Abstract
Twelve monoclonal antibodies neutralizing the CVS strain of rabies virus were used to characterize antigenic site II of the viral glycoprotein. Nineteen antigenic mutants resistant to neutralization by some of these antibodies were selected; some continued to normally or partially bind the antibody, whereas others did not. Mutations conferring resistance to neutralization by site II-specific monoclonal antibodies were localized into two clusters, the first between amino acids 34 and 42 (seven groups of mutants) and the second at amino acids 198 and 200 (three groups of mutants). Two intermediate mutations were identified at positions 147 and 184. Four mutations resulted in reduced pathogenicity after intramuscular inoculation of the virus in adult mice. One of the mutants, M23, was 300 times and the others were 10 to 30 times less pathogenic than CVS. In three cases the attenuated phenotype was related to an important modification of antigenic site II, whereas the other known antigenic sites were unchanged.
Full text
PDF

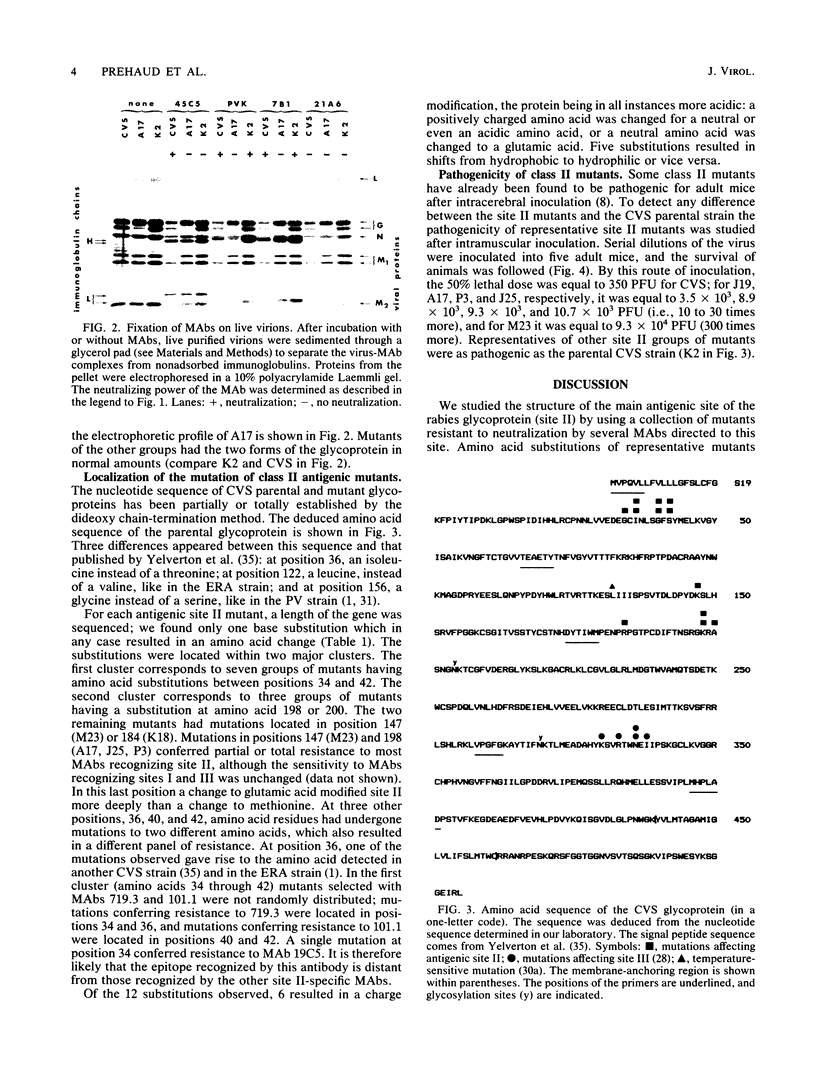


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Wunner W. H., Curtis P. J. Structure of the glycoprotein gene in rabies virus. Nature. 1981 Nov 19;294(5838):275–278. doi: 10.1038/294275a0. [DOI] [PubMed] [Google Scholar]
- Blondel B., Crainic R., Fichot O., Dufraisse G., Candrea A., Diamond D., Girard M., Horaud F. Mutations conferring resistance to neutralization with monoclonal antibodies in type 1 poliovirus can be located outside or inside the antibody-binding site. J Virol. 1986 Jan;57(1):81–90. doi: 10.1128/jvi.57.1.81-90.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bussereau F., Flamand A., Pese-Part D. Reproducible plaquing system for rabies virus in CER cells. J Virol Methods. 1982 May;4(4-5):277–282. doi: 10.1016/0166-0934(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Celis E., Miller R. W., Wiktor T. J., Dietzschold B., Koprowski H. Isolation and characterization of human T cell lines and clones reactive to rabies virus: antigen specificity and production of interferon-gamma. J Immunol. 1986 Jan;136(2):692–697. [PubMed] [Google Scholar]
- Coulon P., Rollin P. E., Flamand A. Molecular basis of rabies virus virulence. II. Identification of a site on the CVS glycoprotein associated with virulence. J Gen Virol. 1983 Mar;64(Pt 3):693–696. doi: 10.1099/0022-1317-64-3-693. [DOI] [PubMed] [Google Scholar]
- Coulon P., Rollin P., Aubert M., Flamand A. Molecular basis of rabies virus virulence. I. Selection of avirulent mutants of the CVS strain with anti-G monoclonal antibodies. J Gen Virol. 1982 Jul;61(Pt 50):97–100. doi: 10.1099/0022-1317-61-1-97. [DOI] [PubMed] [Google Scholar]
- Dalziel R. G., Lampert P. W., Talbot P. J., Buchmeier M. J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986 Aug;59(2):463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond D. C., Jameson B. A., Bonin J., Kohara M., Abe S., Itoh H., Komatsu T., Arita M., Kuge S., Nomoto A. Antigenic variation and resistance to neutralization in poliovirus type 1. Science. 1985 Sep 13;229(4718):1090–1093. doi: 10.1126/science.2412292. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Macfarlan R., Varrichio A. Antigenic structure of rabies virus glycoprotein: ordering and immunological characterization of the large CNBr cleavage fragments. J Virol. 1982 Nov;44(2):595–602. doi: 10.1128/jvi.44.2.595-602.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., el-Zaatari F. A., Stohlman S. A., Weiner L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986 Jun;58(3):869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Marlin S. D., Levine M., Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983 Feb;45(2):672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Selection of unique antigenic variants of Newcastle disease virus with neutralizing monoclonal antibodies and anti-immunoglobulin. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7106–7110. doi: 10.1073/pnas.82.20.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye K. M., Spriggs D. R., Bassel-Duby R., Fields B. N., Tyler K. L. Genetic basis for altered pathogenesis of an immune-selected antigenic variant of reovirus type 3 (Dearing). J Virol. 1986 Jul;59(1):90–97. doi: 10.1128/jvi.59.1.90-97.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera P., Dolivo M., Coulon P., Flamand A. Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. J Virol. 1985 Jul;55(1):158–162. doi: 10.1128/jvi.55.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafon M., Wiktor T. J., Macfarlan R. I. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):843–851. doi: 10.1099/0022-1317-64-4-843. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Wilson P. T., Hawrot E., Speicher D. W. Amino acid sequence similarity between rabies virus glycoprotein and snake venom curaremimetic neurotoxins. Science. 1984 Nov 16;226(4676):847–848. doi: 10.1126/science.6494916. [DOI] [PubMed] [Google Scholar]
- Löve A., Rydbeck R., Kristensson K., Orvell C., Norrby E. Hemagglutinin-neuraminidase glycoprotein as a determinant of pathogenicity in mumps virus hamster encephalitis: analysis of mutants selected with monoclonal antibodies. J Virol. 1985 Jan;53(1):67–74. doi: 10.1128/jvi.53.1.67-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Winn W. C., Bauer S. P. Comparative pathogenesis of rabies and rabies-like viruses: infection of the central nervous system and centrifugal spread of virus to peripheral tissues. Lab Invest. 1973 Jul;29(1):1–16. [PubMed] [Google Scholar]
- Naito S., Matsumoto S. Identification of cellular actin within the rabies virus. Virology. 1978 Nov;91(1):151–163. doi: 10.1016/0042-6822(78)90363-x. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Coulon P., Rollin P. E., Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985 Mar;53(3):926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheshberadaran H., Norrby E. Characterization of epitopes on the measles virus hemagglutinin. Virology. 1986 Jul 15;152(1):58–65. doi: 10.1016/0042-6822(86)90371-5. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Fields B. N. Attenuated reovirus type 3 strains generated by selection of haemagglutinin antigenic variants. Nature. 1982 May 6;297(5861):68–70. doi: 10.1038/297068a0. [DOI] [PubMed] [Google Scholar]
- Tidke R., Préhaud C., Coulon P., Blancou J., Flamand A. Characterization of a double avirulent mutant of rabies virus and its potency as a vaccine, live or inactivated. Vaccine. 1987 Sep;5(3):229–233. doi: 10.1016/0264-410x(87)90106-x. [DOI] [PubMed] [Google Scholar]
- Tordo N., Poch O., Ermine A., Keith G., Rougeon F. Walking along the rabies genome: is the large G-L intergenic region a remnant gene? Proc Natl Acad Sci U S A. 1986 Jun;83(11):3914–3918. doi: 10.1073/pnas.83.11.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., György E., Schlumberger D., Sokol F., Koprowski H. Antigenic properties of rabies virus components. J Immunol. 1973 Jan;110(1):269–276. [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Dietzschold B., Smith C. L., Lafon M., Golub E. Antigenic variants of CVS rabies virus with altered glycosylation sites. Virology. 1985 Jan 15;140(1):1–12. doi: 10.1016/0042-6822(85)90440-4. [DOI] [PubMed] [Google Scholar]
- Yelverton E., Norton S., Obijeski J. F., Goeddel D. V. Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli. Science. 1983 Feb 11;219(4585):614–620. doi: 10.1126/science.6297004. [DOI] [PubMed] [Google Scholar]