Abstract
Our study identifies tyrosine phosphorylation as a novel protein kinase Cδ (PKCδ) activation mechanism that modifies PKCδ-dependent phosphorylation of cardiac troponin I (cTnI), a myofilament regulatory protein. PKCδ phosphorylates cTnI at Ser23/Ser24 when activated by lipid cofactors; Src phosphorylates PKCδ at Tyr311 and Tyr332 leading to enhanced PKCδ autophosphorylation at Thr505 (its activation loop) and PKCδ-dependent cTnI phosphorylation at both Ser23/Ser24 and Thr144. The Src-dependent acquisition of cTnI-Thr144 kinase activity is abrogated by Y311F or T505A substitutions. Treatment of detergent-extracted single cardiomyocytes with lipid-activated PKCδ induces depressed tension at submaximum but not maximum [Ca2+] as expected for cTnI-Ser23/Ser24 phosphorylation. Treatment of myocytes with Src-activated PKCδ leads to depressed maximum tension and cross-bridge kinetics, attributable to a dominant effect of cTnI-Thr144 phosphorylation. Our data implicate PKCδ-Tyr311/Thr505 phosphorylation as dynamically regulated modifications that alter PKCδ enzymology and allow for stimulus-specific control of cardiac mechanics during growth factor stimulation and oxidative stress.
Protein kinase Cδ (PKCδ)2 is a ubiquitous serine/threonine kinase implicated in a wide range of cellular responses (1, 2). PKCδ is conventionally viewed as a lipid-dependent enzyme that is anchored to membranes in close proximity to target substrates through interactions with lipid cofactors. However, there is recent evidence that PKCδ also is dynamically regulated through activation loop (Thr505) phosphorylation (3, 4). For other PKC isoforms, activation loop phosphorylation is a stable “priming” phosphorylation completed during de novo enzyme synthesis (5). In the case of cPKCs, activation loop phosphorylations are mediated by phosphoinositide-dependent kinase-1 and are essential to generate a catalytically competent enzyme. Although newly synthesized PKCδ also undergoes maturational phosphoinositide-dependent kinase-1-dependent Thr505 phosphorylation, PKCδ differs from other PKC isoforms in that 1) PKCδ is a catalytically active enzyme even without Thr505 phosphorylation and 2) PKCδ-Thr505 phosphorylation is dynamically regulated through an autocatalytic mechanism (4). Although there are hints that Thr505 phosphorylation might contribute to the control of PKCδ enzymology, a PKCδ-Thr505 autophosphorylation mechanism that regulates the actions of PKCδ toward a physiologically relevant substrate in a differentiated cell has never been reported.
PKCδ also is regulated through tyrosine phosphorylation. However, the consequences of PKCδ tyrosine phosphorylation remain disputed, because PKCδ tyrosine phosphorylation is variably linked to increased, decreased, or unchanged PKCδ activity (1). Inconsistencies in the literature have been attributed to the presence of multiple tyrosine residues throughout the structure of PKCδ (including Tyr52, Tyr64, Tyr155, Tyr187, Tyr311, Tyr332, Tyr512, and Tyr523, numbering based upon rodent sequence) that are targets for independently regulated phosphorylations by Src family kinases, c-Abl, and potentially other tyrosine kinases. The general consensus in the literature is that PKCδ tyrosine phosphorylation patterns vary according to the nature of the inciting stimulus and dictate the functional properties of the enzyme in cells. Our recent studies show Tyr311 and Tyr332 (residues in the hinge region of PKCδ that links the regulatory and kinase domains) are phosphorylated in vivo in H2O2-treated cardiomyocytes and in vitro in kinase assays performed with Src (4, 6). We also obtained unanticipated evidence that Src and PKCδ tyrosine phosphorylation promote PKCδ-Thr505 autophosphorylation in cells. These results run counter to the prevailing assumption that PKCδ tyrosine and Thr505 phosphorylations are independently regulated mechanisms and suggest that at least some of the dynamically regulated changes in PKCδ-Thr505 phosphorylation in cells can be attributed to tyrosine phosphorylation (i.e. PKCδ-Thr505 autophosphorylation constitutes a final common mechanism to control PKCδ activity, including during oxidative stress).
PKCs induce structural and functional ventricular remodelling both by activating signaling pathways that alter gene expression and by directly phosphorylating myofilament proteins that control cardiac contraction (7). cTnI, the “inhibitory” subunit of the troponin complex, is an important PKC substrate that plays an indispensable role in Ca2+-dependent regulation of myofilament function (8). At low intracellular [Ca2+], cTnI is anchored to the actin filament via peptides (an inhibitory peptide 138GKFKRPTLRRVR149) and a second actin-binding site (residues 161–188) flanking a so-called switch peptide (9). This tethering of cTnI acts together with other regulatory proteins to prevent strong, force-generating interactions of myosin cross-bridges with thin filaments. At high intracellular [Ca2+], the switch peptide binds strongly to the cTnC regulatory domain and induces a movement of cTnI away from actin thereby releasing inhibition (for recent reviews see Refs. 10 and 11 and references within). Myofilament function is further regulated and fine-tuned to hemodynamic load, by cTnI phosphorylation. cTnI contains three phosphorylation clusters (Ser23/Ser24, Ser43/Ser45, and Thr144) that exert distinct effects on cardiac function. Ser23/Ser24 phosphorylation desensitizes myofilaments to Ca2+ and leads to an enhanced rate of relaxation, augmented cross-bridge cycling, and accelerated unloaded shortening velocity. In contrast, Ser43/Ser45 phosphorylation leads to a decrease in maximal actomyosin Mg-ATPase, Ca2+-activated force, and cross-bridge cycling rate. Despite the fact that Thr144 is critically positioned in the inhibitory peptide region, the functional impact of Thr144 phosphorylation has been less intensively studied. Recent studies suggest that Thr144 phosphorylation may influence myofilament Ca2+ sensitivity (12–14).
PKC-dependent cTnI phosphorylation is believed to be particularly important in the development of hypertrophy/failure syndromes, where coordinate increases in PKC isoform expression and cTnI phosphorylation have been linked to reduced actin-myosin interactions and depressed contractile function (7, 15, 16). However, conventional models of PKCδ activation, which attribute PKC isoform specificity entirely to translocation events that localize the enzyme to membranes, do not adequately explain PKC-dependent cTnI phosphorylation in the sarcomere. A role for tyrosine-phosphorylated PKCδ that is released from membranes and recovered as a constitutively active, lipid-independent enzyme in the soluble fraction of H2O2-treated cardiomyocytes would be more plausible (6). This study examines the role of PKCδ as a cTnI kinase, with a particular focus on the role of Src (and PKCδ tyrosine phosphorylation) to regulate PKCδ-dependent phosphorylation of cTnI and transduce physiologically relevant changes in contractile function.
EXPERIMENTAL PROCEDURES
Materials—Antibodies were from the following sources: PKCδ-Thr(P)505, PKCδ-Tyr(P)311, Src-Tyr(P)416, troponin I-Ser(P)23/Ser(P)24, and anti-pTXR from Cell Signaling Technology; GFP (for immunoblot analysis), PKCδ, and PKCδ-Tyr(P)332 from Santa Cruz Biotechnology; Src from Oncogene; anti-Tyr(P) from Upstate Biotechnology; and GFP (for immunoprecipitation) from Invitrogen. Recombinant human PKCδ was from Calbiochem; active Src kinase was from Invitrogen; c-Abl was from Upstate Biotechnology. PMA was from Sigma. 1,2-Dioleoyl-sn-glycerol was from Avanti Polar Lipids, Inc. The other chemicals were reagent grade.
cTnI Phosphorylation in Adult Ventricular Cardiomyocytes—Left ventricular myocytes enzymatically isolated from male Sprague-Dawley rats according to a modification of published methods were plated for 2 h on laminin-coated dishes (10 μg/ml; Invitrogen) at a density of 5 × 104 rod-shaped cells/35-mm dish (27). The cells were washed with fresh media, pretreated with vehicle (Me2SO), GF109203X (5 μm), or PP1 (10 μm) and then stimulated with H2O2 (5 mm) or PMA (100 nm) for 15 min. Cell lysis was in ice-cold radioimmune precipitation assay buffer (with protease/phosphatase inhibitor mixture) and was followed by centrifugation (16,000 × g, 4 °C, 15 min) to pellet myofilament proteins, which were then resuspended in a urea/thiourea sample buffer and separated on 15% acrylamide gels. The gels were stained with Pro-Q Diamond (to detect phosphorylated proteins) followed by Sypro Ruby (to detect total protein), and analysis was by imaging on a Typhoon 9410 molecular imager (GE Healthcare) using ImageQuant TL software.
Cardiomyocyte Culture and PKCδ Immunoprecipitation for Kinase Assays—Cardiomyocytes were isolated from hearts of 2-day-old Wistar rats by a trypsin dispersion procedure that uses a differential attachment procedure followed by irradiation to enrich for cardiomyocytes (6). The cells were plated on protamine sulfate-coated culture dishes at a density of 5 × 106 cells/100-mm dish and grown in minimum essential medium (Invitrogen) supplemented with 10% fetal calf serum for 4 days and then serum-deprived for 24 h prior to experiments. The cells were treated with vehicle, 5 mm H2O2, or 100 nm PMA for 15 min and then lysed in homogenization buffer, and cell extracts were subjected to immunoprecipitation with anti-PKCδ (Santa-Cruz) and used in immunocomplex kinase assays modified only to include cTn complex as substrate according to methods described previously (4, 6, 12).
In Vitro Kinase Assays with Recombinant Enzymes—In vitro kinase assays were performed with 0.032 units (32.4 ng) of recombinant human PKCδ (Calbiochem) for assays of cTn complex phosphorylation (or 0.4 units for peptide mapping studies) or other serine/threonine kinases (0.083 units of PKCα, 0.063 units of PKCβII, 0.566 units of PKCε, 0.026 units of PKD, or 3.84 units of PKA; all from Upstate Biotechnology) in 80 μl of a reaction buffer containing 30 mm Tris-Cl, pH 7.5, 5 mm MgCl2, 10 mm MnCl2, 0.9 mm EDTA, 0.9 mm EGTA, 3 mm dithiothreitol, 0.1 mm sodium vanadate, 76 mm NaCl, 23.5% glycerol, 0.006% Brij-35, 0.023% Triton X-100, 0.04 mm phenylmethylsulfonyl fluoride, 0.2 mm benzamidine, 83 μg/ml phosphatidylserine (PS), 175 nm PMA, 4 μg of troponin complex, and [γ-32P]ATP (25 μCi, 97 μm). Incubations were for 30 min at 30 °C in the absence or presence of Src or c-Abl (0.66 units) and lipid cofactors; PS-PMA was included in assays to allosterically activate PKCδ and render it a better substrate for Src-dependent tyrosine phosphorylation (4).
PKCδ Mutants—pPKCδ-EGFP (pGFP-PKCδ) was obtained as a generous gift from Dr. Mary Reyland (University of Colorado Health Sciences Center, Denver, CO). The pPKCδ-EGFP construct expresses PKCδ protein with enhanced GFP fused to its C terminus. The pPKCδ(T505A)-EGFP and pPKCδ(T505E)-EGFP were generated by site-directed mutagenesis according to the manual for the QuikChange site-directed mutagenesis kit (Stratagene). The PKCδ expression plasmids were introduced into COS-7 cells by Effectene transfection reagent (Qiagen) according to the instruction manual. The cells were grown for 24 h, lysed in homogenization buffer (20 mm Tris-Cl, pH7.5, 0.05 mm EDTA, 0.5 mm dithiothreitol, 0.2% Triton X-100, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 5 μg/ml benzamidine, 1 mm phenylmethylsulfonyl fluoride, 5 μm pepstatin A). Cell extracts were subjected to immunoprecipitation with mouse monoclonal anti-GFP antibody 3E6 (Invitrogen).
Peptide Mapping Studies—For peptide mapping studies, kinase reactions (see above) were stopped by adding 27 μl of 4× SDS-PAGE sample buffer. The proteins were separated by SDS-PAGE and transferred to nitrocellulose, and the band corresponding to PKCδ was excised from the membrane, cut into small pieces, and treated for 30 min at 37 °C with polyvinylpyrrolidone (0.5%, w/v) in acetic acid (100 mm), followed by five water washes (to remove the acid) and a 10-min incubation at room temperature in the dark with 100 mm iodoacetate to carboxymethylate PKCδ. The membrane pieces were then washed three times with water and twice with 50 mm ammonium bicarbonate and incubated overnight at 37 °C in 60 μl of a buffer containing 42 mm ammonium bicarbonate, 17 μm HCl, and 10 μg of sequencing grade trypsin. Digested peptides were eluted from the membrane by sonication and lyophilized, and the residue was reconstituted in 0.1% trifluoroacetic acid and fractionated by reverse phase-HPLC on a Vydac semimicro C18 column (2.1 × 250 mm). The peptides were eluted with a linear gradient from 0.1% trifluoroacetic acid in water to 0.1% trifluoroacetic acid in acetonitrile over 140 min at a flow rate of 1 ml/min. The eluant was monitored at 220 nm, and fractions were collected every 30 s for Cherenkov counting. Radioactive peptides of interest were submitted to the Howard Hughes Medical Institute/Columbia University Protein Chemistry Core Facility for sequencing by MALDITOF mass spectrometry.
Mechanical Measurements in Skinned Rat Ventricular Myocytes—Rat ventricular myocytes were mechanically isolated and mounted in the experimental set-up as described previously (20). Isometric force production and the rate constant of force redevelopment (ktr) were assessed in single, skinned myocytes, as described previously (20), before and after PKCδ treatment alone (as described above) or in combination with Src kinase. All of the PKCδ treatments were in the presence of lipid cofactors (PS/PMA). Lipid cofactors and Src alone had no effect on mechanical parameters.
Statistical Analysis—All of the values are presented as the means ± S.E., and the values of p < 0.05 were the criteria of statistical significance. Statistical evaluation was by Student t test (KaleidaGraph, Synergy Software).
RESULTS
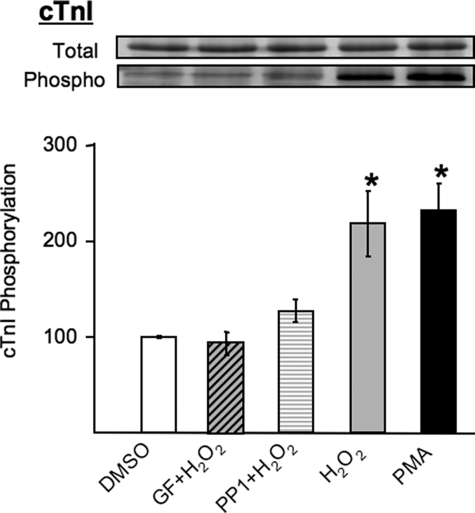
cTnI Phosphorylation via a PKCδ/Src-dependent Mechanism in H2O2-treated Cardiomyocytes—Initial studies examined the mechanisms that control cTnI phosphorylation in situ in the myofilament lattice of cultured adult rat cardiomyocytes subjected to oxidative stress by H2O2 treatment. Fig. 1 shows that PMA (which directly activates PKCs) and H2O2 induce similar increases in cTnI phosphorylation. The H2O2-dependent increment in cTnI phosphorylation is completely abrogated by GF109203X (a relatively nonselective inhibitor of PKC isoforms), and it is markedly blunted by PP1 (an inhibitor of Src family kinases). The observation that H2O2 increases cTnI phosphorylation through a PKC-dependent mechanism that requires tyrosine kinase activity provided the rationale to consider a role for tyrosine-phosphorylated PKCδ.
FIGURE 1.
Stimulus-dependent cTnI phosphorylation in cardiomyocytes. Adult ventricular myocytes were treated with vehicle (dimethyl sulfoxide (DMSO)), GF109203X (5 μm), or PP1 (10 μm) followed by H2O2 (5 mm) or PMA (100 nm) for 15 min. The gels were stained with Pro-Q Diamond (phosphoproteins stain) followed by Sypro Ruby (total protein stain), and the phospho/total protein ratios were determined (n = 4, means ± S.E.; *, p < 0.05). The results are normalized by setting the mean value for vehicle-treated samples to 100.
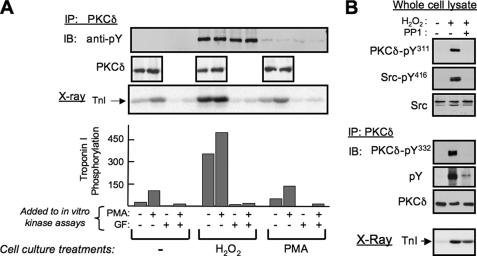
PKCδ was immunopurified from vehicle-, PMA-, and H2O2-treated cardiomyocyte cultures and used in assays with cTn ternary complexes (consisting of equimolar cTnI, cTnC, and cTnT) as substrate to resolve the cellular actions of PKCδ from the actions of other PKC isoforms. Native PKCδ is recovered from quiescent cardiomyocyte cultures with only very low lipid-independent cTnI kinase activity; cTnI phosphorylation is markedly increased when lipid activators, PS/PMA, are added to the in vitro kinase assay (Fig. 2A). PMA treatment of cardiomyocyte cultures, prior to PKCδ immunoprecipitation, leads to a minor increase in basal and lipid-dependent cTnI kinase activity, in association with a trace increase in overall PKCδ tyrosine phosphorylation. Oxidative stress induced by H2O2 treatment results in a marked increase in PKCδ tyrosine phosphorylation and a dramatic increase in PKCδ-dependent phosphorylation of cTnI, including in assays without lipid cofactors (i.e. PKCδ is recovered from cardiomyocytes subjected to oxidative stress as a lipid-independent cTnI kinase). Although H2O2 treatment has been linked to the activation of an array of serine/threonine kinases that may potentially associate with PKCδ during immunoprecipitation, control experiments identify similar pharmacologic profiles for the cTnI kinase activity recovered from resting and H2O2-treated cultures; in both cases, cTnI phosphorylation is fully inhibited by 5 μm GF109203X and 5 μm Gö6983 (broad spectrum PKC inhibitors) but not by 10 μm Gö6976 (a conventional PKC isoform-selective inhibitor that also directly inhibits the catalytic activity of PKD, another potential H2O2-activated cTnI kinase; Fig. 2 and data not shown). These results implicate PKCδ as a lipid-independent cTnI kinase in cardiomyocytes exposed to oxidative stress.
FIGURE 2.
PKCδ is recovered as a lipid-independent kinase from H2O2-treated cardiomyocytes. A, PKCδ immunoprecipitated from cardiomyocytes treated with vehicle (Me2SO), PMA (100 nm), or H2O2 (5 mm), each for 15 min, was used in immune complex kinase assays with cTn complex as substrate (with 100 nm PMA and 5 μm GF109203X also included in kinase assays as indicated). Immunoblotting (IB) was used to validate equal PKCδ recovery from control and PMA- and H2O2-treated cultures and to show that PKCδ is tyrosine-phosphorylated in H2O2-treated cultures (and to a much lesser extent in PMA-treated cultures) but not in control cultures. The autoradiogram showing cTnI phosphorylation and quantification is from a representative experiment, with similar results obtained in separate experiments on four separate culture preparations. B, cardiomyocytes were treated with vehicle, 5 mm H2O2, or 5 mm H2O2 + 10 μm PP1 (each for 15 min); cell extracts were subjected to immunoblotting for PKCδ-Tyr(P)311 and Src-Tyr(P)416 (with Src protein as a loading control, top right), and PKCδ pull-downs were immunoblotted for PKCδ-Tyr(P)332 and Tyr(P) (with PKCδ as a loading control; the anti-PKCδ-Tyr(P)332 antibody displays too much nonspecific immunoreactivity to be used directly on cell extracts, middle right). PKCδ in immune complexes also was used in kinase assays with cTn complex as substrate; a representative autoradiogram of cTnI phosphorylation is illustrated (bottom right). IP, immunoprecipitation.
PKCδ kinase activity may be activated by H2O2 indirectly as a result of Src-dependent tyrosine phosphorylation or directly through cysteine oxidation (17). To assess the importance of tyrosine phosphorylation in H2O2-dependent changes in PKCδ activity, we compared cTnI phosphorylation by PKCδ recovered from myocytes treated with H2O2 alone or H2O2+PP1 (to inhibit PKCδ tyrosine phosphorylation). Fig. 2B shows that H2O2 increases overall PKCδ tyrosine phosphorylation (tracked with a general anti-phosphotyrosine antibody) and PKCδ-Tyr311 and -Tyr332 phosphorylation (tracked by phospho site-specific antibodies (PSSAs)); H2O2-dependent PKCδ tyrosine phosphorylation is blocked by PP1, under conditions that also block H2O2-dependent activation of Src (tracked by immunoblotting for Src activation loop Tyr416 phosphorylation). PP1 attenuates (by 28.4 ± 4%, n = 4, p < 0.05) but does not entirely block the H2O2-dependent increase in cTnI phosphorylation by PKCδ. These results indicate that H2O2 increases PKCδ phosphorylation of cTnI through at least two mechanisms that differ in their requirements for Src-dependent tyrosine phosphorylation. The Src and tyrosine phosphorylation-dependent mechanisms that regulate PKCδ activity are the focus of the remainder of the studies in this manuscript.
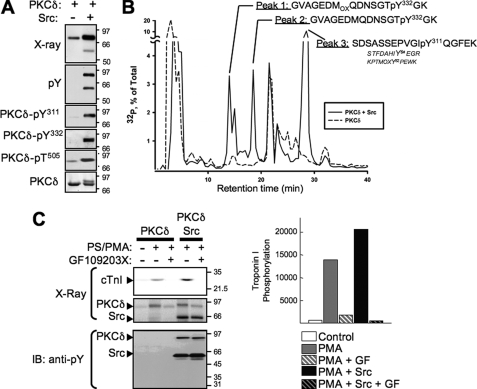
Src Phosphorylates PKCδ at Tyr311 and Tyr332, Leading to Enhanced PKCδ Phosphorylation of cTnI—We recently used PSSAs to identify Tyr311 and Tyr332 as sites for Src-dependent PKCδ phosphorylation (4). Recombinant PKCδ (human sequence) was subjected to in vitro kinase assays without and with active Src in buffers containing [γ-32P]ATP and PS/PMA, and radiolabeled PKCδ was subjected to peptide mapping analysis as an alternative method to map sites for Src-dependent PKCδ phosphorylation sites (without relying on available PSSAs). Fig. 3A shows that Src increases 32P incorporation into PKCδ, leading to the appearance of a PKCδ species that migrates more slowly in SDS-PAGE and displays increased PKCδ-Tyr(P)311 and PKCδ-Tyr(P)332 immunoreactivity. Src also increases PKCδ autophosphorylation at Thr505, consistent with our recent observation that the activation loop of PKCδ is the target of an autophosphorylation reaction that is augmented by Src (4).
FIGURE 3.
Src-dependent PKCδ phosphorylation maps to Tyr311 and Tyr332 and leads to increased PKCδ-dependent cTnI phosphorylation. PKCδ was incubated in a kinase buffer containing [32P]ATP without and with active Src and PKCδ phosphorylation was tracked by immunoblotting (A) and MALDI-TOF mass spectrometry (B) according to “Experimental Procedures.” C, in vitro kinase assays were performed with PKCδ in the absence or presence of Src, 100 nm PMA + 112 μm PS, and 5 μm GF109203X as indicated. Autoradiograms showing 32P incorporation into PKCδ, Src, and cTnI are depicted (top left) with results for cTnI phosphorylation quantified (right). Immunoblotting (IB) with anti-Tyr(P) antibody shows Src autophosphorylation and Src-dependent tyrosine phosphorylation of PKCδ, but no cTnI tyrosine phosphorylation.
PKCδ purified by SDS-PAGE and blotted to nitrocellulose was excised from the membrane and digested with trypsin, and peptide fragments were separated by reverse phase-HPLC (Fig. 3B). Radioactive peptide fragments detected in assays with PKCδ + Src (but not in assays with PKCδ alone) were sequenced by MALDI-TOF mass spectrometry to determine sites for Src-dependent PKCδ phosphorylation. Fig. 3B shows that three distinct radioactive peaks were detected in reverse phase-HPLC chromatograms of phospho-peptide fragments derived from in vitro kinase assays with PKCδ, Src, and [32P]ATP (and not in vitro kinase assays with PKCδ alone). Peaks 1 and 2 were identified by mass spectrometry analysis as the oxidized and reduced forms of a Tyr(P)332-containing fragment (note that differences in oxidation status arise during sample preparation and should not be construed as evidence that this modification occurs in cells). Peak 3 contained a Tyr(P)311 fragment, as well as nonphosphorylated forms of peptides containing Tyr64 and Tyr52 (other sites on PKCδ that have been reported to be phosphorylated under certain stimulatory conditions). Collectively, these results identify Tyr311 and Tyr332 as the major sites for in vitro Src-dependent PKCδ tyrosine phosphorylation.
Having identified Tyr311 and Tyr332 as major targets for Src-dependent PKCδ phosphorylation in vitro and H2O2-dependent PKCδ phosphorylation in vivo, we performed in vitro kinase assays with PKCδ, active Src, a kinase buffer containing [32P]ATP and cTn complex (consisting of wild type (WT) cTnT, WT-cTnC, and WT-cTnI) to determine whether Src-dependent PKCδ tyrosine phosphorylation leads to a change in PKCδ-dependent phosphorylation of cTnI. PKCδ phosphorylates cTnI in a lipid-dependent fashion in vitro, with cTnI phosphorylation markedly increased in assays performed in the presence of active Src (under conditions that lead to PKCδ tyrosine phosphorylation; Fig. 3C). The Src-dependent increment in cTnI phosphorylation cannot be attributed to direct myofibrillar protein phosphorylation by Src, because 1) cTnI phosphorylation is completely blocked by GF109203X and 2) anti-Tyr(P) antibodies detect tyrosine-phosphorylated forms of PKCδ and Src, but no anti-Tyr(P) immunoreactivity comigrating with cTnI was detected. These results indicate that the Src-dependent increment in cTnI phosphorylation results from serine/threonine phosphorylation by PKCδ rather than direct cTnI tyrosine phosphorylation by Src.
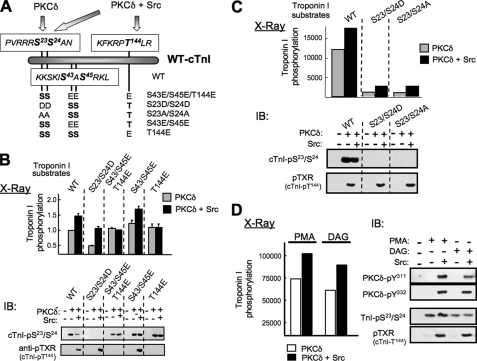
In vitro kinase assays were extended to use cTn complexes containing WT-cTnI or substituted forms of cTnI (cTnI-S23D/S24D, cTnI-S23A/S24A, cTnI-S43E/S45E, cTnI-Thr144E, or cTnI-S43E/S45E/T144E; see Fig. 4A) as substrate to 1) map the sites phosphorylated by allosterically activated PKCδ (in assays with PS/PMA) and 2) determine whether the Src-dependent increment in PKCδ activity is directed toward the same or different sites on cTnI. PhosphorImager analysis of PKCδ-dependent cTnI phosphorylation shows that PKCδ preferentially radiolabels forms of cTnI that can be phosphorylated at Ser23/Ser24 (Fig. 4B, top panel, gray bars). PKCδ induces similar increases in 32P incorporation into WT-cTnI and cTnI mutants harboring Ser → Glu substitutions at Ser43/Ser45 or Thr144 alone or in combination (i.e. cTnI-S43E/S45E, cTnI-T144E, and cTnI-S43E/S45E/T144E), whereas PKCδ-dependent phosphorylation of cTnI-S23D/S24D is markedly reduced in comparison. Quantification of data from three separate experiments shows the S23D/S24D substitution (making Ser23/Ser24 nonphosphorylatable) reduces PKCδ-dependent cTnI phosphorylation by 68.0 ± 2.3% (relative to WT-cTnI; n = 3, p < 0.05). These results indicate that PS/PMA-activated PKCδ phosphorylates cTnI primarily at Ser23/Ser24; Ser43/Ser45 and Thr144 are relatively poor substrates for allosterically activated PKCδ. A similar mutagenesis approach was used to map the Src-de-pendent increment in PKCδ-dependent cTnI phosphorylation to cTnI-Thr144. Src increases PKCδ-dependent phosphorylation of WT-cTnI and forms of cTnI that can be phosphorylated at Thr144 (cTnI-S23D/S24D or cTnI-S43E/S45E), whereas the Src-dependent increment in PKCδ-dependent cTnI phosphorylation is abrogated by T144E or cTnI-S43E/S45E/T144E substitutions (Fig. 4B, top panel, black bars).
FIGURE 4.
Lipid-activated PKCδ is a cTnI-Ser23/Ser24 kinase; tyrosine-phosphorylated-PKCδ is a cTnI-Ser23/Ser24 kinase that also phosphorylates cTnI at Thr144. A, schematic of WT-cTnI and cTnI mutants used in this study. B and C, in vitro kinase assays with PKCδ alone or PKCδ + Src performed with 112 μm PS + 175 nm PMA and cTnI complexes containing WT or mutant cTnIs as substrates. cTnI phosphorylation was quantified by PhosphorImager analysis (mean ± S.E., n = 3 top) and immunoblotting (IB) with anti-cTnI-Ser23/Ser24 and anti-pTXR (that recognizes cTnI-Thr144 phosphorylation; representative experiment is depicted at the bottom). D, in vitro kinase assays with PKCδ alone or PKCδ + Src with complexes containing WT-cTnI and either 175 nm PMA or 7.2 μm 1,2-DAG (each with 112 μm PS) as lipid cofactor. PKCδ tyrosine (Tyr311 and Tyr332) was tracked by immunoblotting; cTnI phosphorylation was tracked by PhosphorImager analysis (top) and immunoblotting (bottom). Similar results were obtained in two additional separate experiments.
Conclusions derived from PhosphorImager studies were validated by immunoblot analysis (Fig. 4B, bottom panel). An anti-cTnI-Ser(P)23/Ser(P)24 PSSA that specifically recognizes the Ser23/Ser24-phosphorylated form of cTnI detects similar PKCδ-dependent phosphorylation of WT-cTnI and cTnI mutants harboring single residue substitutions at Ser43/Ser45 and Thr144 (alone and in combination); this PSSA does not detect phosphorylation of the cTnI-S23D/S24D mutant. Importantly, radiolabeling studies show that Src increases overall WT-cTnI phosphorylation by PKCδ, but direct immunoblotting studies show that Src does not increase PKCδ-dependent cTnI-Ser23/Ser24 phosphorylation (compare Fig. 4B, top and bottom panels). We performed additional studies with an anti-pTXR phospho-motif antibody (Cell Signaling Technology; that recognizes phospho-threonines flanked by an arginine in the +2 position, which is predicted to recognize cTnI phosphorylation at KRPT144LR, but not RS23S24AN or KIS43AS45RK) to localize the Src-dependent increment in PKCδ-dependent cTnI phosphorylation. The anti-pTXR PSSA does not recognize cTnI phosphorylation in assays with PKCδ alone (without Src), where PKCδ acts primarily as a cTnI-Ser23/Ser24 kinase. Rather, this antibody selectively recognizes phosphorylation of WT-cTnI, cTnI-S23D/S24D, and cTnI-S43E/S45E (i.e. forms of cTnI that can be phosphorylated at Thr144) in assays performed with PKCδ + Src (Fig. 4B, bottom panel); the cTnI-S43E/S45E/T144E and cTnI-T144E mutants are not detected. These immunoblotting studies validate the use of the anti-pTXR PSSA to selectively track cTnI-Thr144 phosphorylation and validate conclusions derived from radiolabeling experiments tracking overall cTnI phosphorylation (by PhosphorImager); both techniques identify an effect of Src to selectively increase PKCδ cTnI-Thr144 kinase activity. Additional studies show that PKCδ functions as a cTnI-Thr144 kinase in assays with Src having either cTnI-S23D/S24D or cTnI-S23/24A as substrate (Fig. 4C). These results argue that Thr144 phosphorylation does not require prior phosphorylation (or negative charge) at Ser23/Ser24 (i.e. cTnI phosphorylation does not appear to be a hierarchical process). Collectively, these results provide surprising evidence that PKCδ acquires a new activity, directed toward a different site on cTnI (namely Thr144), when it is tyrosine-phosphorylated by Src; Src does not increase PKCδ-dependent cTnI-Ser23/Ser24 phosphorylation.
In vitro kinase assays were performed in buffers containing PS/PMA, which is included both to activate PKCδ (in assays without Src) and to induce a conformational change required for Src-dependent PKCδ tyrosine phosphorylation (in assays with Src); our previous studies established that Src phosphorylates PKCδ only in assays performed with PS/PMA (4). Given recent evidence that the PKCδ C1 domain is comprised of C1A and C1B domains with markedly different affinities for PMA and the natural lipid cofactor DAG (i.e. PMA binds with high affinity to the PKCδ-C1B domain, whereas DAG anchors full-length PKCδ to membranes via an interaction with the PKCδ-C1A domain (18)), it was important to determine whether the Src-dependent changes in PKCδ-dependent cTnI phosphorylation also are detected in assays with DAG. Fig. 4D shows that DAG effectively substitutes for PMA in these experiments. PS/DAG supports PKCδ-dependent cTnI-Ser23/Ser24 phosphorylation, in assays with PKCδ alone and mimics the effect of PMA to render PKCδ a substrate for Src, leading to PKCδ-Tyr311/Tyr332 phosphorylation and PKCδ-dependent cTnI-Thr144 phosphorylation in assays with Src.
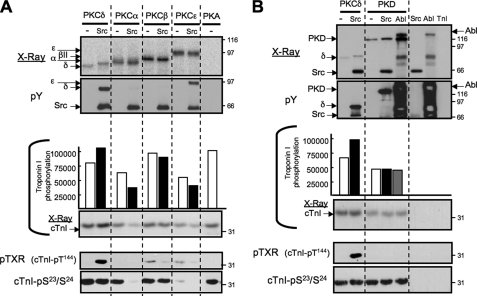
cTnI is a substrate for several physiologically and pathophysiologically relevant serine/threonine kinases that control contractile function, including other PKC isoforms, PKA, and PKD. We performed in vitro kinase assays with PKA, PKD, PKCα, PKCβII, PKCδ, and PKCε (under conditions calibrated to achieve similar levels of autophosphorylation) and used immunoblotting with anti-cTnI-Ser23/Ser24 and anti-TXR (cTnI-Thr144) PSSAs to determine whether Src regulates the cTnI kinase activity of these other enzymes. All of these enzymes increase 32P incorporation into cTnI, tracked by PhosphorImager analysis; immunoblotting studies show that PKCδ, PKCα, PKCβII, PKA, and PKD are all effective cTnI-Ser23/Ser24 kinases (Fig. 5). In contrast, PKCε promotes cTnI phosphorylation (detected by PhosphorImager), but PKCε is a relatively poor cTnI-Ser23/Ser24 kinase. Rather, PKCε and PKCβ are weak cTnI-Thr144 kinases; PKCα, PKD, PKA, and allosterically activated PKCδ (in assays without Src) do not phosphorylate cTnI at Thr144. Of note, PKCε- and PKCβII-dependent increases in cTnI-Thr144 phosphorylation are modest in magnitude compared with the robust increase in cTnI-Thr144 phosphorylation induced by Src-phosphorylated PKCδ. Moreover, the effect of Src to convert PKCδ into a cTnI-Thr144 kinase is quite specific. Although Src also tyrosine phosphorylates PKCε and PKD (but not PKCα or PKCβII), Src has no effect on (or actually decreases, in the case of PKCα) the cTnI kinase activities of these other enzymes (measured by PhosphorImager analysis or by immunoblotting with the anti-cTnI-pSer23/Ser24 PSSA). Src does not convert any of these other enzymes into effective cTnI-Thr144 kinases. Finally, because PKD also is a known substrate for c-Abl (19), c-Abl-dependent modulation of PKD activity also was considered. Fig. 5B shows that PKD undergoes an autophosphorylation reaction; 32P incorporation into PKD is increased slightly by Src (which promotes PKD tyrosine phosphorylation) and to an even greater extent by c-Abl (although the very high nonspecific anti-Tyr(P) immunoreactivity in assays with c-Abl alone thwarted efforts to directly track c-Abl-dependent PKD tyrosine phosphorylation). PKD does not acquire cTnI-Thr144 kinase activity when tyrosine-phosphorylated by Src or c-Abl.
FIGURE 5.
cTnI phosphorylation by PKC isoforms, PKA, and PKD. In vitro kinase assays performed with PKCδ, PKCα, PKCβII, PKCε, or PKA alone or with Src as indicated are depicted in A; assays with PKD (alone or with Src or c-Abl) and control assays with Src and c-Abl alone are depicted in B. All of the reactions contained PS/PMA. Autoradiograms and anti-Tyr(P) blots showing the positions of the various enzymes is on top; cTnI phosphorylation quantified by PhosphorImager analysis (with a representative autoradiogram depicted) and immunoblotting for cTnI-Ser23/Ser24 and Thr144 (detected as anti-pTXR) from a single experiments are illustrated at the bottom; the results were replicated in separate experiments.
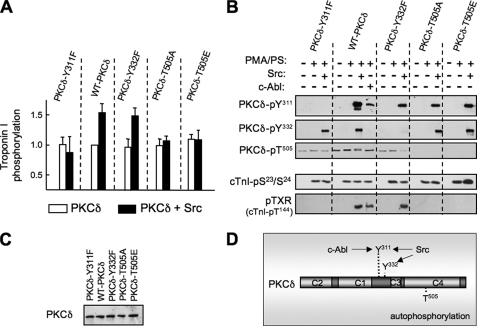
Src-dependent Changes in PKCδ Substrate Specificity Require Tyr311 and Thr505—We used a mutagenesis approach to explore the structural basis for Src-dependent changes in PKCδ substrate specificity. Fig. 6 shows that Tyr → Phe substitutions at Tyr311 or Tyr332 (that selectively abrogate Src-dependent PKCδ phosphorylation at the cognate tyrosine residues) have no effect on PKCδ-dependent cTnI-Ser23/Ser24 phosphorylation. However, the Tyr → Phe substitution at Tyr311 completely abrogates the Src-dependent increment in cTnI phosphorylation by PKCδ (detected by PhosphorImager) and cTnI-Thr144 phosphorylation (detected by immunoblot analysis). In contrast, a Tyr → Phe substitution at Tyr332 does not interfere with these activities.
FIGURE 6.
Tyr311 and Thr505 substitutions prevent Src-dependent changes in PKCδ substrate specificity. COS cells were transfected with plasmids that drive expression of WT and Y311F, Y332F, T505A, and T505E substituted forms of PKCδ fused to GFP. PKCδ was immunoprecipitated with anti-GFP, subjected to immunoblotting with anti-GFP to validate equal protein recovery (C), and subjected to immunocomplex kinase assays without and with lipid cofactor, Src, or c-Abl as indicated. cTnI phosphorylation was quantified by PhosphorImager (and is expressed relative to the basal level of phosphorylation for each PKCδ construct (A). PKCδ-Tyr311, -Tyr332, and -Thr505 phosphorylation and cTnI-Ser23/Ser24 and Thr144 phosphorylation were detected by immunoblot analysis according to the legend for B. The results were replicated in two separate experiments. D, a schematic that marks the various phosphorylation sites of PKCδ and the main kinases implicated in phosphorylating each site.
We used a similar mutagenesis approach to examine the role of activation loop phosphorylation. Although recombinant PKCδ (which displays at most trace Thr505 phosphorylation prior to in vitro kinase assays) undergoes pronounced Thr505 autophosphorylation during in vitro kinase assays (particularly in the presence of Src), WT-PKCδ, PKCδ-Y311F, and PKCδ-Y332F enzymes recovered from COS-7 cells exhibit similar levels of basal Thr505 phosphorylation that do not increase during in vitro kinase assays (with or without Src; compare Figs. 3A and 6). Basal PKCδ-Thr505 phosphorylation (for WT and Tyr → Phe substituted enzymes heterologously overexpressed in COS-7 cells) is presumed to be attributable to a phosphoinositide-dependent kinase-1-dependent mechanism that mediates activation loop phosphorylation in trans during de novo enzyme synthesis (and is not prevented by Y311F or Y332F mutations). T505A or T505E substitutions prevent activation loop phosphorylation, but they do not interfere with Src-dependent PKCδ-Tyr311 (or Tyr332) phosphorylation; T505A or T505E substitutions also do not alter overall cTnI phosphorylation (detected by 32P incorporation into cTnI) or cTnI-Ser23/Ser24 phosphorylation (detected by immunoblot analysis with the anti-cTnI-pSer23/Ser24 PSSA) in assays without Src. However, T505A and T505E substitutions abrogate the Src-dependent increment in PKCδ-dependent overall cTnI phosphorylation (detected by PhosphorImager) and the Src-dependent acquisition of cTnI-Thr144 kinase activity (detected with the anti-TXR PSSA). These results indicate that PKCδ is catalytically active without Thr505 phosphorylation, but that Thr505 phosphorylation is necessary (and that a phospho-mimetic substitution is not sufficient) for Src-dependent regulation of PKCδ substrate specificity. Collectively, these results indicate that Tyr311 and Thr505 cooperate in the Src-activated mechanism that alters the substrate specificity of PKCδ. The conclusion that the substrate specificity of PKCδ is influenced by a phosphorylation event at Tyr311, but not Tyr332, is supported further by studies with c-Abl, which selectively increases PKCδ phosphorylation at Tyr311 (not Tyr332) and supports PKCδ-dependent cTnI-Thr144 phosphorylation (Fig. 6).
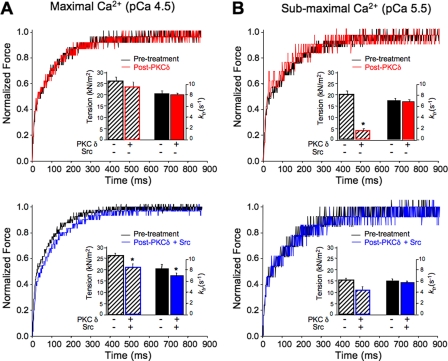
PKCδ Regulates Cardiac Contractile Function in a Src-dependent Manner—We performed mechanical studies on skinned (detergent extracted) ventricular myocytes (20) to determine whether Src (and tyrosine phosphorylation) alters PKCδ-dependent regulation of contraction. PKCδ alone reduces submaximal Ca2+-activated isometric tension (force/cross-sectional area of the myocyte at pCa 5.5), without changing maximal Ca2+-activated tension (at pCa 4.5) in skinned myocytes (Table 1 and Fig. 7). However, PKCδ does not alter cross-bridge cycling kinetics as reflected in the rate constant of force redevelopment (ktr) following a rapid release-restretch maneuver at either submaximum or maximum Ca2+ concentration. Control studies show that lipids (PS/PMA) and Src alone do not significantly alter mechanical function. These results are expected from previous data characterizing cTnI-Ser23/Ser24 phosphorylation as a modification that reduces submaximal tension but does not alter maximal Ca2+-activated tension or ktr (21, 22).
TABLE 1.
Summary of mechanical properties of detergent extracted (skinned) rat ventricular myocytes before and after kinase treatments The data are presented as the means ± S.E. Maximum tension and the rate constant of force redevelopment (ktr) were measured at pCa 4.5 in myocytes before and after treatment with PKCδ alone (n = 4) or with PKCδ in the presence of Src kinase (n = 5). Submaximal tension and ktr were measured at pCa 5.5.
|
[Ca2+]
|
PKCδ
|
Src + PKCδ
|
|||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Tension (kN/m2) | pCa 4.5 | 26.7 ± 1.0 | 23.4 ± 1.4 | 26.2 ± 0.6 | 20.4 ± 1.8a |
| ktr (s–1) | pCa 4.5 | 8.3 ± 0.2 | 8.1 ± 0.2 | 8.6 ± 0.3 | 7.4 ± 0.5a |
| Tension (kN/m2) | pCa 5.5 | 19.7 ± 1.2 | 3.3 ± 0.3a | 15.2 ± 2.0 | 10.6 ± 3.1 |
| ktr (s–1) | pCa 5.5 | 6.8 ± 0.7 | 6.7 ± 0.7 | 6.3 ± 0.3 | 6.0 ± 0.3 |
Significantly different from pre-treatment value as determined by paired Student t-test (p < 0.05)
FIGURE 7.
Mechanical properties of skinned cardiomyocyte preparations before and after PKCδ treatments at maximal and submaximal [Ca2+]. The traces show the recovery of force following a mechanical detachment of cross-bridges, to drop force to near-zero values. A, top panel, representative records used for the determination of ktr rates before (black) and after (red) PKCδ treatment demonstrate no change in rate of force recovery. Histograms (inset) summarize lack of effect of PKCδ on maximum Ca2+-activated parameters. A, bottom panel, force tracings demonstrate that Src (tyrosine-phosphorylated) PKCδ (blue) decreases the rate of recovery of force (i.e. return to equilibrium more slowly) and therefore ktr. Histograms of maximum Ca2+-activated tension and ktr values demonstrate significant decreases in these parameters in myocytes treated with Src (tyrosine-phosphorylated) PKCδ. B, top panel, tracings show no difference between control (black) and PKCδ (red) on the rate of force recovery at submaximal Ca2+, although submaximal tension production was significantly low (histogram). B, bottom panel, force tracings reflect a minor (although nearly significant p = 0.07) shift in force recovery with PKCδ pretreated with Src kinase (blue). In contrast to PKCδ alone, no significant change in submaximal tension production was observed (histogram, bottom). All of the measurements were performed in the presence of lipid activators PS/PMA. The results are reported as the means ± S.E. (* indicates p < 0.05).
The functional response to treatment with Src-phosphorylated PKCδ is quite different. PKCδ + Src lowers tension and the rate of force redevelopment at maximal but not submaximal Ca2+-activated tension in skinned myocytes (Table 1 and Fig. 7). These results are in agreement with previous studies linking cTnI-Thr144 phosphorylation to a decrease in maximum Ca2+-activated tension (12, 23). The observation that Src-phosphorylated PKCδ does not mimic the effect of PKCδ alone to lower submaximal Ca2+-activated isometric tension suggests that a Ser23/Ser24 phosphorylation-induced effect is attenuated when cTnI is simultaneously phosphorylated at Ser23/Ser24 and Thr144 (by Src-phosphorylated PKCδ). These mechanical studies provide a functional correlate to the in vitro biochemical studies and support the conclusion that when allosterically activated by PS/PMA PKCδ selectively phosphorylates cTnI at Ser23/Ser24 and leads to a fall in submaximal tension (without altering contractile function at maximal Ca2+) and that when it is prephosphorylated by Src PKCδ acquires an additional activity to phosphorylate cTnI at Thr144 (which prevents the Ser23/Ser24 phosphorylation-dependent decrease in submaximal Ca2+-activated tension and results in a decrease in force and rate of force redevelopment at maximal Ca2+).
Other laboratories, conducting similar mechanical studies, considered analogous changes in tension and ktr (as reported in Table 1) significant. Patel et al. (22) reported that PKA accelerates the rate of force development in murine skinned myocardium expressing α-or β-tropomyosin. They reported small but significant changes in the submaximal rate of force redevelopment after PKA treatments of non-transgenic myocardium with 100% α-tropomysin (increased by 15%) and trangenic myocardium with 60% β-tropomysin (increased by 18%) myocardium. Korte et al. (28) showed that loaded shortening, power output, and rate of force development (WT = 5.8 ± 0.9 s-1 versus MyBP-C-/- = 7.7 ± 1.7 s-1) are increased with knock-out of cardiac myosin binding protein-C. Moreover similar shifts (to those presented here) in Ca2+ sensitivity of force are closely related to events common in fatal human cardiomyopathies.
DISCUSSION
This study provides novel evidence that cTnI phosphorylation by PKCδ is controlled by Src and tyrosine phosphorylation. These observations are highly significant for several reasons. First, previous studies have implicated tyrosine phosphorylation as a mechanism that alters the activity of PKCδ, but there is still no consensus as to the precise nature of this regulatory control. Tyrosine phosphorylation has variably been implicated as a mechanism that increases or decreases PKCδ activity. Although there have been isolated reports that link tyrosine phosphorylation to changes in PKCδ activity toward only selected substrates, studies of substrate specificity have been confined to assays with peptides or proteins with little-to-no physiologic significance. The results reported herein link a tyrosine phosphorylation-dependent change in PKCδ activity to a physiologically important event in the cell, namely the phosphorylation of cTnI. Second, this study is the first to map tyrosine phosphorylation sites that control PKCδ activity. Our experiments implicate Tyr311 (which is a major target for Src-dependent phosphorylation during both stimulation by PMA and oxidative stress) and Thr505 (a site that is phosphorylated via an autocatalytic reaction that is increased by Src) in this form of regulatory control. In contrast, Tyr332 (which also is phosphorylated by Src during oxidative stress but is not a substrate for c-Abl and is not phosphorylated during stimulation with PMA) does not mediate Src-dependent changes in PKCδ substrate specificity. Third, an effect of PKCδ-Tyr311/Thr505 phosphorylation to regulate the phosphorylation of only selected sites on a single substrate is unprecedented in the literature. Post-translational modifications typically induce more general changes in enzyme activity measured as a change in maximal catalytic activity (Kcat) or affinity for substrate or ATP (Km). Although a recent study using a proteomic approach provided intriguing hints that activation loop phosphorylation can influence PKCδ substrate specificity (24), a post-translational modification that selectively regulates the phosphorylation of selected sites on a single substrate has not previously been identified.
The role of PKA-dependent cTnI phosphorylation at Ser23/Ser24 in the control of cardiac dynamics is well documented (10). Although PKC phosphorylation of cTnI was originally mapped to Ser43/Ser45 and Thr144, recent studies also implicate Ser23/Ser24 as an alternate (and perhaps more important) phosphorylation site for certain PKC isoforms (and PKC-activated effectors). Our results also show that PKCα, PKCβ, PKCδ, and PKD act in a similar manner to phosphorylate cTnI at Ser23/Ser24; only PKCε is uniquely identified as a poor cTnI-Ser23/Ser24 kinase. We also link PKCδ-dependent cTnI-Ser23/Ser24 phosphorylation to a decrease in force development at submaximal Ca2+, but no change in maximal Ca2+-activated tension or cross-bridge cycling kinetics in skinned cardiomyocytes (the predicted response based upon studies examining the regulatory actions of PKA, the prototypical cTnI-Ser23/Ser24 kinase).
The data summarized in Table 1 show that at submaximal Ca2+ concentrations Ser23/Ser24 phosphorylation leads to an 83% fall in tension (from 19.7 ± 1.2 to 3.3 ± 0.3 kN/m2). This significant change in tension is in agreement with previous studies detailing the effect of PKA-dependent phosphorylation of cTnI-Ser23/Ser24 on cardiac muscle. The Ser23/Ser24 phosphorylation effect on tension (3.3 ± 0.3 kN/m2) is largely reversed under oxidative stress conditions through additional phosphorylation of Thr144 (10.6 ± 3.1 kN/m2). This 68% increase in tension indicates improved sarcomeric Ca2+ responsiveness.
Allosterically activated PKCδ is not a cTnI-Thr144 kinase. In fact, whereas cTnI-Thr144 phosphorylation originally was attributed to PKCs, the physiologically relevant cTnI-Thr144 kinase (and the functional consequences of cTnI-Thr144 phosphorylation) remains uncertain. cTnI-Thr144 phosphorylation mechanisms have largely been inferred from studies with T144A or T144E-substituted cTnI mutants, because a PSSA that directly tracks cTnI-Thr144 phosphorylation (similar to the cTnI-pSer23/Ser24 PSSA) is not available. The identification of the anti-pTXR phospho-motif antibody as a reagent that can be used to track cTnI-Thr144 phosphorylation in immunoblotting experiments allowed a comparison of cTnI-Thr144 phosphorylation by individual PKC isoforms that was not previously possible. Our studies identify a low level of cTnI-Thr144 phosphorylation by PKCβ and PKCε (consistent with recent studies that attribute cTnI-Thr144 phosphorylation to PKC-βII (14)) but no cTnI-Thr144 phosphorylation by related kinases such as PKCα, PKA, PKD, and allosterically activated PKCδ (i.e. the form of PKCδ that is anchored to membranes by lipid cofactors). In contrast, tyrosine-phosphorylated PKCδ is a robust cTnI-Thr144 kinase. These results suggest that tyrosine-phosphorylated PKCδ is the most physiologically relevant cTnI-Thr144 kinase and that cTnI-Thr144 phosphorylation would be most prominent in vivo in conditions such as oxidative stress (where activation of Src leads to PKCδ tyrosine phosphorylation). Current concepts regarding the functional consequences of cTnI-Thr144 phosphorylation are based largely on studies that use mutagenesis approaches or genetic models, with evidence that cTnI-Thr144 phosphorylation accelerates myofilament relaxation in adult rat myocytes, decreases Ca2+ sensitivity in sliding filament assays, or sensitizes myofilaments to Ca2+ (without changing maximum tension) in skinned myocytes from transgenic mice that express the cTnI-Ser23/Ser24A mutant (12–14). Importantly, the studies reported herein examine the physiologic consequences of a coordinate increase in cTnI phosphorylation at Ser23/Ser24 and Thr144 induced by Src-phosphorylated PKCδ. Here, the Ca2+-desensitizing effect of PKCδ alone is no longer detected. Rather, PKCδ + Src lowers tension and the rate of force redevelopment at maximal Ca2+, an effect that is predicted to contribute to oxidative stress-induced myocardial dysfunction. On the other hand, at submaximal Ca2+ concentrations, where the myocardium typically operates, oxidative stress events that activate Src-PKCδ signaling pathways could enhance cardiac function. This effect on one hand might be beneficial in maintaining systolic contractile function of the failing myocardium. On the other hand this might be detrimental during β-adrenergic stimulation hindering the expected increase of relaxation rate. These data underscore the importance of understanding events that modulate troponin I phosphorylation and ultimately fine-tune sarcomeric function.
Recent NMR data suggest that cTnI phosphorylation at Ser23/Ser24 weakens the interaction of the cTnI N-terminal residues 1–30 with cTnC, induces a lever-like bending around residues 33–42, and facilitates its repositioning to bind the inhibitory region of cTnI (9). This association between N-terminal and inhibitory regions of cTnI is stabilized by favorable electrostatic interactions between an acidic patch consisting of residues Asp3, Glu4, Asp7, Glu11, and a basic patch Arg142, Arg146, and Arg149, surrounding Thr144. Interestingly, the R146G mutation (linked to familial hypertrophic cardiomyopathy in humans) leads to myofilaments with increased Ca2+ sensitivity and lack of responsiveness to phosphorylation at Ser23/Ser24 (25, 26). In the case of the R146G mutant, replacement of an Arg with Gly presumably induces its effect by disrupting the electrostatic interactions between the acidic N terminus and the inhibitory region of cTnI. In our case, the addition of a bulky, negatively charged phosphate group to the basic patch, through Thr144 phosphorylation, could induce a similar effect. Our mechanical data are consistent with this model, because Thr144 phosphorylation alleviates the Ca2+-desensitizing effect of Ser23/Ser24 phosphorylation. Our data further suggest that phosphorylation at Thr144 (on a Ser23/Ser24 phosphorylation background) prevents the cTnI inhibitory region from properly interacting with actin-tropomyosin, leading to a decrease in maximum force.
Collectively, our studies identify distinct signaling modes for PKCδ, a single PKC isoform whose substrate specificity can be dynamically regulated through tyrosine phosphorylation. This study focuses on a dual role for PKCδ as both an allosterically activated and a tyrosine-phosphorylated enzyme leading to the phosphorylation of functionally distinct sites on cTnI, potentially underlying differences in PKC-dependent regulation of myofilament contraction in the normal heart and in the context of diseases associated with oxidative stress. This novel tyrosine phosphorylation-dependent change in PKCδ substrate specificity is likely to represent a general regulatory mechanism that also influences PKCδ phosphorylation of other cellular substrates and might be targeted in the future for therapeutic advantage.
This work was supported, in whole or in part, by National Institutes of Health, United States Public Service Grants HL 77860, HL 62426, HL 64035, HL 71865, T32 007692, and AHA-SDG 0335199N. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PKC, protein kinase C; cTnI, cardiac troponin I; GFP, green fluorescent protein; EGFP, enhanced GFP; PMA, phorbol 12-myristate 13-acetate; PKA, cAMP-dependent protein kinase; PS, phosphatidylserine; HPLC, high pressure liquid chromatography; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; WT, wild type; PSSA, phospho site-specific antibody; DAG, diacylglycerol; PKD, protein kinase D.
References
- 1.Steinberg, S. F. (2004) Biochem. J. 384 449-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitaler, M., and Cantrell, D. A. (2004) Nat. Immunol. 5 785-790 [DOI] [PubMed] [Google Scholar]
- 3.Rybin, V. O., Sabri, A., Short, J., Braz, J. C., Molkentin, J. D., and Steinberg, S. F. (2003) J. Biol. Chem. 278 14555-14564 [DOI] [PubMed] [Google Scholar]
- 4.Rybin, V. O., Guo, J., Gertsberg, Z., Elouardighi, H., and Steinberg, S. F. (2007) J. Biol. Chem. 282 23631-23638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton, A. C. (2004) Trends Pharmacol. Sci. 25 175-177 [DOI] [PubMed] [Google Scholar]
- 6.Rybin, V. O., Guo, J., Sabri, A., Elouardighi, H., Schaefer, E., and Steinberg, S. F. (2004) J. Biol. Chem. 279 19350-19361 [DOI] [PubMed] [Google Scholar]
- 7.Dorn, G. W., and Force, T. (2005) J. Clin. Investig. 115 527-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang, X., Pi, Y., Lee, K. J., Henkel, A. S., Gregg, R. G., Powers, P. A., and Walker, J. W. (1999) Circ. Res. 84 1-8 [DOI] [PubMed] [Google Scholar]
- 9.Howarth, J. W., Meller, J., Solaro, R. J., Trewhella, J., and Rosevear, P. R. (2007) J. Mol. Biol. 373 706-722 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi, T., and Solaro, R. J. (2005) Annu. Rev. Physiol. 67 39-67 [DOI] [PubMed] [Google Scholar]
- 11.Metzger, J. M., and Westfall, M. V. (2004) Circ. Res. 94 146-158 [DOI] [PubMed] [Google Scholar]
- 12.Burkart, E. M., Sumandea, M. P., Kobayashi, T., Nili, M., Martin, A. F., Homsher, E., and Solaro, R. J. (2003) J. Biol. Chem. 278 11265-11272 [DOI] [PubMed] [Google Scholar]
- 13.Westfall, M. V., Lee, A. M., and Robinson, D. A. (2005) J. Biol. Chem. 280 41324-41331 [DOI] [PubMed] [Google Scholar]
- 14.Wang, H., Grant, J. E., Doede, C. M., Sadayappan, S., Robbins, J., and Walker, J. W. (2006) J. Mol. Cell Cardiol. 41 823-833 [DOI] [PubMed] [Google Scholar]
- 15.Sumandea, M. P., Burkart, E. M., Kobayashi, T., de Tombe, P. P., and Solaro, R. J. (2004) Ann. N. Y. Acad. Sci. 1015 39-52 [DOI] [PubMed] [Google Scholar]
- 16.Lewinter, M. M., and Vanburen, P. (2002) J. Card Fail 8 S271-S275 [DOI] [PubMed] [Google Scholar]
- 17.Gopalakrishna, R., and Jaken, S. (2000) Free Radic. Biol. Med. 28 1349-1361 [DOI] [PubMed] [Google Scholar]
- 18.Stahelin, R. V., Digman, M. A., Medkova, M., Ananthanarayanan, B., Rafter, J. D., Melowic, H. R., and Cho, W. (2004) J. Biol. Chem. 279 29501-29512 [DOI] [PubMed] [Google Scholar]
- 19.Storz, P., Doppler, H., and Toker, A. (2004) Mol. Cell Biol. 24 2614-2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinken, A. C., and McDonald, K. S. (2004) Am. J. Physiol. 287 C500-C507 [DOI] [PubMed] [Google Scholar]
- 21.Pi, Y., Zhang, D., Kemnitz, K. R., Wang, H., and Walker, J. W. (2003) J. Physiol. 552 845-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel, J. R., Fitzsimons, D. P., Buck, S. H., Muthuchamy, M., Wieczorek, D. F., and Moss, R. L. (2001) Am. J. Physiol. 280 H2732-H2739 [DOI] [PubMed] [Google Scholar]
- 23.Noland, T. A., Guo, X., Raynor, R. L., Jideama, N. M., Averyhart-Fullard, V., Solaro, R. J., and Kuo, J. F. (1995) J. Biol. Chem. 270 25445-25454 [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y., Belkina, N. V., Graham, C., and Shaw, S. (2006) J. Biol. Chem. 281 12102-12111 [DOI] [PubMed] [Google Scholar]
- 25.Gomes, A. V., and Potter, J. D. (2004) Mol. Cell Biochem. 263 99-114 [DOI] [PubMed] [Google Scholar]
- 26.Kruger, M., Zittrich, S., Redwood, C., Blaudeck, N., James, J., Robbins, J., Pfitzer, G., and Stehle, R. (2005) J. Physiol. 564 347-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolska, B. M., Kitada, Y., Palmiter, K. A., Westfall, M. V., Johnson, M. D., and Solaro, R. J. (1996) Am. J. Physiol. 270 H24-H32 [DOI] [PubMed] [Google Scholar]
- 28.Korte, F. S., McDonald, K. S., Harris, S. P., and Moss, R. L. (2003) Circ. Res. 93 752-758 [DOI] [PubMed] [Google Scholar]