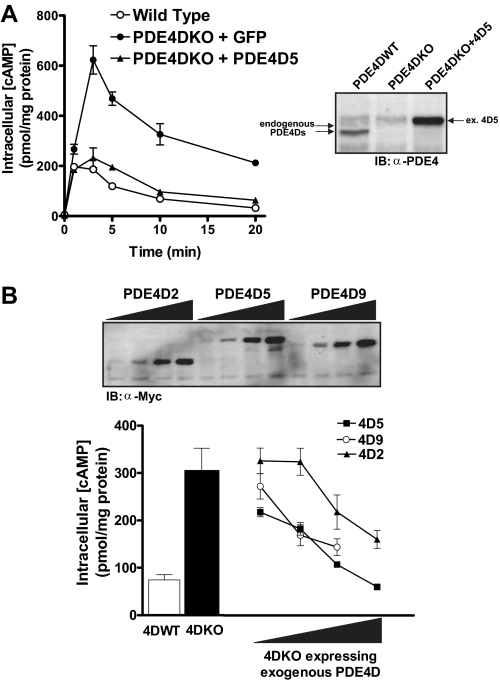
Expression of exogenous PDE4D rescues the phenotype of PDE4DKO MEFs. A, PDE4DKO MEFs were infected with adenoviruses encoding a Myc-tagged PDE4D5 or the green fluorescent protein as a control at a multiplicity of infection of 20 for 36 h. Cells were then stimulated with 10 μm ISO for the indicated times, and cAMP concentrations were measured by RIA. Data represent the means ± S.E. of three experiments and are compared with the cAMP accumulation of uninfected wild type cells. The inset reports a Western blot of extracts prepared from PDE4DWT MEFs, PDE4DKO MEFs, and PDE4DKO cells infected with the PDE4D5 adenovirus using an anti-PDE4 antibody. Similar amounts of protein extract were loaded in all lanes. B, wild type, PDE4DKO, and PDE4DKO MEFs infected with adenoviruses encoding PDE4D5, PDE4D9, or PDE4D2 were stimulated with 10 μm ISO for 5 min. Incubations were then terminated, and cAMP concentrations were measured by RIA. Cyclic AMP accumulation in the presence of exogenous PDE4Ds is compared at different levels of overexpression; at each point the three PDE4D splice forms are expressed at similar amounts as determined by Western blotting (see inset). Data represent the means ± S.E. of three experiments (4D2 and 4D5) or the average and range of two experiments (4D9).
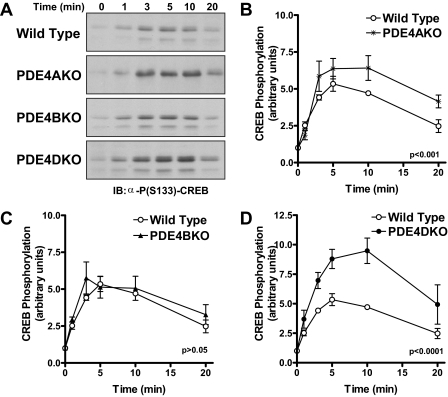
Isoproterenol-dependent Phosphorylation of CREB in PDE4KO MEFs—Next, we determined whether the altered pattern of cAMP accumulation in PDE4KO MEFs is reflected in similar changes in the activation of downstream targets of cAMP signaling in wild type and PDE4KO cells. The PKA-mediated phosphorylation of the cAMP response element-binding protein (CREB) was used as a read-out (32). The magnitude of ISO-dependent PKA phosphorylation of CREB (Ser-133) in PDE4DKO MEFs was significantly altered compared with wild type controls (Fig. 5A), and CREB phosphorylation closely follows the pattern of cAMP accumulation, with increased but delayed maximal stimulation and a delayed return to basal levels compared with wild type controls. Also consistent with the pattern of cAMP accumulation is the observation that PDE4A ablation had a modest effect, and inactivation of PDE4B had no effect on the level of CREB phosphorylation (Fig. 5, A–C). These results indicate that the altered cAMP accumulation caused by PDE4D ablation is reflected in changes of downstream effectors.
FIGURE 5.
Isoproterenol-dependent phosphorylation of CREB at Ser-133 in PDE4KO MEFs. MEFs deficient in PDE4A, PDE4B, or PDE4D as well as wild type controls were incubated with 10 μm ISO for the indicated times and then harvested in SDS sample buffer. Equal amounts of cell extract were separated on SDS-PAGE, and Western blot analysis that was performed usingα-phospho-CREB(Ser-133) and α-CREB antibodies to probe the level of CREB phosphorylation and CREB protein expression, respectively. A, shown are representative pCREB blots for experiments performed using the different MEFs. B–D, the graphs depict the quantification of the time course of CREB(Ser-133) phosphorylation in each of the different MEFs. All data represent means ± S.E. of three experiments performed. Statistical significance was determined by two-way ANOVA.
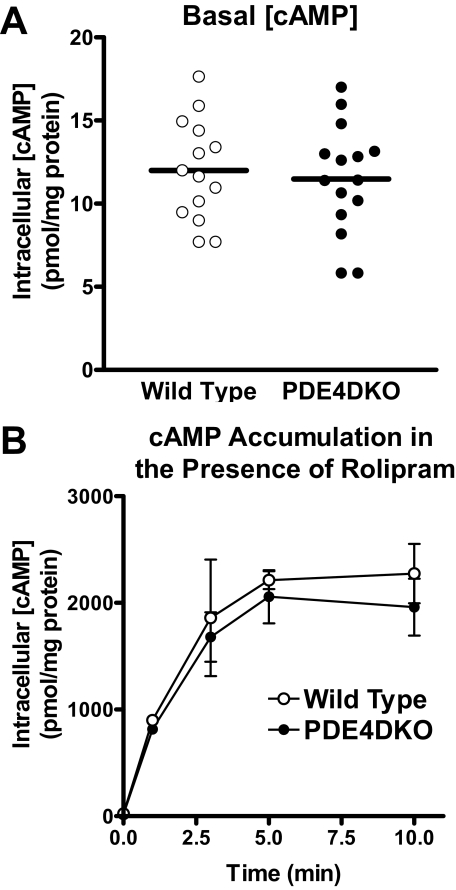
Intrinsic cAMP-generating Capacity Is Unchanged in PDE4DKO MEFs—Whereas cAMP accumulation in response to ISO is markedly affected, basal cAMP levels in PDE4DKO and wild type MEFs were identical (WT = 11.99 ± 0.81; PDE4DKO = 11.48 ± 0.84 pmol cAMP/mg protein; Fig. 6A), suggesting that PDE4D did not contribute appreciably to basal cAMP hydrolysis. Moreover, ISO-induced cAMP accumulation was similar in wild type and PDE4DKO MEFs when assayed in the presence of the PDE4-selective inhibitor rolipram (WT = 2396.4 ± 389.4; PDE4DKO = 2252.8 ± 332.1 pmol cAMP/mg protein; Fig. 6B). Collectively, these findings suggest that all components of the signaling pathway, such as β-adrenergic receptors, G proteins, and adenylyl cyclases, were present at similar levels and function properly in the two cell lines. Thus, there were no major compensatory events induced by the absence of PDE4D that might have altered β2AR signaling per se. This possibility was further verified with a rescue experiment in which an exogenous PDE4D was reintroduced in PDE4DKO cells. Infection of PDE4DKO MEFs with an adenovirus encoding the PDE4D splice form, PDE4D5, reduced cAMP accumulation to levels similar to those in wild type MEFs (Fig. 7A). Although maximal cAMP accumulation (cAMPmax) and the rate of return to basal cAMP levels (tdecay½) returned to wild type levels, the time required to reach maximum accumulation (tmax½) still appeared to be slightly delayed. When overexpressed in PDE4DKO MEFs, both PDE4D5 and PDE4D9, the two splice forms that are expressed endogenously in wild type MEFs, as well as PDE4D2, a short form that lacks the PKA consensus site, reduced ISO-dependent cAMP accumulation (Fig. 7B). However, PDE4D5 and PDE4D9 were significantly more efficient than PDE4D2 at reducing cAMP transients.
FIGURE 6.
Intrinsic cAMP signaling capacity is not affected in PDE4DKO MEFs. A, the basal intracellular cAMP concentration in wild type and PDE4DKO MEFs in all experiments performed is reported. The horizontal lines represent the average of at least 14 separate experiments. cAMP levels in wild type and PDE4DKO cells are not significantly different (p > 0.05; Student's t test). B, PDE4DKO and matched wild type MEFs were pretreated for 5 min with 10 μm rolipram. Cells were then stimulated with 10 μm ISO for the indicated times, after which the incubations were terminated and cAMP concentrations were determined by RIA. Treatment with rolipram elevates cAMP accumulation in both wild type and PDE4DKO MEFs (compare panel B with Fig. 4A). In the presence of rolipram, the genetic inactivation of PDE4D has no effect on ISO-induced cAMP accumulation compared with wild type controls (p > 0.05, two-way ANOVA).
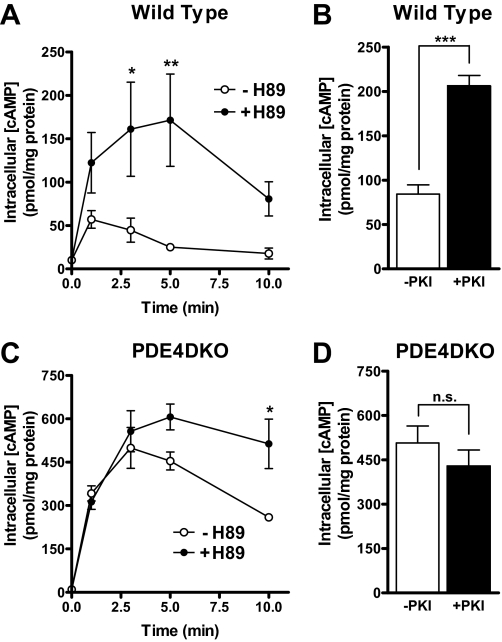
The Effect of PKA on cAMP Accumulation Is Lost in PDE4DKO MEFs—In view of the above data, the marked increase in cAMP accumulation in the PDE4DKO MEFs may have been caused by the loss of PDE4 activation during β2AR signaling as well as the overall decrease in PDE activity. In an alternative strategy to define the impact of PKA-mediated PDE activation on cAMP accumulation in MEFs, cells were pretreated with or without the PKA inhibitor H89 and then stimulated with ISO (Fig. 8, A and C). Inhibition of PKA in wild type MEFs elicited a rapid and prolonged increase in cAMP accumulation compared with untreated controls (Fig. 8A). The increase was detected immediately, and maximal cAMP accumulation in H89-treated cells was 4–6-fold that of untreated MEFs. Conversely, in PDE4DKO MEFs, PKA inhibition had no effect on cAMP accumulation during the first 3 min of ISO treatment, and a significant difference in H89-treated versus untreated cells was not seen until 10 min after the addition of ISO (Fig. 8C). Notice that PKA inhibition also caused a delay in the time required to reach maximum cAMP levels, in a manner similar to that observed with PDE4D inactivation. Overexpression of the PKA inhibitor peptide, PKI, also had an effect comparable to H89 in wild type cells and no significant effect on PDE4DKO cells (Fig. 8, B and D). The effects of H89 and PKI on ISO-induced cAMP transients in wild type and PDE4DKO cells strongly suggest that PKA-mediated activation of PDE4D is a major determinant of the pattern of cAMP accumulation in response to β-adrenergic stimulation.
FIGURE 8.
Effect of PKA on β2-adrenergic cAMP signals is blunted in PDE4DKO MEFs. A and C, wild type (A) and PDE4DKO (C) MEFs were pretreated with the PKA inhibitor H89 (10 μm) or vehicle (DMSO) for 15 min. Cells were then stimulated with 10 μm ISO for the indicated times. Incubations were terminated by the addition of 0.1% trichloroacetic acid in 95% ethanol and intracellular cAMP was measured by RIA. Data shown represent means ± S.E. of three experiments. PKA inhibition with H89 has a greater effect on cAMP accumulation in wild type MEFs (**, p < 0.001; two-way ANOVA) compared with PDE4DKO cells (*, p < 0.05). B and D, wild type (B) and PDE4DKO (D) MEFs infected with an adenovirus encoding the protein kinase A inhibitor peptide (+PKI) or with green fluorescent protein as a control (–PKI) were stimulated for 3 min with 10 μm ISO. Incubations were then terminated, and intracellular cAMP was measured by RIA. Data shown represent means ± S.E. of three experiments. Statistical significance was determined using Student's t test. ***, p < 0.001; n.s., not significant.
Desensitization and Coupling of β2AR to Gαi Is Disrupted in PDE4DKO MEFs—Receptor desensitization is the result of a complex set of changes that include the phosphorylation of the β2AR by PKA and/or GRKs, recruitment of β-arrestins, and receptor sequestration, all mechanisms contributing to uncoupling of the receptor from Gαs (33). We proposed previously that PKA-mediated phosphorylation and activation of PDE4 is an additional component that contributes, together with receptor uncoupling and internalization, to the overall “cell desensitization” process (34). To further investigate how β2AR desensitization develops in the absence of PDE4D, wild type and PDE4DKO MEFs were preincubated with ISO for 15 min, after which the cells were washed and then rechallenged a second time with ISO (Fig. 9). As shown previously, the initial incubation of PDE4DKO MEFs with ISO resulted in a 3–4-fold higher increase in cAMP accumulation compared with that of wild type cells. A short washing step restored intracellular cAMP back to basal levels (Fig. 9A). Restimulation of both wild type and PDE4DKO MEFs with ISO produced a second cAMP transient; however, cAMP accumulation did not reach the levels of the first stimulation. Thus, both cell types displayed a desensitized response (Fig. 9B). Upon restimulation, wild type MEFs accumulated cAMP to levels that were only 28 ± 4% compared with that of the original stimulation, whereas PDE4DKO cells responded to the second stimulation with an increase of intracellular cAMP that was 62 ± 7% compared with the original stimulation. Thus, ablation of PDE4D results in a reduced β2AR desensitization in MEFs.
FIGURE 9.
β2AR desensitization is altered in the absence of PDE4D. A and B, wild type and PDE4DKO MEFs were stimulated with 10 μm ISO for the indicated times. A separate group of cells were incubated with ISO for 15 min and were then quickly washed four times with PBS and restimulated for the indicated times with 10 μm ISO. All incubations were terminated by the addition of 0.1% trichloroacetic acid in 95% ethanol, and cAMP concentrations were measured by RIA. Data reported are the means ± S.E. of three to six experiments. B, maximal cAMP accumulation during the second ISO stimulation is compared with that during the initial ISO stimulation. Statistical significance between cAMP accumulation in wild type and PDE4DKO MEFs was determined using Student's t test. *, p < 0.05.
A proposed model for a rapid β2AR desensitization suggests that PKA phosphorylation of the receptor uncouples the β2AR from its initial signaling through Gαs and promotes the sequential coupling to Gαi (3, 35). In addition, PDE4D recruited to the β2AR by β-arrestin has been implicated in controlling the switch from Gαs to Gαi signaling (17). To determine the impact of Gαi coupling in MEFs deficient in PDE4D, MEFs were treated overnight with or without pertussis toxin (PTX), which inhibits Gαi signaling. Cells were then stimulated with ISO and monitored for cAMP accumulation (Fig. 10). PTX pretreatment caused a 2-fold increase in cAMP accumulation in wild type MEFs, presumably because of the inhibition of Gαi coupling (Fig. 10A). Surprisingly, PDE4DKO MEFs were insensitive to Gαi inhibition (Fig. 10B). The different sensitivity to the toxin is not due to altered protein expression of Gαs and Gαi or to an effect of PTX treatment on PKA-mediated PDE4D activation, because expression levels of the two G proteins were identical in wild type and PDE4DKO cells (Fig. 10, C and D) and ISO-induced PDE4 activation was unaffected by PTX treatment (Fig. 10E).
FIGURE 10.
β2AR coupling to Gαi is impaired in PDE4DKO MEFs. A and B, after a 16-h pretreatment with 100 ng/ml PTX, wild type (A) and PDE4DKO (B) MEFs were stimulated with 10 μm ISO for the indicated times. Incubations were then terminated, and intracellular cAMP was determined by RIA. All data represent the means ± S.E. of three independent experiments. Statistical significance was determined with two-way ANOVA. C and D, similar amounts of detergent extracts from wild type and PDE4DKO MEFs were separated on SDS-PAGE. The expression level of Gαs and Gαi proteins was determined by Western blotting, and the signal intensities were subsequently quantified (D). E, after a 16-h pretreatment with 100 ng/ml PTX, wild type MEFs were stimulated with 10 μm ISO for 5 min. At the end of the incubation, cells were lysed and PDE activity was measured in the presence or absence of 10 μm rolipram. The change in PDE4 activity, which is defined as the PDE activity inhibited by rolipram, is reported. Data shown in D and E represent the means ± S.E. of three independent experiments. Statistical analysis was performed using Student's t test. n.s., not significant.
To better dissect the dynamics of β2AR uncoupling in PDE4DKO cells, MEFs were preincubated for different times with ISO, and then rapidly washed four times over a period of 2 min. This short period of washing was not expected to produce substantial resensitization of the receptor, as PKA and GRK phosphorylation are essentially unchanged 2 min after removal of the agonist (36). During the last wash, a high concentration of the nonselective PDE inhibitor IBMX (1 mm) was added to completely block all PDE activity in the cells. Finally, the cells were restimulated with saturating concentrations of ISO for 2 min (see scheme in Fig. 11A). As cAMP hydrolysis by PDEs is prevented, cAMP synthesis is linear over a period of 2–3 min (data not shown). Thus, this assay measures the rate of cAMP synthesis and reflects Vmax of β2AR-mediated activation of adenylyl cyclase. This approach of measuring cAMP synthesis in an intact cell has an advantage over cell-free cyclase assays, because it avoids possible artifacts due to membrane preparation. In wild type MEFs (Fig. 11B), the response to ISO decreased in a time-dependent manner with two discernible phases: an initial fast component (t½ ∼ 0.5 min when data are fitted using a two-phase exponential decay equation) and a subsequent slower phase (t½ ∼ 5 min). This time course is compatible with published data on the time course of β2AR receptor phosphorylation and desensitization (30, 31). The time course of desensitization in PDE4DKO cells was significantly different (p < 0.001, ANOVA) from that of wild type controls, with the fast component being difficult to detect. Thus, data from PDE4DKO cells could only be fitted with a one-phase exponential model of decay. Because desensitization in PDE4DKO cells proceeds with a slow time course, PDE4DKO cells responded significantly better than wild type cells even after prolonged preincubation with ISO (see Fig. 11). Again, this difference was not due to reduced cAMP hydrolysis in PDE4DKO cells during the second stimulation, as the experimental design removed any impact of PDEs by its use of high concentrations of IBMX, which inhibited PDE activity more than 90%. Conversely, the slower β2AR desensitization was likely due to the absence of PDE4D activity during the first preincubation. Indeed, acute inhibition of PDE4 during pretreatment with the PDE4-selective inhibitor rolipram caused a similar delay in the time course of β2AR desensitization compared with PDE4D ablation (see supplemental Fig. 2). Notice also that PTX preincubation, although increasing the maximal β2AR-stimulated cAMP levels, did not affect the time course of desensitization in wild type MEFs (data not shown), suggesting that the Gαs/Gαi switch is not a primary component of desensitization in these cells.
DISCUSSION
It is well established that catecholamine binding to the β2AR produces a rapid but transient increase in cAMP in a variety of cells ranging from neurons to cardiomyocytes and airway smooth muscle cells. The transient nature of this response is thought to be physiologically relevant (37) and is a distinctive property of the β2AR compared with cognate receptors such as the β1AR, which generate longer lasting cAMP signals (38, 39). The mechanisms underlying this transient response have been studied extensively and are thought to include the rapid phosphorylation of the receptor by different kinases, including PKA and GRK, and the binding of adaptor molecules such as β-arrestins, promoting the uncoupling and desensitization of the receptor as well as its sequential coupling to Gαi. Here we have demonstrated that PDE4D, and to a lesser extent PDE4A phosphorylation, is an additional component responsible for shaping and controlling the transient nature of the cAMP signal produced by β2AR stimulation. Ablation of PDE4D expression in MEFs causes a major change in the kinetics of the β2AR cAMP response, most notably a profound delay of signal dissipation, i.e. return of cAMP to basal level. Given the findings that PDE4D is rapidly phosphorylated and activated by PKA upon β-adrenergic stimulation, that little PDE activation is left after ablation of the PDE4D gene, and that the effects of PKA inhibition on cAMP kinetics are marginal in PDE4DKO cells, we propose that the PKA-mediated activation of PDE4D is an important feedback regulation contributing to the transient nature of the β2AR response and represents a major adaptive mechanism required for physiological β2AR signaling. Both the increase in maximal cAMP accumulation and the delayed time to reach the transient steady state of cAMP accumulation have been predicted as a consequence of PDE inactivation by mathematical modeling (40–42).
Except for the loss of the PDE targeted by genetic inactivation, the expression and activity of other PDEs were similar in all PDE4KO cells. More importantly, basal cAMP levels were comparable under the culture conditions used, and maximal responses to ISO in the presence of PDE4-selective or nonselective PDE inhibitors were identical in wild type and PDE4DKO cells. These data suggest that the overall signal transduction machinery is comparable in the different cell lines and that there are no compensatory adjustments following PDE4D ablation that affect expression of β2AR, Gαs, Gαi, or adenylyl cyclases. Thus, these genetically engineered MEFs can be used as a model in which the role of different PDEs in β-adrenergic signaling or other signaling pathways can be studied. Consistent with this view, reintroduction of exogenous PDE4D in PDE4DKO MEFs produces an almost complete rescue of the phenotype (Fig. 7), confirming that the primary cause of the observed differences is the absence of PDE4D. When expressed at similar levels, PDE4D5 and -9, the two PDE4D splice forms expressed endogenously in wild type MEFs, are more efficient in reducing ISO-induced cAMP accumulation in PDE4DKO MEFs compared with PDE4D2 (Fig. 7B). This difference may be due to PKA activation of PDE4D5 and -9, whereas PDE4D2 lacks the PKA phosphorylation site, once more emphasizing the importance of the PKA-mediated PDE4 activation as a negative feedback mechanism controlling cAMP transients. Alternatively, PDE4D5 and PDE4D9 may be tethered to the β2AR macromolecular signaling complex, whereas PDE4D2 is not; hence the difference in their efficiency in regulating β2-adrenergic cAMP signals.
It should be noted that absence of major compensation is not always the outcome of PDE4D removal, as we have shown previously that PDE4D ablation in lung fibroblasts is associated with enhanced cAMP accumulation (similar to the MEFs shown here) but also with a substantial down-regulation of the β2AR in vivo (43). The different phenotype in lung fibroblasts is likely because these cells are exposed to catecholamines in vivo and the ablation of PDE4D causes a chronic, prostaglandin E receptor or βAR-driven increase in cAMP, hence the down-regulation of the βARs. This compensation does not seem to occur in proliferating MEFs in culture, most likely because catecholamines are absent from the culture medium and paracrine signals are probably minimized during the exponential growth phase. A different set of adaptive changes has been also observed after PDE4D ablation in granulosa cells of the ovary (44). Responses to LH (luteinizing hormone) are blunted after PDE4D ablation likely because of the constitutive uncoupling of the LH receptors from Gαs and cyclase. Thus, each cell may adapt to ablation of PDE4D differently by resetting the function of distinct components of cAMP signaling.
That PDE4B ablation has no significant effect on β2AR signaling measured either as cAMP accumulation or CREB phsophorylation strongly suggests that PDE4B isoforms are not involved in the control of β2AR signaling. The observation that down-regulation of PDE4B does not affect the PKA-mediated phosphorylation of β2AR in HEK293 cells (45) is consistent with our data. In the same vein, in vivo analysis of the phenotype of PDE4-deficient mice has led to the conclusion that PDE4B but not PDE4D is involved in the control of Toll-like receptor signaling and tumor necrosis factor-α production by inflammatory cells (12). Taken together, these genetic manipulations suggest that PDE4B functions in a manner distinct from PDE4D in cellular signaling. Thus, the role of PDE4B in MEFs is yet to be defined, begging the question of why PDE4B is expressed in these cells and with which signaling pathway it is involved. Similarly, PDE3 isoenzymes are not involved in regulating β2AR responses in these cells.
Ablation of PDE4D causes sustained cAMP levels for up to 20 min after addition of the β-adrenergic agonist (Fig. 7). This indicates that PDE4D contributes to generating the transient response and strongly suggests that cAMP-hydrolysis by PDE4D, in particular the increased rate of cAMP hydrolysis following phosphorylation of PDE4D by PKA, is an important component of dissipation of the β2AR cAMP signal and, in extension, of β2AR desensitization. Indeed, the second β2AR response after prestimulation with ISO is less affected in cells deficient in PDE4D (Fig. 9). Therefore, we propose that PDE4D is a component of the β2AR desensitization machinery. The relative contribution of PDE4D and, for instance, β-arrestin recruitment on β2AR signaling can be deduced by comparing the effect of ablation of these two components on cAMP accumulation after ISO stimulation. PDE4D ablation causes an average 5-fold increase in cAMP accumulation after 5 min, whereas β-arrestin ablation leads to a 2–3-fold increase in cAMP (7). Thus, the two mechanisms limiting β2AR signals have a comparable impact on the overall β2AR signaling.
A more detailed analysis of the desensitization time course suggests that desensitization still occurs in PDE4DKO MEFs but at a significantly slower rate than in wild type control cells. In addition, we observed a decrease in the sensitivity of the β2AR response to PTX treatment, suggesting that β2AR signaling through Gαi per se may be altered after PDE4D ablation. This apparent decrease in β2AR interaction with Gαi may account for part of the increased response in the PDE4DKO MEFs, even though PTX pretreatment does not affect the time course of desensitization in wild type cells. Thus, unlike other cell types where the Gαs/Gαi switch plays a prominent role in the dissipation of the β2AR signal, this was not the case in these MEFs. The biochemical basis for the decreased uncoupling after PDE4D ablation is unclear at present and may be the consequence of altered macromolecular signaling complexes. Regardless of the exact mechanism, PDE4D appears to limit β2AR signals in two ways: a direct effect due to cAMP hydrolysis and an indirect action by affecting other mechanisms of desensitization.
The delay in β2AR desensitization shown here in PDE4DKO MEFs is somewhat surprising, as it has been reported previously that down-regulation of PDE4D using antisense nucleotides in HEK293-β2AR cells or co-expression of dominant-negative PDE4D constructs together with the β2AR in rat cardiomyocytes promotes increased PKA phosphorylation of the β2AR, thus resulting in an accelerated switch from Gαs to Gαi signaling measured as the rate of Erk1/2 activation (17). One would expect that if this increased β2AR coupling to Gαi played a major role, an accelerated cAMP decay should have been observed in MEFs deficient in PDE4D. On the contrary, overall cAMP accumulation is stabilized in the MEF model, desensitization is slowed, and PTX effects are decreased after PDE4D ablation. Taken together, these findings strongly suggest that, if PDE4D does promote or accelerate β2AR coupling to Gαi, this has a minimal impact on the overall β2AR signal in our model. Possible explanations that reconcile the divergent conclusions include the inherent differences in the properties of the cell lines used. An additional explanation for the divergent results may lie in different behavior of the endogenous β2AR (in MEFs) compared with the exogenous green fluorescent protein-tagged receptor expressed in HEK293-β2AR cells. Receptor overexpression may have altered the stochiometry of the components participating in β2AR signaling and skewed toward Gαi regulation while minimizing other mechanisms. Indeed, in agreement with our findings in MEFs, recent work using native HEK293 cells suggests that knock-down of PDE4D, but not PDE4B, limits rather than enhances β2AR desensitization (46).
We have previously observed a loss of PTX efficacy on β2AR signaling in a model of cultured neonatal cardiomyocytes (39). The rate of spontaneous contraction of these cells responds to β2-adrenergic stimulation in a biphasic manner. An initial increase is followed by a reduction of contraction rate below basal levels, the latter being driven by β2AR signaling through Gαi. Whereas PTX blocks the down-regulation of contraction rate in wild type cells, PTX treatment had a reduced effect after inhibition of PDE4 with rolipram. We previously assumed that in these contraction assays, the overall increase in cAMP due to PDE4D ablation becomes predominant (i.e. through activation of downstream targets of cAMP such as the activation of Ca2+ channels) and may mask the Gαs/Gαi switch in β2AR signaling. However, the reduced β2AR coupling to Gαi observed in PDE4DKO MEFs in the present study opens the possibility that limited signaling through Gαi may have also contribute to the altered β2-adrenergic response in rolipram-treated cardiomyocytes. Protein expression levels of Gαs and Gαi were the same in wild type and PDE4DKO MEFs (Fig. 10, C and D); thus, a decrease in Gαi expression is not the cause of the limited Gαi coupling in the PDE4DKO. In view of the numerous PDE4D protein-protein interactions that have been reported, it is possible that ablation of PDE4D disrupts the function of these signaling complexes and, indirectly, Gαi coupling. An interaction of a PDE with a G protein has been established for the retina and is reported in frog ventricular myocytes (40).
In summary, our data demonstrate that of the different PDE isoenzymes expressed, PDE4D is the major form contributing to the shape of β2AR signals in MEFs. Although present, PDE3, PDE4A, and PDE4B did not impact substantially the β2AR-dependent response. PDE4D functions at different levels during the β2AR signal. Because of its activation by PKA, it controls the maximal response and the time in which cAMP accumulates in the cell. More importantly, PDE4D plays a major role in the dissipation of the β2AR signal, directly and indirectly, by controlling the receptor coupling to G proteins. Although the exact mechanisms need to be elucidated further, we conclude that PDE4D regulation is a major component of cell desensitization.
Supplementary Material
Acknowledgments
We are indebted to Dr. Brian Kobilka for helpful discussions and critical advice and to Sara Panigone for performing the Western blots for CREB phosphorylation. We acknowledge the generous gift of α-Gαs and α-Gαi antibodies from Dr. Teresa L. Z. Jones.
Note Added in Proof—During the revision of this manuscript, a study consistent with a critical role of PDE in β2-adrenergic signaling was published (Violin, J. D., DiPilato, L. M., Yildirim, N., Elston, T. C., Zhang, J., and Lefkowitz, R. J. (2008) J. Biol. Chem. 283, 2949–2961).
This work was supported, in whole or in part, by National Institutes of Health Grant HD20788 (to M. C.). This work was also supported by a grant from the Leducq Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: βAR, β-adrenergic receptor; ANOVA, analysis of variance; CREB, cAMP response element-binding protein; DMSO, dimethyl sulfoxide; Erk, extracellular signal-regulated kinase; GRK, G protein-coupled receptor kinase; IBMX, 3-isobutyl-1-methylxanthine; IP, immunoprecipitation; ISO, isoproterenol; KO, knock-out; MEF, mouse embryonic fibroblast; PDE, phosphodiesterase; PKA, cAMP-stimulated protein kinase; PKI, PKA inhibitor peptide; PTX, pertussis toxin; PBS, phosphate-buffered saline; RIA, radioimmunoassay; WT, wild type.
References
- 1.Xiao, R. P. (2001) Sci. STKE 2001, RE15. [DOI] [PubMed]
- 2.Johnson, M. (1998) Am. J. Respir. Crit. Care Med. 158 S146–S153 [DOI] [PubMed] [Google Scholar]
- 3.Zamah, A. M., Delahunty, M., Luttrell, L. M., and Lefkowitz, R. J. (2002) J. Biol. Chem. 277 31249–31256 [DOI] [PubMed] [Google Scholar]
- 4.Benovic, J. L., Pike, L. J., Cerione, R. A., Staniszewski, C., Yoshimasa, T., Codina, J., Caron, M. G., and Lefkowitz, R. J. (1985) J. Biol. Chem. 260 7094–7101 [PubMed] [Google Scholar]
- 5.Drake, M. T., Shenoy, S. K., and Lefkowitz, R. J. (2006) Circ. Res. 99 570–582 [DOI] [PubMed] [Google Scholar]
- 6.Conner, D. A., Mathier, M. A., Mortensen, R. M., Christe, M., Vatner, S. F., Seidman, C. E., and Seidman, J. G. (1997) Circ. Res. 81 1021–1026 [DOI] [PubMed] [Google Scholar]
- 7.Kohout, T. A., Lin, F.-T., Perry, S. J., Conner, D. A., and Lefkowitz, R. J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischmeister, R., Castro, L. R., Abi-Gerges, A., Rochais, F., Jurevicius, J., Leroy, J., and Vandecasteele, G. (2006) Circ. Res. 99 816–828 [DOI] [PubMed] [Google Scholar]
- 9.Mongillo, M., and Zaccolo, M. (2006) Biochem. Soc. Trans. 34 510–511 [DOI] [PubMed] [Google Scholar]
- 10.Conti, M., and Beavo, J. (2007) Annu. Rev. Biochem. 76 481–511 [DOI] [PubMed] [Google Scholar]
- 11.Conti, M., Richter, W., Mehats, C., Livera, G., Park, J. Y., and Jin, C. (2003) J. Biol. Chem. 278 5493–5496 [DOI] [PubMed] [Google Scholar]
- 12.Jin, S.-L. C., Richter, W., and Conti, M. (2007) in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo, J., Francis, S., and Houslay, M., eds) pp 323–346, CRC Press, Inc., Boca Raton, FL
- 13.Oki, N., Takahashi, S. I., Hidaka, H., and Conti, M. (2000) J. Biol. Chem. 275 10831–10837 [DOI] [PubMed] [Google Scholar]
- 14.Houslay, M. D., and Adams, D. R. (2003) Biochem. J. 370 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodge, K. L., Khouangsathiene, S., Kapiloff, M. S., Mouton, R., Hill, E. V., Houslay, M. D., Langeberg, L. K., and Scott, J. D. (2001) EMBO J. 20 1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasken, K. A., Collas, P., Kemmner, W. A., Witczak, O., Conti, M., and Tasken, K. (2001) J. Biol. Chem. 276 21999–22002 [DOI] [PubMed] [Google Scholar]
- 17.Baillie, G. S., Sood, A., McPhee, I., Gall, I., Perry, S. J., Lefkowitz, R. J., and Houslay, M. D. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Perry, S. J., Baillie, G. S., Kohout, T. A., McPhee, I., Magiera, M. M., Ang, K. L., Miller, W. E., McLean, A. J., Conti, M., Houslay, M. D., and Lefkowitz, R. J. (2002) Science 298 834–836 [DOI] [PubMed] [Google Scholar]
- 19.Richter, W., Day, P., Agrawal, R., Bruss, M. D., Granier, S., Wang, Y. L., Rasmussen, S. G., Horner, K., Wang, P., Lei, T., Patterson, A. J., Kobilka, B., and Conti, M. (2008) EMBO J. 27 384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenoy, S. K., Drake, M. T., Nelson, C. D., Houtz, D. A., Xiao, K., Madabushi, S., Reiter, E., Premont, R. T., Lichtarge, O., and Lefkowitz, R. J. (2006) J. Biol. Chem. 281 1261–1273 [DOI] [PubMed] [Google Scholar]
- 21.Jin, S. L., and Conti, M. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 7628–7633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, S. L., Lan, L., Zoudilova, M., and Conti, M. (2005) J. Immunol. 175 1523–1531 [DOI] [PubMed] [Google Scholar]
- 23.Jin, S. L., Richard, F. J., Kuo, W. P., D'Ercole, A. J., and Conti, M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 11998–12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper, J. F., and Brooker, G. (1975) J. Cyclic Nucleotide Res. 1 207–218 [PubMed] [Google Scholar]
- 25.Thompson, W. J., and Appleman, M. M. (1971) Biochemistry 10 311–316 [PubMed] [Google Scholar]
- 26.Richter, W., and Conti, M. (2002) J. Biol. Chem. 277 40212–40221 [DOI] [PubMed] [Google Scholar]
- 27.Richter, W., Jin, S. L., and Conti, M. (2005) Biochem. J. 388 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193 265–275 [PubMed] [Google Scholar]
- 29.Sette, C., and Conti, M. (1996) J. Biol. Chem. 271 16526–16534 [DOI] [PubMed] [Google Scholar]
- 30.Roth, N. S., Campbell, P. T., Caron, M. G., Lefkowitz, R. J., and Lohse, M. J. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 6201–6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran, T. M., Friedman, J., Qunaibi, E., Baameur, F., Moore, R. H., and Clark, R. B. (2004) Mol. Pharmacol. 65 196–206 [DOI] [PubMed] [Google Scholar]
- 32.Parker, D., Ferreri, K., Nakajima, T., LaMorte, V. J., Evans, R., Koerber, S. C., Hoeger, C., and Montminy, M. R. (1996) Mol. Cell. Biol. 16 694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefkowitz, R. J., and Shenoy, S. K. (2005) Science 308 512–517 [DOI] [PubMed] [Google Scholar]
- 34.Conti, M., Nemoz, G., Sette, C., and Vicini, E. (1995) Endocr. Rev. 16 370–389 [DOI] [PubMed] [Google Scholar]
- 35.Daaka, Y., Luttrell, L. M., and Lefkowitz, R. J. (1997) Nature 390 88–91 [DOI] [PubMed] [Google Scholar]
- 36.Tran, T. M., Friedman, J., Baameur, F., Knoll, B. J., Moore, R. H., and Clark, R. B. (2007) Mol. Pharmacol. 71 47–60 [DOI] [PubMed] [Google Scholar]
- 37.Xiao, R. P. (2000) Circ. Res. 87 635–637 [DOI] [PubMed] [Google Scholar]
- 38.Devic, E., Xiang, Y., Gould, D., and Kobilka, B. (2001) Mol. Pharmacol. 60 577–583 [PubMed] [Google Scholar]
- 39.Xiang, Y., Naro, F., Zoudilova, M., Jin, S. L., Conti, M., and Kobilka, B. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 909–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brechler, V., Pavoine, C., Hanf, R., Garbarz, E., Fischmeister, R., and Pecker, F. (1992) J. Biol. Chem. 267 15496–15501 [PubMed] [Google Scholar]
- 41.Rich, T. C., Xin, W., Mehats, C., Hassell, K. A., Piggott, L. A., Le, X., Karpen, J. W., and Conti, M. (2007) Am. J. Physiol. 292 C319–C331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin, W., Tran, T. M., Richter, W., Clark, R. B., and Rich, T. C. (2008) J. Gen. Physiol. 131 349–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehats, C., Jin, S. L., Wahlstrom, J., Law, E., Umetsu, D. T., and Conti, M. (2003) FASEB J. 17 1831–1841 [DOI] [PubMed] [Google Scholar]
- 44.Park, J. Y., Richard, F., Chun, S. Y., Park, J. H., Law, E., Horner, K., Jin, S. L., and Conti, M. (2003) Mol. Endocrinol. 17 1117–1130 [DOI] [PubMed] [Google Scholar]
- 45.Lynch, M. J., Baillie, G. S., Mohamed, A., Li, X., Maisonneuve, C., Klussmann, E., van Heeke, G., and Houslay, M. D. (2005) J. Biol. Chem. 280 33178–33189 [DOI] [PubMed] [Google Scholar]
- 46.Willoughby, D., Baillie, G. S., Lynch, M. J., Ciruela, A., Houslay, M. D., and Cooper, D. M. (2007) J. Biol. Chem. 282 34235–34249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.