Abstract
Human respiratory syncytial virus (RSV) constitutes a highly pathogenic virus that infects lung epithelial cells to cause a wide spectrum of respiratory diseases. Our recent studies have revealed the existence of an interferon-α/β-independent, innate antiviral response against RSV that was dependent on activation of NF-κB. We demonstrated that NF-κB inducing pro-inflammatory cytokines like tumor necrosis factor-α (TNF) confers potent antiviral function against RSV in an NF-κB-dependent fashion, independent of interferon-α/β. During our efforts to study this pathway, we identified HBD2 (human β-defensin-2), a soluble secreted cationic protein as an antiviral factor induced during NF-κB-dependent innate antiviral activity in human lung epithelial cells. Our results demonstrated that HBD2 is induced by TNF and RSV in an NF-κB-dependent manner. Induction of HBD2 in infected cells was mediated by the paracrine/autocrine action of TNF produced upon RSV infection. HBD2 plays a critical role during host defense, because purified HBD2 drastically inhibited RSV infection. We also show that the antiviral mechanism of HBD2 involves blocking of viral cellular entry possibly because of destabilization/disintegration of the viral envelope. The important role of HBD2 in the innate response was also evident from loss of antiviral activity of TNF upon HBD2 silencing by short interfering RNA. The in vivo physiological relevance of HBD2 in host defense was apparent from induction of murine β-defensin-4 (murine counterpart of HBD2) in lung tissues of RSV-infected mice. Thus, HBD2 functions as an antiviral molecule during NF-κB-dependent innate antiviral immunity mediated by the autocrine/paracrine action of TNF.
Nonsegmented negative strand RNA viruses (superfamily Mononegaviridae) constitute highly pathogenic human viruses that cause high morbidity. Human respiratory syncytial virus (RSV),2 a nonsegmented negative strand virus, is an important human lung tropic respiratory tract pathogen causing high morbidity and mortality among infants, children, and elderly by manifesting disease states, including pneumonia, and bronchiolitis (1, 2). To date, no effective vaccine or antiviral therapy exists for RSV. Therefore, elucidation of innate immune antiviral response induced by RSV holds significant potential for development of effective antiviral therapies in the near future.
The innate immune antiviral response against viruses represents an important host defense mechanism (3). Innate immunity includes the first line of defense by the host to combat virus infection before an orchestrated adaptive immune response involving immune cell priming, and antibody production is launched. Two key molecules regulating the innate antiviral function are interferon regulatory factors (IRFs) and NF-κB (4). These two transcription factors are activated either individually or together in infected cells, resulting in the expression and production of interferon-α/β (IFN), which are potent antiviral cytokines (5, 6). IFN produced from infected cells binds to their cognate IFN receptors on uninfected cells to induce the JAK/STAT antiviral pathway. Thus the paracrine action of IFN is absolutely critical during innate antiviral defense (4–6). Although the paracrine action of IFNs plays a critical role in innate immune antiviral response, we have recently identified an IFN-independent antiviral pathway that was directly dependent on NF-κB activation (7).
We demonstrated that similar to the potent antiviral action of IFN, pretreatment of cells with NF-κB inducing cytokines, tumor necrosis factor-α (TNF), and interleukin-1β (IL-1β) resulted in drastic inhibition of RSV replication (7). The cytokine-mediated antiviral action was directly dependent on NF-κB activation, because inhibition of NF-κB induction abolished the antiviral property of TNF and IL-1β. Moreover, we found that NF-κB-dependent antiviral action against RSV was independent of IFN and the IFN-induced JAK/STAT pathway (7). These results have suggested that NF-κB-dependent antiviral factor(s) are produced (independent of IFN-induced JAK/STAT pathway) by TNF and IL-1β. In an effort to better understand the NF-κB-dependent innate antiviral pathway, it is important to identify the antiviral factor(s) induced by TNF in an NF-κB-dependent fashion because of the following: (a) apart from our studies, a protective role of TNF in RSV infection in vitro and in vivo (mouse model) has been reported previously (8, 9); (b) RSV is nonresponsive to the antiviral action of IFN and fails to induce/express IRF3/IFN in lung epithelial cells; and (c) administration of IFN to human infants with established RSV infection has not been associated with a reduced severity of illness (10). Toward that end, we have identified human β-defensin-2 (HBD2 is a cysteine-rich protein belonging to the β-defensin family of peptides possessing antimicrobial activity) (11–13) as an anti-RSV molecule that is induced during the NF-κB-dependent innate immune response in human lung epithelial cells.
We show induction of HBD2 by RSV and TNF in human lung epithelial A549 cells, the cells utilized by RSV during the productive infection of human host. HBD2 expression was dependent on NF-κB activation and autocrine/paracrine action of TNF produced from RSV-infected cells. The induction of HBD2 is important for innate antiviral response, because HBD2 possessed antiviral activity against RSV. The antiviral mechanism constitutes disruption of lipid bilayer membrane of the virion envelope leading to inhibition in RSV cellular entry. The importance of HBD2 during innate response was further established by observing loss of antiviral activity of TNF following silencing of the HBD2 gene in A549 cells. The in vivo relevance of HBD2 induction was also validated in RSV-infected mice, which expressed high levels of murine β-defensin-4 or mBD4 (the murine counterpart of HBD2) in lung tissues following RSV infection. Our current studies have therefore identified HBD2 as an anti-RSV factor and demonstrated the importance of HBD2 during NF-κB-dependent innate antiviral response.
EXPERIMENTAL PROCEDURES
Virus and Cells—RSV (A2 strain) and adenoviruses (expressing either GFP or IκB-super-repressor) were propagated in CV-1 cells and 293 cells, respectively (7, 14, 15). RSV was purified by centrifugation on discontinuous sucrose gradients as described previously (16). Human lung epithelial A549 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, and glutamine. The RSV titer was monitored by plaque assay analysis with CV-1 cells as described earlier (7, 14, 15). Vaccinia virus (WR strain) was propagated as described earlier (17).
RNA Isolation, PCR Amplification, and Reverse Transcription (RT)-PCR—Total RNA isolation from cells was performed using a monophasic solution of phenol and guanidine thiocyanate (TRIzol) (Sigma), as recommended by the suppliers. Similarly, lung tissue obtained from mock-infected and RSV-infected mice was directly homogenized in TRIzol reagent. Total cellular RNA (∼1 μg) was used to generate cDNA using Moloney murine leukemia virus reverse transcriptase (Applied Biosystems). PCR was routinely performed using 0.25 units of Taq polymerase, 10 pmol of each oligonucleotide primer, 1 mm MgCl2, and 100 μm deoxynucleotide triphosphates in a final reaction volume of 25 μl. The amplification cycle was as follows. An initial denaturing step (95 °C for 3–5 min) was followed by either 25, 30, or 35 cycles of denaturing (94 °C for 30 s), annealing (60 °C for 30 s), and extending (72 °C for 30 s), followed by either 5 or 10 min at 72 °C for elongation. Following amplification, the PCR products were analyzed on 2% agarose gels, and band intensities were quantified by densitometry (Syngene gel documentation system). Equal loading in each well was confirmed by analyzing expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used to detect the indicated genes by RT-PCR are shown as follows: HBD1-forward (5′–3′) GATCATTACAATTGCGTCAGCAGTGG, and reverse (5′–3′) CTCACTTGCAGCACTTGGCCTTC (product size 111 bp); HBD2-forward (5′–3′) GGTATAGGCGATCCTGTTACCTGC, and reverse (5′-3′) TCATTGGCTTTTTTGCAGCATTTTGTTC (product size 126 bp); GAPDH-forward (5′–3′) GTCAGTGGTGGACCTGACCT, and reverse (5′–3′) AGGGGTCTACATGGCAACTG (product size 421 bp); mBD3-forward (5′–3′) GCATTGGCAACACTCGTCAGA, and reverse (5′–3′) CGGGATCTTGGTCTTCTCTA (product size 85 bp); mBD4-forward (5′–3′) GCAGCCTTTACCCAAATTATC, and reverse (5′–3′) ACAATTGCCAATCTGTCGAA (product size 102 bp).
Real Time Quantitative RT-PCR (qPCR)—RNA was extracted from A549 cells (infected/treated with RSV or TNF) using TRIzol. Human keratin 5 RNA was used to normalize RNA content in each preparation. Intron-spanning primers and PCR conditions utilized for these reactions have been described previously (18, 19). Each 25-μl PCR mixture consisted of 125 ng of RNA, primers (0.4 μm each), 0.4 mm dNTPs, 5mm MgCl2, a mixture of reverse transcriptase and Taq DNA polymerase, 1× PCR buffer, RNase inhibitor (5 units), and SYBR Green dye diluted 1:2500. Standard curves were constructed using RNA generated by transcribing HBD1 or HBD2 plasmids using the RiboProbe in vitro transcription system (Promega). Concentration of mRNAs was determined by spectrometry at 260 nm. Single stock solutions of serial dilutions from 107 to 10 RNA copies were prepared and stored at –80 °C. All real time RT-PCR amplifications, data acquisition, and analysis were performed using the Smart Cycler System, software version 1.2d (Cepheid, Sunnyvale, CA).
Cell Surface Biotinylation—A549 cells incubated with 1 μgof HBD2 for 0–24 h were washed gently with PBS prior to cell surface biotinylation (20). Cells were chilled at 4 °C for 2 h and were washed once with chilled 1× PBS, and NHS-Biotin (Pierce) (250 μg/ml) was added to the washed cells in the presence of cold 1× PBS. The cells were incubated with biotin at 4 °C for 90 min with the addition of fresh biotin after the first 45 min. The biotinylation reaction was quenched following the washing of the cells (four times, 10 min each, 4 °C) with 1× PBS (cold) containing 100 mm glycine. The cells were then washed twice with 1× PBS and lysed. Avidin conjugated to agarose beads was added to the cell lysates, and following 16 h of incubation at 4 °C, the beads were washed extensively with 1× PBS, and SDS-PAGE sample buffer was directly added to the washed beads for Western blot analysis with anti-HBD2 antibody (Abcam, Cambridge, MA).
Attachment and Internalization of 35S-Methionine-labeled RSV (35S-RSV)—Radiolabeled 35S-RSV was prepared and purified as described previously (14, 16, 20, 21). The 35S-RSV attachment and internalization assays were performed as described previously for human parainfluenza virus type 3 (14, 20, 21). The attachment kinetics of 35S-RSV was examined by adding 0.25 m.o.i. (1.5 × 105 cpm), 0.5 m.o.i. (3 × 105 cpm), 1 m.o.i. (6 × 105 cpm), and 2 m.o.i. (12 × 105 cpm) of 35S-RSV to chilled A549 cells. Following 2 h of incubation at 4 °C (the temperature that supports attachment but not internalization), the cells were washed extensively with chilled 1× PBS, and the cell lysate (the cell-associated radioactivity representing the attached 35S-RSV) was counted with a scintillation counter.
For the attachment assay in the presence of HBDs, A549 cells were incubated with HBDs (4 μg/ml) for 3 h at 4 °C. The cells were then washed gently (only once) with chilled PBS, and 35S-RSV (1 m.o.i. = 6 × 105 cpm) was added (in the absence of HBDs). After 2 h at 4°C, the cells were washed extensively with chilled 1× PBS; the washed cells were lysed, and the lysate radioactivity representing the attached 35S-RSV was counted with a scintillation counter. Similarly, 35S-RSV was preincubated with HBDs for 2 h at 37 °C. The virus was then added to chilled A549 cells, and following 2 h of incubation (at 4 °C), the cell lysate was analyzed for cell-associated radioactivity.
For the internalization assay (14, 20, 21), the cells were treated similarly to the attachment assay described above, but following attachment of 35S-RSV (1 m.o.i. = 6 × 105 cpm) for 2 h, the cells were washed with chilled PBS, and the temperature was shifted to 37 °C to allow internalization of the attached virus. At 0.5-, 1-, and 2-h post-internalization time frames, cells were washed extensively with 1× PBS and trypsinized for 15 min at 37 °C to remove cell surface-attached viruses as described previously (14, 20, 21). The protease activity was neutralized with complete Dulbecco's modified Eagle's medium, and the cells were washed twice with 1× PBS. The washed cells were lysed, and the lysates representing the internalized 35S-RSV were counted (counts/min) with a scintillation counter. A similar internalization assay was performed with 35S-RSV preincubated with HBDs.
For the assays described above, the background counts were determined by counting cell lysates obtained from cells not incubated with 35S-RSV, and the background was subtracted from all of the counts derived from cells incubated with the radiolabeled virus. The efficiency of attachment, expressed as a percentage of attachment, was calculated based on a ratio that is detailed in the applicable legend.
ELISA to Detect HBD2—A549 cells treated/infected with TNF and RSV for 0–16 h were used to prepare cell lysate and collect medium supernatants. The amount of HBD2 protein in cell lysates (cell-associated) and culture supernatant was analyzed by HBD2-specific ELISA kit (Phoenix Pharmaceuticals, Burlingame, CA). The ELISA was performed according to the manufacturer's specifications, and the results were expressed as nanograms/ml of HBD2. In another experiment, 1 μg of purified HBD2 was added (exogenously) to each well of A549 cells, and following 0–16 h of incubation, the medium supernatants and cell lysates were used to quantify cell-associated versus soluble HBD2 by ELISA analysis.
Virus Infection—To study the antiviral function of TNF and HBD2, A549 cells were pretreated with either TNF (10 ng/ml for 20 h) or purified HBDs (0–6 μg/ml for 2 h). Following incubation, purified RSV (0.2 m.o.i.) (wild type or RSV expressing GFP) was added to the washed cells, and the adsorption was continued for 1.5 h at 37 °C in the presence of TNF or HBDs. The cells were then washed to remove unbound viruses, and the infection was continued for an additional 36 h in the absence of TNF or HBDs. Thirty six hours post-infection, the culture supernatants were collected to measure virus yield by plaque assay analysis. Similarly, RSV (0.2 m.o.i.) preincubated with HBDs (0–6 μg/ml for 2 h) were added to A549 cells and the infection proceeded as described above. For experiments with GFP-RSV, the GFP expression was monitored by fluorescence microscopy. The homogeneous preparations of purified HBDs utilized in these studies were either obtained as a gift from Dr. Miguel E. Quinones-Mateu (Cleveland Clinic Foundation, OH), who used them to study anti-HIV-1 activity (22), or purchased from PeproTech (Rocky Hill, NJ). For the HBD2 studies, serum-free Dulbecco's modified Eagle's medium was used to treat cells and RSV.
Adenoviruses expressing GFP (control) or IκB-super repressor (IκB-SR) (2 m.o.i.) was used to infect A549 cells (7). At 48 h post-adenovirus infection, RSV (0.5 m.o.i.) was added to cells to measure HBD2 expression at 16 and 24 h post-infection. Similarly, UV-inactivated RSV was used to analyze HBD2 expression at 16 h post-infection.
The antiviral effect of HBD2 against vaccinia virus (WR strain) was tested by incubating 10 μg/ml HBD2 with the virus for 3 h at 37 °C, followed by addition of the virus to Vero cells (17). The cells were overlaid with methylcellulose, and the plaques were visualized after crystal violet staining. As a control, virus was also treated similarly with PBS and neutralizing antibody against vaccinia virus.
Treatment with TNF-neutralizing Antibody—A549 cells were infected with RSV in the presence of either control antibody or TNF-specific neutralizing antibody (600 ng/ml) (R & D Systems) (7). The expression of HBD2 was studied at 36 h post-infection.
Treatment of Cells with siRNAs—siRNAs against HBD2 and HBD1 were designed based on the siRNA designing program provided on line by Qiagen. During these experiments, scrambled siRNA (control) served as a negative control. The high pressure liquid chromatography-purified siRNAs (purchased from Qiagen) were transfected using the siRNA transfection kit (Qiagen) according to the manufacturer's protocol. At 36 h post-transfection, cells were treated with TNF (for 8 and 16 h), and HBD2 expression was monitored by RT-PCR. Similarly, HBD1 expression was monitored in cells transfected with control and HBD1 siRNA. In some experiments, at 36 h post-transfection, cells were treated with TNF for 20 h, followed by RSV infection for 36 h. Viral titer in HBD2, HBD1, and control siRNA transfected cells (±TNF) was monitored by plaque assay.
Transmission Electron Microscopic Analysis of Virion Particles—Virus stock was concentrated by ultracentrifugation at 40,000 rpm at 4 °C for 1 h. The medium was removed, and the pellet was washed with 1.0 ml of 0.01× TSB, 10 mm phosphate buffer and spun again. The final pellet was resuspended in a 0.2-ml final volume in buffer containing peptides (HBD1 and HBD2) at final concentrations of 6 μg/ml. The mixture was incubated at 37 °C for 8 h, followed by the addition of an equal volume of 3% glutaraldehyde. The samples were stored at 4 °C until transmission electron microscopy (23, 24) was performed with Phillip's CM-10 (University of Texas Health Science Center, San Antonio, Electron Microscope Core Facility).
Intra-nasal Infection of Mice with RSV—Female, 7–8-week-old pathogen-free BALB/c mice (Charles River Laboratories, Wilmington, MA) were anesthetized using inhaled methoxyfluorane and intranasally inoculated with 105 pfu/animal of purified RSV in 100 μl of Opti-MEM (25). Uninfected control animals were sham-inoculated with 100 μl of Opti-MEM. Animals were allowed 30 s to aspirate the inoculum while held upright until fully recovered from the anesthesia. Mice were anesthetized with an intraperitoneal injection of ketamine and acepromazine before euthanasia by exsanguination. Whole lung samples were collected for determination of defensin expression. Specimens were obtained on days 0 (within 2 h of inoculation) and 24, 48, and 96 h post-infection. Lungs were homogenized in TRIzol with a tissue homogenizer to collect total RNA. RT-PCR analysis with the RNA samples was performed to examine expression of murine β-defensin-3 (mBD3) and -4 (mBD4).
Cesium Chloride (CsCl) Gradient Centrifugation—CsCl solutions were prepared with 1 g of CsCl per g of Tris buffer, and 100 μl of 35S-RSV (HBD1- or HBD2-treated virus) was layered on top of 10 ml of this solution. Following centrifugation (using SW41 rotor) for 36 h at 40,000 rpm, 0.7-ml fractions were collected. Aliquots of each fraction were used for measuring radioactivity (cpm corresponding to 35S-RSV) by scintillation counter and density as described earlier (26).
RESULTS
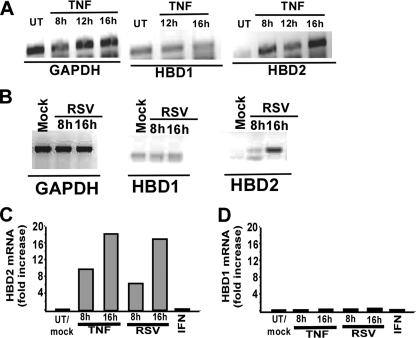
Induction of HBD2 by RSV and TNF—Our focus on HBD2 as a candidate NF-κB-dependent (IFN-independent) antiviral factor was aroused from several known properties of HBD2, including the following: (a) requirement of NF-κB for HBD2 induction, especially by NF-κB inducing cytokines like TNF and IL-1β (27, 28); (b) inducible expression of HBD2 in airway cells (29, 30); (c) potent antiviral activity of defensins (including β-defensins like HBD2) against HIV-1 and herpes simplex virus (HSV) (22, 31–35); and (d) requirement of NF-κB binding to its consensus elements in the promoter region of the HBD2 gene for HBD2 gene expression (36, 37). Based on these earlier studies, we rationalized that HBD2 may constitute an NF-κB-dependent antiviral gene. To study the innate antiviral property of HBD2, we first examined the ability of RSV and TNF to induce HBD2 in human lung epithelial A549 cells. Treatment of cells with TNF (10 ng/ml) for 8–16 h led to induction of HBD2 as assessed by RT-PCR analysis (Fig. 1A). In contrast to HBD2, induction of HBD1 was not observed, because it was expressed constitutively. Similar to TNF, RSV (0.5 m.o.i.) also induced HBD2 at 8–16 h post-infection, although it failed to induce HBD1 (Fig. 1B). The RT-PCR results were further confirmed by real time qPCR analysis, which revealed induction of HBD2 (but not HBD1) by both RSV (0.5 m.o.i.) and TNF (10 ng/ml) (Fig. 1, C and D). In contrast to TNF, interferon-α (IFN-α) failed to induce HBD2 in A549 cells (Fig. 1, C and D). We also observed significant induction of HBD2 with low concentrations of TNF (0.5–1 ng/ml) (data not shown). These results demonstrated the ability of RSV and TNF to efficiently induce HBD2 in A549 cells.
FIGURE 1.
Induction of HBD2 by TNF and RSV. A, total RNA collected from untreated (UT) and TNF-treated (10 ng/ml TNF treatment for 8–16 h) A549 cells were subjected to RT-PCR to detect HBD1, HBD2, and GAPDH (loading control). B, total RNA collected from mock-infected and RSV-infected (0.5 m.o.i. for 8–16 h) A549 cells were subjected to RT-PCR to detect HBD1, HBD2, and GAPDH (loading control). C, total RNA collected from untreated/mock-treated and RSV-infected (0.5 m.o.i. RSV infection for 8–16 h), TNF-treated (10 ng/ml for 8–16 h), and interferon-α-treated A549 cells (cells were incubated with 2000 units/ml IFN-α for 16 h) were subjected to real time qPCR to detect HBD2 expression. D, total RNA collected from untreated/mock-treated, RSV-infected (0.5 m.o.i. RSV infection for 8–16 h), TNF-treated (10 ng/ml for 8–16 h), and IFN-treated A549 cells were subjected to qPCR to detect HBD1 expression. For the qPCR, the transcript levels were normalized to human keratin 5, and the results are representative of three independent experiments with similar values.
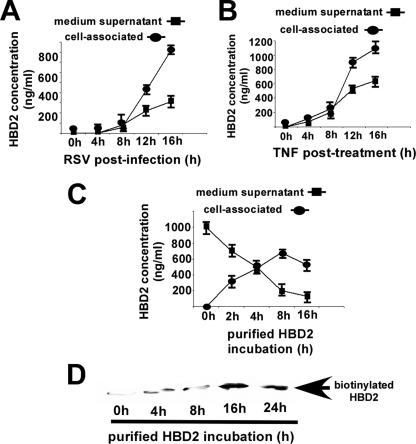
RSV- and TNF-mediated Expression of Cell-associated and Secreted HBD2 Protein—The results in Fig. 1 showed the ability of RSV and TNF to induce HBD2 gene, which prompted us to investigate the HBD2 protein status following TNF treatment and RSV infection. Previous studies have demonstrated that β-defensins (including HBD2) are secreted into the cultured supernatant following induction (38). To examine the localization (intracellular versus extracellular) of induced HBD2 protein, cells were treated/infected with TNF or RSV for 0–16 h. At each time point, the medium supernatant and cell lysate were collected to analyze HBD2 protein by ELISA (Fig. 2, A and B). It is important to note that the cell lysates were obtained following only one gentle wash, because we were also interested in quantifying the amount of secreted HBD2 that may be bound (via weak noncovalent interaction) to the cell surface. Surprisingly, we found that with increasing treatment/infection time periods, the majority of HBD2 is cell-associated compared with its presence in cultured medium (Fig. 2, A and B). The presence of high amounts of HBD2 protein (in the micro- to nanogram range) following TNF and RSV treatment/infection was also noticeable. It is well known that β-defensins, because of their cationic nature, possess high affinity for charged polar head groups of lipids (11–13, 22, 39–44). In that scenario, one may envision that secreted HBD2 will rapidly interact with the extracellularly exposed lipids of the plasma membrane. This phenomenon was tested by adding purified HBD2 to A549 cells and measuring HBD2 protein in cell lysates (to measure cell-associated HBD2) and cultured medium (to measure unbound soluble HBD2) at different postincubation times by ELISA. As shown in Fig. 2C, incubation with HBD2 for 4 h resulted in enrichment of cell-associated protein compared with soluble HBD2 present in the cultured medium. In addition, cell surface biotinylation revealed that during this time frame the majority of HBD2 is bound to the extracellular portion of the cellular plasma membrane (Fig. 2D). These results suggested that upon induction secreted HBD2 rapidly interacts with extracellular lipids, thus resulting in “coating” of the plasma membrane with HBD2.
FIGURE 2.
Cell surface association of soluble HBD2. A, HBD2-specific ELISA analysis of medium supernatant and cell lysate collected from RSV-infected (0–16 h post-infection) A549 cells. B, HBD2-specific ELISA analysis of medium supernatant and cell lysate collected from TNF-treated (0–16 h treatment) A549 cells. C, medium supernatant and cell lysate collected from A549 cells incubated with 1 μg of purified HBD2 for 0–16 h were subjected to ELISA to detect cell-associated versus soluble HBD2. Amount of HBD2 deduced from the ELISA was expressed as ng/ml, and each value represents the mean ± S.D. for three determinations. D, A549 cells incubated with 1 μg of purified HBD2 for 0–24 h were biotinylated (cell surface biotinylation), followed by precipitation with avidin-agarose and blotting with anti-HBD2 antibody.
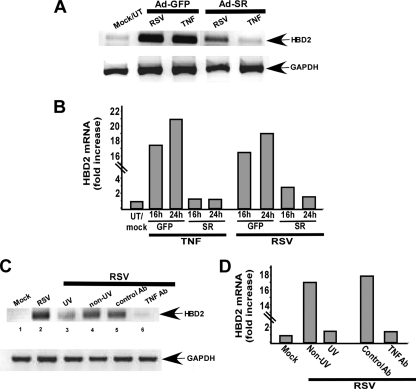
Requirement of NF-κB Activity for HBD2 Induction—Previous studies have demonstrated that the HBD2 gene possesses NF-κB-binding sites, and NF-κB activation is crucial for HBD2 induction (27, 28, 36, 37). We and others (7, 45) have shown previously that RSV activates NF-κB in A549 cells at 6–16 h post-infection. Therefore, we speculated that NF-κB activation in infected cells may play an important role in HBD2 induction. To examine this possibility, A549 cells were infected with noninfectious recombinant adenovirus expressing either NF-κB inhibitor IκB super-repressor (Ad-SR) or GFP (control, Ad-GFP). At 48 h post-adenovirus infection, cells were infected with RSV (0.5 m.o.i.) for 16 h, and HBD2 expression was analyzed by RT-PCR. At the 16-h post-infection time period, RSV failed to efficiently induce HBD2 in NF-κB inhibited cells (i.e. cells expressing IκB-SR) compared with control cells (cells expressing GFP) (Fig. 3A). qPCR results also confirmed that at 16 and 24 h post-infection, expression of IκB-SR reduced HBD2 induction by 81 and 92%, respectively (Fig. 3B). As expected, TNF (16 h treatment with 10 ng/ml TNF) also failed to induce HBD2 in IκB-SR-expressing cells (Fig. 3A). Inhibition of NF-κB drastically diminished TNF-mediated HBD2 induction by 92–94% as deduced by qPCR (Fig. 3B).
FIGURE 3.
Requirement of replicating RSV, NF-κB induction, and paracrine/autocrine action of TNF in HBD2 induction. A, total RNA collected from mock-infected/untreated (UT) and TNF-treated (treated with 10 ng/ml TNF for 16 h) or RSV-infected (infected with 0.5 m.o.i. virus for 16 h) A549 cells infected with recombinant adenovirus expressing either GFP (control) (Ad-GFP) or IκB-super-repressor (Ad-SR) were subjected to RT-PCR to detect HBD2 and GAPDH (loading control). B, total RNA collected from untreated (UT)/mock-, TNF (10 ng/ml for 16–24 h)-treated and RSV-infected (infected with 0.5 m.o.i. virus for 16 and 24 h) A549 cells expressing either GFP or IκB-SR (SR) were subjected to real time qPCR to detect HBD2 expression. C, total RNA collected from mock-infected and RSV-infected cells (infected with either live/non-UV or UV-irradiated RSV) were subjected to RT-PCR to detect HBD2. HBD2 mRNA expression was also monitored in cells infected with RSV in the presence of control antibody (control Ab) or TNF-neutralizing antibody (TNF Ab). D, total RNA collected from mock, live RSV (non-UV), and UV inactivated (UV) RSV-infected A549 cells either treated with control antibody (control Ab) or TNF-neutralizing antibody (TNF Ab) were subjected to qPCR to detect HBD2 expression. For the qPCR, the transcript levels were normalized to human keratin 5, and the results are representative of three independent experiments with similar values.
The reduced HBD2 induction in IκB-SR cells is not because of reduced RSV infection, because RSV infection efficiency (monitored by RT-PCR and plaque assay analysis) was unaltered at 18 or 24 h post-infection in GFP- or IκB-SR-expressing cells infected with RSV at 0.5 m.o.i. (supplemental Fig. S1). In contrast, infection with 0.05 m.o.i. of RSV resulted in enhanced virus infectivity in NF-κB-inhibited cells (supplemental Fig. S2). The differences in viral titers between GFP- and IκB-SR-expressing cells are more pronounced 24–48 h post-infection (supplemental Fig. S2). Replication advantage of RSV in NF-κB-inhibited cells could be due to the absence of NF-κB-dependent antiviral factors (like HBD2) in these cells. Previously, poly(IC) (double-stranded RNA) was shown to induce HBD2 in human uterine epithelial cells (46). Because poly(IC) can activate the transcription factor IRF3, we examined whether IRF3 plays a role in HBD2 induction in RSV-infected human lung epithelial A549 cells. For these experiments, we utilized dominant negative IRF3 (DN-IRF3), which significantly inhibited poly(IC)-mediated activation of IRF3-dependent luciferase reporter gene in A549 cells (supplemental Fig. S3). However, expression of DN-IRF3 in RSV-infected A549 cells had no effect on HBD2 induction (supplemental Fig. S3). Thus, HBD2 induction in infected cells is not mediated by IRF3, whereas our results demonstrated the crucial requirement of NF-κB activity for HBD2 induction following RSV infection of A549 cells. It is also important to note that HBD2 induction at 8–16 h post-infection (Fig. 1, B and C) coincides with NF-κB activation (at 6–16 h post-infection) (7, 45) in RSV-infected cells.
Replicating Virus and Paracrine/Autocrine Action of TNF Is Critical for HBD2 Induction in RSV-infected Cells—The requirement of NF-κB activation for HBD2 induction in RSV-infected cells suggested that the mechanism for HBD2 induction may involve autocrine/paracrine action of NF-κB activating pro-inflammatory cytokines like TNF. This possibility exists because HBD2 induction at 8–16 h post-infection (Fig. 1, B and C) coincides with NF-κB activation (at 6–16 h post-infection) (7, 45) and TNF expression/production (7–10 h post-infection) (47, 48) in RSV-infected cells. To examine the role of TNF in HBD2 induction, A549 cells were infected with RSV (0.2 m.o.i.) in the presence of TNF-specific neutralizing antibody. At 16 h post-infection, HBD2 expression was analyzed by RT-PCR. Although the presence of control antibody had no effect on HBD2 induction (Fig. 3C, lane 5), TNF-specific neutralizing antibody significantly reduced the ability of RSV to induce HBD2 (Fig. 3C, lane 6). These results demonstrated the major role of autocrine/paracrine action of TNF in HBD2 induction following RSV infection.
It is well established that replicating RSV is required for NF-κB activation in lung epithelial cells (7, 45). NF-κB activation in infected cells is important to produce TNF, thus the inability of nonreplicating virus to activate NF-κB may lead to loss of TNF production and resulting noninduction of HBD2. Therefore, we next examined the ability of UV-inactivated (nonreplicating) virus to induce HBD2. A549 cells were infected with UV-inactivated and live RSV for 16 h, followed by RT-PCR analysis of HBD2 expression. UV-inactivated RSV failed to induce NF-κB (measured by electrophoretic mobility shift assay) and TNF (measured by ELISA) following infection (data not shown). Accordingly, HBD2 induction was significantly diminished in cells infected with UV-inactivated RSV (Fig. 3C, lane 3), which once again confirms the importance of NF-κB and TNF in HBD2 induction following virus infection. The qPCR results also demonstrated failure of UV-inactivated RSV to induce HBD2 (Fig. 3D). Similarly, the presence of anti-TNF neutralizing antibody inhibited HBD2 induction following RSV infection by 90% (Fig. 3D). These results show that replicating RSV and the paracrine action of TNF are critical for HBD2 induction during infection.
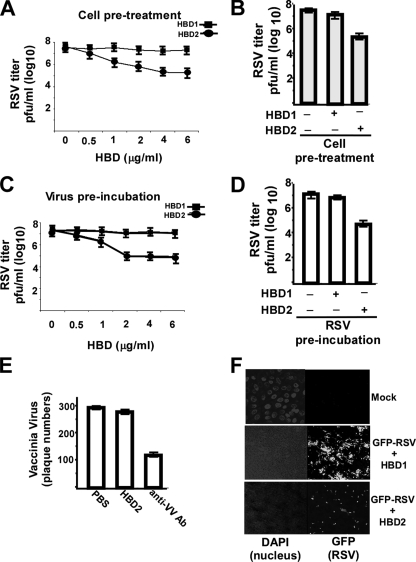
Antiviral Activity of HBD2—Our results have demonstrated the ability of RSV and TNF to induce HBD2 in an NF-κB-dependent fashion. These results suggested that HBD2 may constitute an NF-κB-dependent antiviral factor that operates during NF-κB-dependent (IFN-independent) innate immune antiviral response. To examine whether HBD2 possesses antiviral activity, we studied the ability of purified HBD2 to inhibit RSV infection. A549 cells were incubated with various concentrations (1–6 μg/ml) of HBD2 and HBD1 for 2 h (at 37 °C), followed by addition of RSV (0.2 m.o.i.). Virus adsorption was performed for 1.5 h at 37 °C in the presence of HBDs. Following a 1.5-h absorption, the cells were washed (to remove unbound viruses), and fresh medium (in the absence of HBDs) was added. At 36 h post-infection, the medium supernatants were analyzed for viral titer by plaque assay analysis. A dose-response curve revealed 4 μg/ml HBD2 as the optimal concentration required for antiviral action (Fig. 4A), because higher concentrations (e.g. 6 μg/ml) of HBD2 did not inhibit virus infection further (Fig. 4A). Plaque assay analysis demonstrated significant reduction (more than 100-fold) in RSV titer in cells treated with 4 μg/ml of HBD2 compared with the untreated and HBD1-treated cells (Fig. 4B).
FIGURE 4.
Antiviral activity of HBD2 against RSV infection. A, A549 cells pretreated with either HBD1 or HBD2 (0.5–6 μg/ml) were infected with RSV (in the absence of HBDs) and at 36 h post-infection, and viral titer was measured by plaque assay analysis. B, bar graph showing significant reduction in RSV infection following pretreatment of cells with 4 μg/ml HBD2. C, RSV virion particles preincubated with either HBD1 or HBD2 (0.5–6 μg/ml) were used to infect A549 cells (in the absence of HBDs), and at 36 h post-infection, viral titer was measured by plaque assay analysis. D, bar graph showing significant reduction in infection of A549 cells with RSV preincubated with 2 μg/ml HBD2. The plaque assay values for RSV are expressed as pfu/ml, and each value represents the mean ± S.D. for three determinations. E, vaccinia virus incubated with either PBS (control), HBD2 (10 μg/ml), or neutralizing antibody against vaccinia virus (anti-VV Ab) was used to infect Vero cells, and the plaque numbers were counted at 36 h post-infection. Vaccinia virus infection is represented by the number of plaques, and each value represents the mean ± S.D. for three determinations. F, A549 cells pretreated with either HBD1 or HBD2 (4 μg/ml) were infected (in the absence of HBDs) with RSV expressing green fluorescent protein (GFP-RSV), and at 24 h post-infection, virus infection was monitored by visualizing GFP expression. DAPI, 4,6-diamidino-2-phenylindole.
Apart from the ability of defensins to restrict virus entry presumably by coating the cell surface following incubation of cells with them (Fig. 2), it was also shown that incubation of HIV-1 virion particles with HBD2 and HBD3 renders less infectious virus presumably because of permeabilization of the viral envelope lipid bilayer by the cationic β-defensin proteins (22, 34). Indeed, HBD2 and HBD3 are capable of disrupting both artificial lipid bilayers and bacterial cell wall (41–44). To examine the effect of HBDs on viral envelope integrity, purified RSV virions (0.2 m.o.i.) were incubated with HBD1 and HBD2 (1–6 μg/ml) for 4 h at 37 °C followed by infection for 36 h (in the absence of HBDs). Infection was performed as discussed above for cells pretreated with HBDs. Similar to the dose-response curve obtained from cell pretreatment studies (Fig. 4A), we observed that 2 μg/ml HBD2 was optimal for inhibiting virus infection following incubation of virions with purified HBD2 (Fig. 4C). Incubation of RSV (Fig. 4, C and D) virions with 2 μg/ml HBD2, but not HBD1 resulted in loss of infectivity of A549 cells as evident by the significant reduction in viral titer (by more than 100-fold). The antiviral effect of HBD2 was specific against RSV, because high concentration (up to 10 μg/ml) of HBD2 failed to inhibit vaccinia virus infection, whereas neutralizing antibody to vaccinia virus restricted virus infection (Fig. 4E). In contrast to vaccinia virus, HBD2 retained its antiviral activity against another paramyxovirus (RSV is a paramyxovirus) human parainfluenza virus type 3 (HPIV-3) (7, 14, 20, 21). Treatment of cells or HPIV-3 virion particles with HBD2 resulted in significant loss of infectivity (by 100–300-fold) (supplemental Fig. S4). Similar to RSV, HPIV-3 is also a respiratory-enveloped virus that infects the lung epithelial cells. The retention of antiviral function of HBD2 against two respiratory paramyxoviruses (RSV and HPIV-3) shows the important role of HBD2 in innate immunity, because innate immunity is directed “nonspecifically” against a broad spectrum of pathogens.
The antiviral function of HBD2 was further confirmed by visualizing infection of GFP-expressing RSV (GFP-RSV) (49). A549 cells pretreated with either HBD1 or HBD2 for 2 h were infected with GFP-RSV (0.5 m.o.i.) in the absence of HBDs. At 24 h post-infection, infection status was monitored by visualizing GFP expression by fluorescence microscopy. Pretreatment of cells with HBD2 leads to drastic inhibition in RSV infectivity as evident from diminished GFP expression in these cells, compared with cells pretreated with HBD1 (Fig. 4F). Similar result was obtained following infection of cells with GFP-RSV that was preincubated with HBD2 (data not shown).
The above studies have demonstrated an antiviral function of HBD2 against RSV. The antiviral activity of HBD2 against RSV is specific because of the following: (a) HBD1 failed to inhibit virus infection, and (b) HBD2 did not inhibit vaccinia virus infection. The inability of HBD1 to restrict virus infection was also noted for HIV-1 and herpes simplex virus (22, 34, 35), and the reason for lack of antiviral activity of HBD1 is unknown. It is important to mention that we failed to detect cytotoxicity (measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assay) against A549 cells treated with high concentrations (10–60 μg/ml) of HBD2 for as long as 60 h at 37 °C (supplemental Fig. S5). Loss of cell viability was only observed when very high concentrations (80–100 μg/ml) of HBD2 were used to treat A549 cells (supplemental Fig. S5). Our results suggested that HBD2 may exert its antiviral activity by interacting with the viral lipid envelope leading to destabilization of the viral envelope (and associated altered nonfunctional confirmation of viral envelope proteins), because incubation of virion particles with HBD2 yielded virus with diminished infectivity. In addition, the reduced infectivity of cells pretreated with HBD2 indicates a scenario where the viral envelope is destabilized when the virion particles initiate contact (during entry) with the plasma membrane-associated HBD2.
HBD2 Inhibits RSV Cellular Entry—We speculated that HBD2 may restrict infection at the stage of RSV cellular entry because of the following. (a) HBD2 is secreted extracellularly, and secreted HBD2 possesses high affinity for plasma membrane (Fig. 2), thereby coating the cell surface, which could lead to direct interaction of HBD2 with the virion particle in the extracellular fluid and on the plasma membrane leading to restriction in virus entry. (b) HBDs were shown to inhibit entry of other viruses like HIV-1 and HSV (22, 34, 35). (c) During our antiviral experiments (Fig. 4), HBD2 was present only during adsorption stage, and infection was performed in the absence of HBD2.
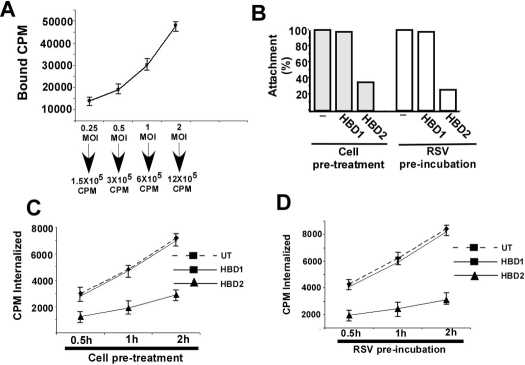
To investigate the ability of HBD2 to block viral entry, we examined the attachment and internalization stages of RSV in the absence or presence of HBD2. The attachment and internalization assays were performed with purified (16) [35S]methionine-labeled RSV (35S-RSV). The kinetics of 35S-RSV attachment was studied by addition of increasing amounts of 35S-RSV (0.25 to 2 m.o.i. corresponding to 1.5 × 105 to 12 × 105 cpm) to chilled A549 cells. Following RSV attachment at 4 °C (the temperature that supports attachment but not internalization) for 2 h, cells were washed extensively (to dissociate unbound virus). The cell lysates representing cell-associated/attached virus were measured (expressed in counts/min) with a scintillation counter. Dose-dependent attachment of 35S-RSV was observed, because virus binding increased linearly with increasing amounts (0.25 to 2 m.o.i.) of 35S-RSV (Fig. 5A). The attachment kinetics demonstrated that ∼4–9% of the input virus binds to the cell surface during our experimental time frame, and addition of 1 m.o.i. virus does not lead to saturable binding. Thus, we chose to use 1 m.o.i. of 35S-RSV (6 × 105 cpm) for our subsequent assays as it represents nonsaturable concentration of viral attachment. Previously, a nonsaturable amount of virus was used to perform attachment and internalization assays (20, 50), because a nonsaturable amount closely mimics the physiological concentration of virus that binds and enters the cells during productive infection.
FIGURE 5.
HBD2 inhibits cellular entry of RSV. A, kinetics of 35S-methionine-labeled RSV (35S-RSV) attachment to A549 cells was examined by adding different amounts of virus (0.25 to 2 m.o.i. or 1.5 × 105 to 12 × 105 cpm) to chilled A549 cells. Following attachment at 4 °C for 2 h, the cells were washed extensively, and the cell-associated radioactivity (in counts/min) representing the attached virus was measured by counting the cell lysate with a liquid scintillation counter. Each value represents the mean ± S.D. for three determinations. B, attachment of 35S-RSV (1 m.o.i., or 6 × 105 cpm) to chilled A549 cells pretreated with HBDs (HBD1 or HBD2) or the virus particle preincubated with the HBDs was determined following attachment of the virus at 4 °C for 2 h. Following adsorption, the cells were washed extensively, and the cell-associated radioactivity (in counts/min) representing the attached virus was measured by counting the cell lysate with a liquid scintillation counter. The percentage of attachment was calculated as a ratio of the amount of radioactivity present in cells incubated with 35S-RSV in the presence of HBDs to the amount of radioactivity present in cells incubated with 35S-RSV alone. C, internalization of 35S-RSV (1 m.o.i., or 6 × 105 cpm) into untreated (UT) or A549 cells pretreated with HBDs was determined following incubation of attached (2 h, 4 °C) virus at 37 °C for 0.5, 1, and 2 h. The cell-associated radioactivities (in counts/min) representing the internalized virus at different time points were measured by counting the cell pellet with a liquid scintillation counter. Each value represents the mean ± S.D. for three determinations. D, internalization of 35S-RSV (1 m.o.i., or 6 × 105 cpm) into A549 cells following preincubation of the virus particle with HBDs was determined following incubation of attached (2 h, 4 °C) virus at 37 °C for 0.5, 1, and 2 h. The cell-associated radioactivities (in counts/min) representing the internalized virus at different time points were measured by counting the cell pellet with a liquid scintillation counter. Each value represents the mean ± S.D. for three determinations.
For the attachment assay in the presence of HBDs, chilled A549 cells were preincubated with either HBD1 or HBD2 for 3 h at 4 °C, followed by the addition of 35S-RSV (1 m.o.i. or 6 × 105 cpm). Similarly, 35S-RSV preincubated with HBDs was added to chilled cells. The virus was incubated at 4 °C for 2 h, and following virus attachment, the cells were washed extensively, and the lysed cells were measured for bound radioactivity with a scintillation counter. As shown in Fig. 5B, the attachment assay revealed no change in RSV cellular binding in the presence of HBD1. However, significant inhibition (70% inhibition) in RSV attachment was observed in the presence of HBD2.
We next investigated whether HBD2 also blocks RSV internalization. Logically, inhibition of attachment should also result in decreased internalization. However, there is a possibility that enhanced internalization kinetics in HBD2-treated cells may compensate for decreased attachment. The internalization assay was performed similarly to the attachment assay, but following attachment at 4 °C, the cells were washed extensively, and the temperature was shifted to 37 °C to promote the internalization of the attached virus particles. At 0.5, 1, and 2 h post-internalization at 37 °C, the extensively washed cells were trypsinized. Following washing of trypsinized cell pellet with PBS, the pellet was counted for radioactivity (the internalized counts/min values represent the internalized virus) with a scintillation counter. Concomitant with the attachment assay, HBD2 inhibited (by 55–66%) RSV internalization following pretreatment of cells with HBD2 (Fig. 5C) and preincubation of virion particles with HBD2 (Fig. 5D). In contrast, HBD1 had no effect on virus internalization.
We further confirmed that the antiviral effect of HBD2 was conferred at the stage of RSV entry because addition of HBD2 during post-entry time periods did not result in RSV infection inhibition (data not shown). Based on previous studies showing disruption of lipid bilayers and bacterial cell wall by β-defensins (41–44), we speculated that direct interaction of HBD2 with RSV particles early during infection may lead to disruption of virion envelope lipid bilayer yielding viruses that are incompetent in efficient cellular entry.
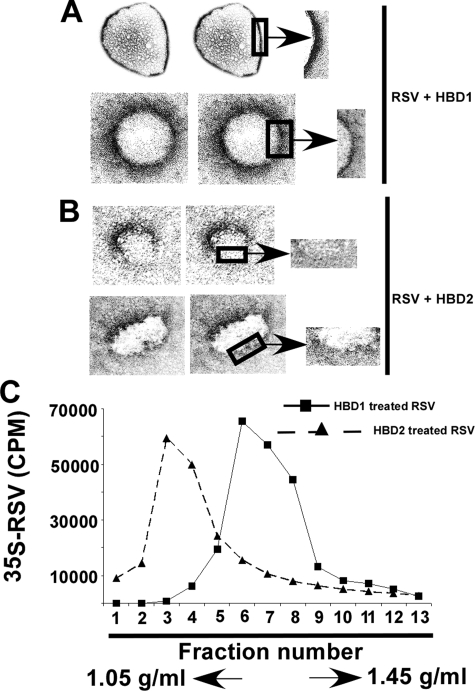
HBD2-mediated Disruption of Virion Envelope—The viral envelope plays an important role in maintaining functional folding of envelope proteins (and homotypic/heterotypic interaction among them) required for virus attachment/fusion (51). Thus, disruption of the envelope lipid dynamics alters envelope protein conformation required for entry. As discussed above, we speculated that direct interaction of HBD2 with the viral envelope may result in its disintegration. The envelope integrity of RSV particles was investigated by incubating purified RSV virion particles with either HBD1 (control) or HBD2 (6 μg/ml) for 8 h at 37 °C. Following incubation, the virion particles were processed for transmission electron microscopy (23, 24). Incubation of virion particles with HBD2 resulted in disruption/disintegration of the viral envelope (Fig. 6B). The absence of continued envelope lining is clearly visible in HBD2-treated virion particle. In contrast, HBD1 had no effect on viral envelope integrity (Fig. 6A). These results suggested that HBD2 confers its antiviral activity by disrupting the virion envelope.
FIGURE 6.
HBD2-mediated disintegration of RSV envelope. Transmission electron micrograph of RSV virion particles following incubation with either HBD1 (A) or HBD2 (B) for 8 h. The boxed area representing a section of the viral envelope was magnified (denoted with arrows) to show the disruption of RSV envelope in the presence of HBD2. C, density gradient centrifugation of HBD1-treated (black squares and solid line) and HBD2 (black triangles and dashed line)-treated purified 35S-RSV virions on CsCl gradient (density gradient of 1.05–1.45 g/ml). Radioactivity (counts/min) present in the fractions was measured by a scintillation counter. Fraction 1 represents the lowest density and was recovered from the top of the gradient.
The ability of HBD2 to disrupt viral particle shape/size by virtue of lipid destabilization was further validated by monitoring buoyant density of virus particles in the CsCl gradient. For these studies, we utilized purified 35S-RSV (16). The 35S-RSV was either untreated or incubated with β-defensins (HBD1 or HBD2) for 8 h at 37 °C. Following incubation, the virus particle was used for density gradient centrifugation (isopycnic centrifugation) using CsCl (density gradient of 1.05–1.45 g/ml) (26). After centrifugation for 36 h at 40,000 rpm, fractions were collected, and the radioactivity present in each fraction was measured by scintillation counter. The buoyant density profile of the viruses revealed that majority of HBD2-treated virion particle was present in the low density fractions (fractions 2–4) of the gradient compared with the virus particle incubated with HBD1, which was predominantly distributed in fractions 5–8 (Fig. 6C). The buoyant density profiles of HBD1-treated particles were similar to the untreated virions (data not shown). These results suggested that HBD2-treated viruses indeed possess different morphology (size and shape) compared with untreated or HBD1-treated virions. Moreover, the presence of HBD2-treated particles in lower density fractions suggested that these virion particles were disrupted. These results indicated loss of virion particle size/shape and morphology following interaction of the virion with HBD2.
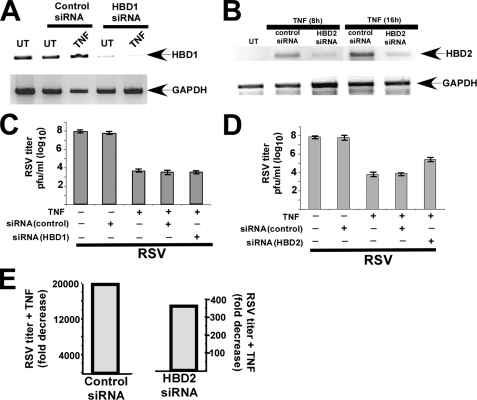
Reduced Antiviral Activity of TNF Following Silencing of HBD2 Expression—Based on our results, we speculated that HBD2 may constitute a TNF/NF-κB-inducible antiviral protein, because TNF induced HBD2 expression in an NF-κB-dependent fashion, and HBD2 possessed potent anti-RSV function. To verify the role of HBD2 during innate antiviral action of TNF, we examined the antiviral efficiency of TNF in HBD2-silenced A549 cells. Silencing of HBD2 expression was achieved by transfecting HBD2-specific siRNA (designed against the N-terminal portion of HBD2) in A549 cells. As a control we also transfected scrambled and HBD1-specific siRNAs. Following 36 h post-siRNA transfection, cells were treated with TNF, and induction of HBD2 and HBD1 was monitored by RT-PCR analysis. Transfection of HBD1-specific siRNA resulted in a drastic decline in endogenous HBD1 expression (Fig. 7A). We also noted significant reduction in HBD2 expression levels in TNF-treated cells (8 and 16 h treatment) transfected with HBD2-specific siRNA compared with control (scrambled) siRNA-transfected cells (Fig. 7B). Silencing of HBD1 had no effect on TNF-mediated HBD2 induction (data not shown). We next utilized HBD1-(Fig. 7C) and HBD2 (Fig. 7D)-silenced cells to measure antiviral activity of TNF. For these studies, untransfected and siRNA-transfected cells were either untreated or treated with TNF (20 h pretreatment). Following TNF pretreatment, cells were infected with RSV. At 36 h post-infection, viral titer was measured by plaque assay analysis of medium supernatants. Based on our previous studies (7), pretreatment of cells with TNF led to a dramatic decline in infectivity (more than 4 logs, around 20,000-fold) (Fig. 7, C and D). Similar antiviral activity was observed in TNF-treated control siRNA (Fig. 7, C and D) and HBD1 siRNA (Fig. 7C)-transfected cells. In contrast, the antiviral efficiency of TNF was significantly reduced in HBD2-silenced cells (Fig. 7D). Only 350–400-fold reduction in virus infection was noted in TNF-treated cells transfected with HBD2-specific siRNA (Fig. 7D). A comparison of the fold decrease in viral titer (as determined by plaque assay results from Fig. 7D) following TNF treatment of HBD2 siRNA and control siRNA-transfected cells is shown in Fig. 7E. The specificity of HBD2 targeting siRNAs is borne out by the observation that two additional siRNAs designed against the central and C-terminal portions of HBD2 also successfully silenced HBD2 expression and reduced the antiviral efficiency of TNF (supplemental Fig. S6).
FIGURE 7.
Role of HBD2 in innate antiviral function of TNF. A, total RNA collected from either untreated (UT) or TNF-treated (10 ng/ml TNF treatment for 16 h) A549 cells transfected with either control or HBD1-specific siRNA were subjected to RT-PCR to detect HBD1 and GAPDH (loading control). B, total RNA collected from untreated (UT) and TNF-treated (10 ng/ml TNF treatment for 8 or 16 h) A549 cells transfected with either control or HBD2-specific siRNA were subjected to RT-PCR to detect HBD2 and GAPDH. C, A549 cells transfected with either control or HBD1-specific siRNAs were pretreated with TNF (20 ng/ml) for 20 h, followed by RSV infection for 36 h. Viral titer at 36 h post-infection was measured by plaque assay analysis. D, A549 cells transfected with either control or HBD2-specific siRNA were pretreated with TNF (20 ng/ml) for 20 h, followed by RSV infection for 36 h. Viral titer at 36 h post-infection was measured by plaque assay analysis. E, plaque assay values from D were tabulated to demonstrate the antiviral efficiency of TNF in control and HBD2-silenced cells. The values are represented as fold decrease in viral titer following treatment of control and HBD2-silenced cells with TNF. The plaque assay values for the above experiments are expressed as pfu/ml, and each value represents the mean ± S.D. for three determinations.
We observed that transfection of control siRNA in A549 cells had no effect on RSV infection as reported earlier (52, 53), although several studies have eluted the production of interferon from siRNA-treated cells (54). Moreover, RSV is not sensitive to the antiviral action of interferon (10, 55). Our results indicated that HBD2 plays an important role during NF-κB-dependent (IFN-independent) innate antiviral action mediated by TNF.
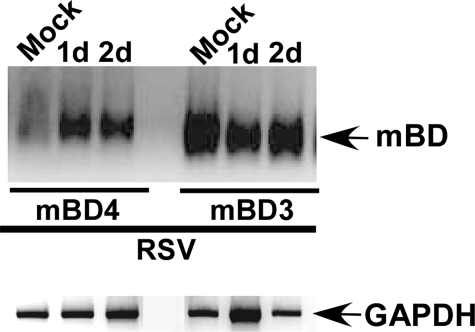
Expression of β-Defensins in the Lungs of RSV-infected Mice—To provide evidence for in vivo relevance of important innate antiviral function of HBD2, we studied murine β-defensin expression in the lungs of RSV-infected mice. BALB/c mice were intranasally infected with RSV (1 × 105 pfu/animal) and at 1, 2, and 4 days post-infection, and the lung tissues were collected from the mock-infected and RSV-infected mice. The tissues were used to detect murine β-defensin expression by RT-PCR. Murine β-defensin-3 (mBD3) was expressed constitutively in mock-infected animals (Fig. 8). In contrast, we observed drastic induction of murine β-defensin-4 (mBD4) at 1 and 2 days post-infection (Fig. 8), whereas mBD4 expression was lost at 4 days post-infection (data not shown). Among the murine β-defensins, only mBD4 and mBD3 possess high amino acid sequence identity (mBD3 and mBD4 are 39.7 and 40% identical to HBD2, respectively) with HBD2 (56, 57) and are therefore considered homologous to HBD2. It is interesting to note that although both mBD3 and mBD4 are murine counterparts of HBD2, RSV specifically induced mBD4 in the lung tissue. Thus, these results demonstrated the ability of RSV to induce β-defensins in vivo, and its induction early (at 1 and 2 days post-infection) during infection (and loss of expression at later post-infection time periods) clearly demonstrates the role of β-defensins in innate immunity, because innate immune molecules have to be induced early during infection to restrict spread of the virus. Moreover, the mBD4 induction time frame (1–2 days post-infection) correlated with high amounts of mouse TNF that are produced from RSV-infected mice lungs during early (0.5–3 days post-infection) (58) infection time periods.
FIGURE 8.
Expression of murine β-defensins in the lungs of RSV-infected mice. Mice were infected intranasally with RSV, and at 1 or 2 days post-infection, total RNA was collected from mock-infected and RSV-infected lung tissues for RT-PCR analysis to detect mBD4 and mBD3 expression.
DISCUSSION
Our interest in identifying TNF-induced antiviral factors stemmed from our studies demonstrating NF-κB-dependent antiviral action of TNF against RSV infection of human lung epithelial cells (7). The TNF-mediated antiviral action was mediated in an IFN-independent fashion; thus, we rationalized that identifying and characterizing TNF/NF-κB-dependent antiviral molecules may shed important light into the innate immune antiviral response. Indeed, the importance of TNF during anti-RSV innate response is clear from production of high levels of TNF in RSV-infected infants/children (59–61). During our effort to identify NF-κB-dependent antiviral factors, we focused our attention on HBD2 due to the following reasons. (a) HBD2 is an inducible secreted protein harboring NF-κB-binding sequences in its gene (36, 37). (b) Induction of HBD2 by NF-κB-inducing cytokines, TNF and IL-1β, has been noted previously (27, 28). (c) Antiviral activity of HBD2 has been documented against HIV-1 (22, 34). (d) HBD2 is specifically expressed in epithelial cells, including airway epithelial cells (29, 30). Our current studies have demonstrated that indeed HBD2 is induced following TNF treatment and RSV infection of human lung epithelial A549 cells (Fig. 1). The induction was dependent on NF-κB activation, and during infection autocrine/paracrine action of TNF (produced from infected cells) plays a major role in HBD2 induction (Fig. 3). HBD2 also possessed potent antiviral activity against RSV by virtue of inhibiting RSV entry probably as a result of disintegrating the lipid bilayer architecture of the virion envelope (Figs. 4, 5, 6). The important role of HBD2 during innate response was established by using siRNA to silence HBD2 expression. HBD2 silencing resulted in loss of antiviral activity of TNF (Fig. 7). Moreover, we observed robust induction of murine β-defensin-4 (murine counterpart of HBD2) in lungs of RSV-infected mice at early (1 and 2 days post-infection) infection time periods (Fig. 8). These results have demonstrated that HBD2 is an important component of innate antiviral response that acts early during infection to limit RSV spread. The importance of HBD2 is also borne out by the observation that 2–4 μg/ml peptide is required for antiviral action (Fig. 4), which is within the physiological limit of β-defensins (including HBD2) secreted/produced from human lung tissues under stress; for example, 10–15 μg/ml HBD2 has been detected in airway aspirates (lavage) of children with pulmonary dysfunction and infection (30, 62–66). Identification of HBD2 as a TNF-dependent antiviral gene has wider implications, because HBD2 may also play a critical role during TNF-mediated innate antiviral action against viruses like influenza, which is sensitive to the antiviral action of TNF (67, 68). Antiviral activity of HBD2 may also be important during NF-κB-dependent (and IFN-independent) antiviral response against hepatitis B virus and gammaherpesviruses (69, 70). It is also important to mention that apart from lung epithelial cells, RSV infection results in high TNF production from immune cells (macrophages, monocytes) present in the airway lumen (71–75). Thus, TNF produced from a variety of cell lines could induce HBD2 robustly to limit spread of RSV in the respiratory tract.
Our current studies have unfolded several insights into the NF-κB-dependent antiviral mechanism mediated by TNF as follows: (a) identification of soluble secreted antiviral proteins like HBD2 during TNF-mediated antiviral response; (b) HBD2-mediated inhibition of RSV cellular entry; and (c) production of antiviral proteins like HBD2 independent of JAK/STAT pathway. In that regard, HBD2 is a unique antiviral protein that is directly activated by NF-κB. However, we speculate that TNF can activate a wide spectrum of antiviral proteins, because silencing of HBD2 (Fig. 7) did not completely abolish the antiviral activity of TNF. In that respect, secreted proteins like HBD2 may constitute one arm of the antiviral defense mechanism of TNF. Production of secreted molecules is advantageous, because these factors could counteract virus infection early during infection (at the cellular entry stages). Thus, orchestrated action of multiple TNF-induced antiviral factors (both extracellular and cytoplasmic) may be required for complete inhibition of virus infection. It is important to note that antiviral action of TNF (and HBD2) may constitute a critical mechanism to protect RSV infection in human lung epithelial cells, because RSV is insensitive to the antiviral action of IFN-α/β (55), and RSV does not efficiently induce IRF3/IFN-α/β activation/expression in these cells (76). Moreover, IFN-α/β therapy in RSV-infected children failed to restrict virus infection (10).
It is important to note that although both RSV and TNF activate NF-κB (7), TNF is the major inducer of HBD2 expression (Fig. 3). NF-κB complex-specific transactivation of specific target genes is well known (77). NF-κB is composed of five subunits (p65 (RelA), p50 (NF-κB1), c-Rel, RelB, and p52 (NF-κB2)), and upon activation, the NF-κB-dependent gene expression is regulated by interaction of NF-κB subunits with other isoforms (to form either homo- or heterodimers), co-activators, and other transcriptional factors. In addition, several studies have also shown the importance of NF-κB subunit phosphorylation in controlling expression of genes (77). Thus, the specificity of NF-κB-dependent gene expression is controlled and regulated at various levels. In that scenario, the promoter region of HBD2 may prefer specific NF-κB subunit complex (and other associated transcriptional factors) for HBD2 expression. However, such correct complex is not activated directly by RSV. In contrast, TNF activates the correct NF-κB complex necessary for HBD2 expression. Indeed earlier studies have reported differential NF-κB complex formation in A549 cells by RSV and TNF. RSV infection results in activation of noncanonical (p52/RelB) and canonical (p65/p50) pathways during early and late infections, respectively (78). However, TNF treatment resulted in activation of p65/p50/c-Rel subunits (79). In another study it was demonstrated that although both TNF and RSV activate p65 in A549 cells, RSV fails to activate p50 strongly compared with TNF (80). These studies clearly show a difference in NF-κB subunit utilization/activation by RSV and TNF, which may explain differential HBD2 expression following RSV infection and TNF treatment.
Defensin family of polypeptides, constituting of α-, β-, and θ-defensins are cysteine-rich peptides possessing anti-bacterial and antiviral activity (11–13). Among them, human β-defensins (HBDs) are compact biologically active polypeptides of 40–50 amino acids (4–7 kDa) with distinctive molecular framework characterized by six cysteine residues paired in three disulfide bridges. HBDs are known to play an important role in innate immune response by virtue of their potent antimicrobial activity against Gram-negative and Gram-positive bacteria. HBDs are highly cationic, and it is suggested that the amphipathic character of HBDs is responsible for their antimicrobial activity, because they can interact with the bacterial membrane (by interacting with the negatively charged phospholipids of the bacterial membrane) leading to permeabilization (disruption of cell wall) and release of cellular contents (11–13, 41–44). The three well established HBDs belonging to the β-defensin family of polypeptides are HBD1, HBD2, and HBD3. Apart from the antimicrobial activity of HBDs, recent studies have demonstrated potent antiviral function of HBD2 and HBD3 against HIV-1 (22, 34). Similar to our observation, HBDs inhibited HIV-1 infection at the stages of cellular entry. In addition, HBD3 was shown to inhibit HSV infection by block multiple stages of the virus life cycle (35). Nevertheless, these studies have established a potent antiviral activity of defensins against a positive-sense single-stranded RNA (HIV-1) virus and a DNA (HSV) virus. Our current studies have extended these observations to a negative stranded nonsegmented RNA virus, because RSV infection was significantly inhibited by HBD2.
We postulate that the mechanism employed by HBD2 to inhibit virus entry constitutes high affinity interaction of HBD2 with the lipid bilayer of the plasma membrane and the virion envelope. Secreted HBD2 associates with the extracellular domain of the plasma membrane and destabilizes the viral envelope when the virion particle establishes contact with plasma membrane during entry. In another scenario, the soluble extracellular HBD2 may directly bind to the virion particles, causing disintegration of viral envelope and altered nonfunctional confirmation (envelope proteins incapable of recognizing cellular receptors and/or to promote fusion) of viral envelope proteins. Indeed, we observed high affinity of HBD2 for extracellular lipids of the plasma membrane (Fig. 2) and the ability of HBD2 to disintegrate RSV envelope and alter virion particle shape/size (Fig. 6). Similar properties of HBD2 and HBD3 were previously reported because they disrupted bacterial cell wall and lipid bilayers (41–44). In case of HIV-1, HBD3 also conferred its antiviral activity by down-regulating cell surface expression of HIV-1 receptor CXCR4 (81). Therefore, it could be speculated that additional antiviral mechanism of HBD2 may constitute masking of the plasma membrane localized cellular receptor(s) utilized by RSV for entry and/or its down-regulation from the cell surface. Further studies addressing these issues are only possible after identification of the specific RSV entry receptor. Although heparan sulfate serves as the attachment receptor for RSV (82), HBD2 does not bind to heparan sulfate (35). However, the ability of HBD2 to directly target and disintegrate viral envelope (Fig. 6) in the absence of cellular components clearly shows the importance of this mechanism in yielding virus particles that are significantly lacking infectivity.
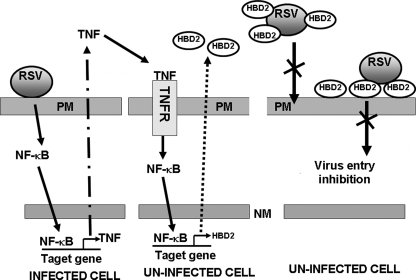
In summary, we have identified HBD2 as an NF-κB-dependent innate antiviral factor that is induced by TNF. Based on our results, we propose a model for the role of HBD2 in innate antiviral response (Fig. 9). Infection of lung epithelial cells with RSV results in activation of NF-κB and production of TNF, which via paracrine action activates HBD2 in uninfected cells. HBD2 secreted from uninfected cells restrict RSV infection by destabilizing the virus envelope following its interaction with the virion particles either on the cell surface (following coating of the plasma membrane with HBD2) or by its direct interaction (as soluble HBD2) with the virion particles. The resulting interactions lead to inhibition in cellular entry. Future studies aimed at characterizing additional NF-κB-dependent antiviral molecules will lead to a better understanding of the innate immune antiviral function in lung epithelial cells.
FIGURE 9.
A schematic diagram depicting a model for HBD2-mediated innate antiviral response. RSV infection of lung epithelial cells results in induction of NF-κB and extracellular secretion of TNF. TNF via paracrine fashion will engage with TNF receptor (TNFR) of uninfected cells to activate NF-κB leading to induction and secretion of HBD2. The progeny viruses released from the infected cells will fail to infect neighboring cells (thus limiting virus spread) productively because of the restriction in viral entry by extracellular HBD2. Entry is inhibited because of direct binding of soluble extracellular HBD2 with the virion particles, causing destabilization of viral envelope. The HBD2 bound to the extracellular domain of plasma membrane may also interact with the virion particles to destabilize the virion envelope and thus inhibit entry. PM, plasma membrane; NM, nuclear membrane; TNFR, TNF receptor.
Supplementary Material
Acknowledgments
We greatly appreciate the gift of purified human β-defensins from Dr. Miguel E. Quinones-Mateu (Cleveland Clinic, Cleveland, OH, and Diagnostic HYBRIDS, Athens, OH). We also thank Dr. Mark E. Peeples (Columbus Children's Hospital and Ohio State University) and Dr. Peter L. Collins (NIAID, National Institutes of Health) for providing the GFP-expressing RSV. We also thank Dr. Adolfo Garcia-Sastre (Mount Sinai School of Medicine) and Dr. Z. Kevin Pan (University of Toledo Medical School) for providing the IRF3-luciferase and dominant negative IRF3 constructs, respectively. We acknowledge the Electron Microscopic Core facility of University of Texas Health Science Center, San Antonio, for assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AI069062 and CA129246 (to S. B.) and T32-DE14318 (to S. B. and A. S.). This work was also supported by American Lung Association National Biomedical Research Grant RG-49629-N (to S. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
Footnotes
The abbreviations used are: RSV, respiratory syncytial virus; IFN, interferon-α/β; HBD, human β-defensin; TNF, tumor necrosis factor-α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; siRNA, short interfering RNA; IRF, interferon regulatory factors; RT, reverse transcription; GFP, green fluorescent protein; qPCR, quantitative PCR; m.o.i., multiplicity of infection; PBS, phosphate-buffered saline; pfu, plaque-forming unit; ELISA, enzyme-linked immunosorbent assay; HSV, herpes simplex virus; Ab, antibody; mBD, murine β-defensin; IL-1β, interleukin-1β; HPIV-3, human parainfluenza virus type 3; HIV-1, human immunodeficiency virus, type 1.
References
- 1.Collins, P. L., Chanock, R. M., and Murphy, B. R. (2001) Fields Virology (Knipe, D. M. and Howley, P. M., eds) 4th Ed., pp. 1443–1486, Lippincott/Williams & Wilkins, Philadelphia
- 2.Falsey, A. R., Hennessey, P. A., Formica, M. A., Cox, C., and Walsh, E. E. (2005) N. Engl. J. Med. 352 1749–1759 [DOI] [PubMed] [Google Scholar]
- 3.Garofalo, R. P., and Haeberle, H. (2000) Am. J. Respir. Cell Mol. Biol. 23 581–585 [DOI] [PubMed] [Google Scholar]
- 4.Bose, S., and Banerjee, A. K. (2003) J. Interferon Cytokine Res. 23 401–412 [DOI] [PubMed] [Google Scholar]
- 5.Sen, G. C. (2001) Annu. Rev. Microbiol. 55 255–281 [DOI] [PubMed] [Google Scholar]
- 6.Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H., and Schreiber, R. D. (1998) Annu. Rev. Biochem. 67 227–264 [DOI] [PubMed] [Google Scholar]
- 7.Bose, S., Kar, N., Maitra, R., DiDonato, J. A., and Banerjee, A. K. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 10890–10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merolla, R., Rebert, N. A., Tsiviste, P. T., Hoffmann, S. P., and Panuska, J. R. (1995) Am. J. Respir. Crit. Care Med. 152 1358–1366 [DOI] [PubMed] [Google Scholar]
- 9.Neuzil, K. M., Tang, Y., and Graham, B. S. (1996) Am. J. Med. Sci. 311 201–204 [DOI] [PubMed] [Google Scholar]
- 10.Chipps, B. E., Sullivan, W. F., and Portnoy, J. M. (1993) Pediatr. Infect. Dis. 12 653–658 [DOI] [PubMed] [Google Scholar]
- 11.Schutte, B. C., and McCray, P. B. (2002) Annu. Rev. Physiol. 64 709–748 [DOI] [PubMed] [Google Scholar]
- 12.Lehrer, R. I., Lichtenstein, A. K., and Ganz, T. (1993) Annu. Rev. Immunol. 11 105–128 [DOI] [PubMed] [Google Scholar]
- 13.Raj, P. A., and Dentino, A. R. (2002) FEMS Microbiol. Lett. 206 9–18 [DOI] [PubMed] [Google Scholar]
- 14.Bose, S., Malur, A., and Banerjee, A. K. (2001) J. Virol. 75 1984–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu, M., Maitra, R. K., Xiang, Y., Meng, X., Banerjee, A. K., and Bose, S. (2006) J. Gen. Virol. 87 2653–2662 [DOI] [PubMed] [Google Scholar]
- 16.Ueba, O. (1978) Acta Med. Okayama 32 265–272 [PubMed] [Google Scholar]
- 17.Meng, X., and Xiang, Y. (2006) Virology 353 220–233 [DOI] [PubMed] [Google Scholar]
- 18.Krisanaprakornkit, S., Weinberg, A., Perez, C. N., and Dale, B. A. (1998) Infect. Immun. 66 4222–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krisanaprakornkit, S., Kimball, J. R., Weinberg, A., Darveau, R. P., Bainbridge, B. W., and Dale, B. A. (2000) Infect. Immun. 68 2907–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bose, S., Basu, M., and Banerjee, A. K. (2004) J. Virol. 78 8146–8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bose, S., and Banerjee, A. K. (2002) Virology 298 73–83 [DOI] [PubMed] [Google Scholar]
- 22.Quinones-Mateu, M. E., Lederman, M. M., Feng, Z., Chakraborty, B., Weber, J., Rangel, H. R., Marotta, M. L., Mirza, M., Jiang, B., Kiser, P., Medvik, K., Sieg, S. F., and Weinberg, A. (2003) AIDS 17 F39–F48 [DOI] [PubMed] [Google Scholar]
- 23.Arns, C. W., Campalans, J., Costa, S. C., Domingues, H. G., D'Arce, R. C., Almeida, R. S., and Coswig, L. T. (2003) Braz. J. Med. Biol. Res. 36 213–218 [DOI] [PubMed] [Google Scholar]
- 24.Brown, G., Aitken, J., Rixon, H. W., and Sugrue, R. J. (2002) J. Gen. Virol. 83 611–621 [DOI] [PubMed] [Google Scholar]
- 25.Mejías, A., Chávez-Bueno, S., Ríos, A. M., Aten, M. F., Raynor, B., Peromingo, E., Soni, P., Olsen, K. D., Kiener, P. A., Gómez, A. M., Jafri, H. S., and Ramilo, O. (2005) Antimicrob. Agents Chemother. 49 4700–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camerini-Otero, R. D., and Franklin, R. M. (1975) Eur. J. Biochem. 53 343–348 [DOI] [PubMed] [Google Scholar]
- 27.McDermott, A. M., Redfern, R. L., Zhang, B., Pei, Y., Huang, L., and Proske, R. J. (2003) Investig. Ophthalmol. Vis. Sci. 44 1859–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neil, D. A., Porter, E. M., Elewaut, D., Anderson, G. M., Eckmann, L., Ganz, T., and Kagnoff, M. F. (1999) J. Immunol. 163 6718–6724 [PubMed] [Google Scholar]
- 29.Tomita, T., Nagase, T., Ohga, E., Yamaguchi, Y., Yoshizumi, M., and Ouchi, Y. (2002) Respirology 7 305–310 [DOI] [PubMed] [Google Scholar]
- 30.Singh, P. K., Jia, H. P., Wiles, K., Hesselberth, J., Liu, L., Conway, B. A., Greenberg, E. P., Valore, E. V., Welsh, M. J., Ganz, T., Tack, B. F., and McCray, P. B., Jr. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 14961–14966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, L., Yu, W., He, T., Yu, J., Caffrey, R. E., Dalmasso, E. A., Fu, S., Pham, T., Mei, J., Ho, J. J., Zhang, W., Lopez, P., and Ho, D. D. (2002) Science 298 995–1000 [DOI] [PubMed] [Google Scholar]
- 32.Munk, C., Wei, G., Yang, O. O., Waring, A. J., Wang, W., Hong, T., Lehrer, R. I., Landau, N. R., and Cole, A. M. (2003) AIDS Res. Hum. Retroviruses 19 875–881 [DOI] [PubMed] [Google Scholar]
- 33.Sinha, S., Cheshenko, N., Lehrer, R. I., and Harold, B. C. (2003) Antimicrob. Agents Chemother. 47 494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun, L., Finnegan, C. M., Kish-Catalone, T., Blumenthal, R., Garzino-Demo, P., La Terra Maggiore, G. M., Berrone, S., Kleinman, C., Wu, Z., Abdelwahab, S., Lu, W., and Garzino-Demo, A. (2005) J. Virol. 79 14318–14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazrati, E., Galen, B., Lu, W., Wang, W., Ouyang, Y., Keller, M. J., Lehrer, R. I., and Herold, B. C. (2006) J. Immunol. 177 8658–8666 [DOI] [PubMed] [Google Scholar]
- 36.Mineshiba, J., Myokai, F., Mineshiba, F., Matsuura, K., Nishimura, F., and Takashiba, S. (2005) FEMS Immunol. Med. Microbiol. 45 37–44 [DOI] [PubMed] [Google Scholar]
- 37.Tsutsumi-Ishii, Y., and Nagaoka, I. (2002) J. Leukocyte Biol. 71 154–162 [PubMed] [Google Scholar]
- 38.Hao, H., Zhao, J., Lotoczky, G., Grever, W., and Lyman, W. D. (2001) J. Neurochem. 77 1027–1035 [DOI] [PubMed] [Google Scholar]
- 39.Lichtenstein, A. K., Ganz, T., Nguyen, T. M., Selsted, M. E., and Lehrer, R. I. (1988) J. Immunol. 140 2686–2694 [PubMed] [Google Scholar]
- 40.Shiba, H., Mouri, Y., Komatsuzawa, H., Ouhara, K., Takeda, K., Sugai, M., Kinane, D. F., and Kurihara, H. (2003) Biochem. Biophys. Res. Commun. 306 867–871 [DOI] [PubMed] [Google Scholar]
- 41.Bohling, A., Hagge, S. O., Roes, S., Podschun, R., Sahly, H., Harder, J., Schroder, J. M., Grotzinger, J., Seydel, U., and Gutsmann, T. (2006) Biochemistry 45 5663–56670 [DOI] [PubMed] [Google Scholar]
- 42.Harder, J., Bartels, J., Christophers, E., and Schroder, J. M. (2001) J. Biol. Chem. 276 5707–5713 [DOI] [PubMed] [Google Scholar]
- 43.Rivas-Santiago, B., Schwander, S. K., Sarabia, C., Diamond, G., Klein-Patel, M. E., Hernandez-Pando, R., Ellner, J. J., and Sada, E. (2005) Infect. Immun. 73 4505–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veldhuizen, E. J., Rijnders, M., Claassen, E. A., van Dijk, A., and Haagsman, H. P. (2008) Mol. Immunol. 45 386–394 [DOI] [PubMed] [Google Scholar]
- 45.Bitko, V., Velazquez, A., Yang, L., Yang, Y. C., and Barik, S. (1997) Virology 232 369–378 [DOI] [PubMed] [Google Scholar]
- 46.Schaefer, T. M., Fahey, J. V., Wright, J. A., and Wira, C. R. (2005) J. Immunol. 174 992–1002 [DOI] [PubMed] [Google Scholar]
- 47.Tsutsumi, H., Takeuchi, R., Ohsaki, M., Seki, K., and Chiba, S. (1999) J. Leukocyte Biol. 66 99–104 [PubMed] [Google Scholar]
- 48.Sakai, S., Ochiai, H., Kawamata, H., Kogure, T., Shimada, Y., Nakajima, K., and Terasawa, K. (1997) J. Med. Virol. 53 145–149 [PubMed] [Google Scholar]
- 49.Hallak, L. K., Collins, P. L., Knudson, W., and Peeples, M. E. (2000) Virology 271 264–275 [DOI] [PubMed] [Google Scholar]
- 50.Tamura, M., Natori, K., Kobayahi, M., Miyamura, T., and Takeda, N. (2000) J. Virol. 74 11589–11597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison, T. G. (2003) Biochim. Biophys. Acta 1614 73–84 [DOI] [PubMed] [Google Scholar]
- 52.Bitko, V., and Barik, S. (2001) BMC Microbiol. 1 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bitko, V., Musiyenko, A., Shulyayeva, O., and Barik, S. (2005) Nat. Med. 11 50–55 [DOI] [PubMed] [Google Scholar]
- 54.Pebernard, S., and Iggo, R. D. (2004) Differentiation 72 103–111 [DOI] [PubMed] [Google Scholar]
- 55.Atreya, P. L., and Kulkarni, S. (1999) Virology 261 227–241 [DOI] [PubMed] [Google Scholar]
- 56.Jia, H. P., Wowk, S. A., Schutte, B. C., Lee, S. K., Vivado, A., Tack, B. F., Bevins, C. L., and McCray, P. B., Jr. (2000) J. Biol. Chem. 275 33314–33320 [DOI] [PubMed] [Google Scholar]
- 57.Bals, R., Wang, X., Meegalla, R. L., Wattler, S., Weiner, D. J., Nehls, M. C., and Wilson, J. M. (1999) Infect. Immun. 67 3542–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chávez-Bueno, S., Mejías, A., Gómez, A. M., Olsen, K. D., Ríos, A. M., Fonseca-Aten, M., Ramilo, O., and Jafri, H. S. (2005) Virol. J. 2 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNamara, P. S., Flanagan, B. F., Selby, A. M., Hart, C. A., and Smyth, R. L. (2004) Eur. Respir. J. 23 106–112 [DOI] [PubMed] [Google Scholar]
- 60.Garofalo, R. P., Patti, J., Hintz, K. A., Hill, V., Ogra, P. L., and Welliver, R. C. (2001) J. Infect. Dis. 184 393–399 [DOI] [PubMed] [Google Scholar]
- 61.Sheeran, P., Jafri, H., Carubelli, C., Saavedra, J., Johnson, C., Krisher, K., Sánchez, P. J., and Ramilo, O. (1999) Pediatr. Infect. Dis. J. 18 115–122 [DOI] [PubMed] [Google Scholar]
- 62.Schaller-Bals, S., Schulze, A., and Bals, R. (2002) Am. J. Respir. Crit. Care Med. 165 992–995 [DOI] [PubMed] [Google Scholar]
- 63.Chen, C. I., Schaller-Bals, S., Paul, K. P., Wahn, U., and Bals, R. (2004) J. Cyst. Fibros. 3 45–50 [DOI] [PubMed] [Google Scholar]
- 64.Proud, D., Sanders, S. P., and Wiehler, S. (2004) J. Immunol. 172 4637–4645 [DOI] [PubMed] [Google Scholar]
- 65.Ross, D. J., Cole, A. M., Yoshioka, D., Park, A. K., Belperio, J. A., Laks, H., Strieter, R. M., Lynch, J. P., III, Kubak, B., Ardehali, A., and Ganz, T. (2004) Transplantation 78 1222–1224 [DOI] [PubMed] [Google Scholar]
- 66.Hiratsuka, T., Nakazato, M., Date, Y., Ashitani, J., Minematsu, T., Chino, N., and Matsukura, S. (1998) Biochem. Biophys. Res. Commun. 249 943–947 [DOI] [PubMed] [Google Scholar]
- 67.Seo, S. H., and Webster, R. G. (2002) J. Virol. 76 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seo, S. H., Hoffmann, E., and Webster, R. G. (2002) Nat. Med. 8 950–954 [DOI] [PubMed] [Google Scholar]
- 69.Biermer, M., Puro, R., and Schneider, R. J. (2003) J. Virol. 77 4033–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown, H. J., Song, M. J., Deng, H., Wu, T. T., Cheng, G., and Sun, R. (2003) J. Virol. 77 8532–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franke-Ullmann, G., Pfortner, C., Walter, P., Steinmuller, C., Lohmann-Matthes, M. L., Kobzik, L., and Freihorst, J. (1995) J. Immunol. 154 268–280 [PubMed] [Google Scholar]
- 72.Midulla, F., Villani, A., Panuska, J. R., Dab, I., Kolls, J. K., Merolla, R., and Ronchetti, R. (1993) J. Infect. Dis. 168 1515–1519 [DOI] [PubMed] [Google Scholar]
- 73.Becker, S., Quay, J., and Soukup, J. (1991) J. Immunol. 147 4307–4312 [PubMed] [Google Scholar]
- 74.Panuska, J. R., Midulla, F., Cirino, N. M., Villani, A., Gilbert, I. A., McFadden, E. R., and Huang, Y. T. (1990) Am. J. Physiol. 259 L396–L402 [DOI] [PubMed] [Google Scholar]
- 75.Soukup, J. M., and Becker, S. (2003) Clin. Immunol. 107 178–185 [DOI] [PubMed] [Google Scholar]
- 76.Spann, K. M., Tran, K. C., and Collins, P. L. (2005) J. Virol. 79 5353–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wietek, C., and O'Neill, L. A. (2007) Trends Biochem. Sci. 32 311–319 [DOI] [PubMed] [Google Scholar]
- 78.Choudhary, S., Boldogh, S., Garofalo, R., Jamaluddin, M., and Brasier, A. R. (2005) J. Virol. 79 8948–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brasier, A. R., Jamaluddin, M., Casola, A., Duan, W., Shen, Q., and Garofalo, R. P. (1998) J. Biol. Chem. 273 3551–3561 [DOI] [PubMed] [Google Scholar]
- 80.Carpenter, L. R., Moy, J. N., and Roebuck, K. A. (2002) BMC Infect. Dis. 28 2:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng, Z., Dubyak, G. R., Lederman, M. M., and Weinberg, A. (2006) J. Immunol. 177 782–786 [DOI] [PubMed] [Google Scholar]
- 82.Feldman, S. A., Audet, S., and Beeler, J. A. (2000) J. Virol. 74 6442–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.