Abstract
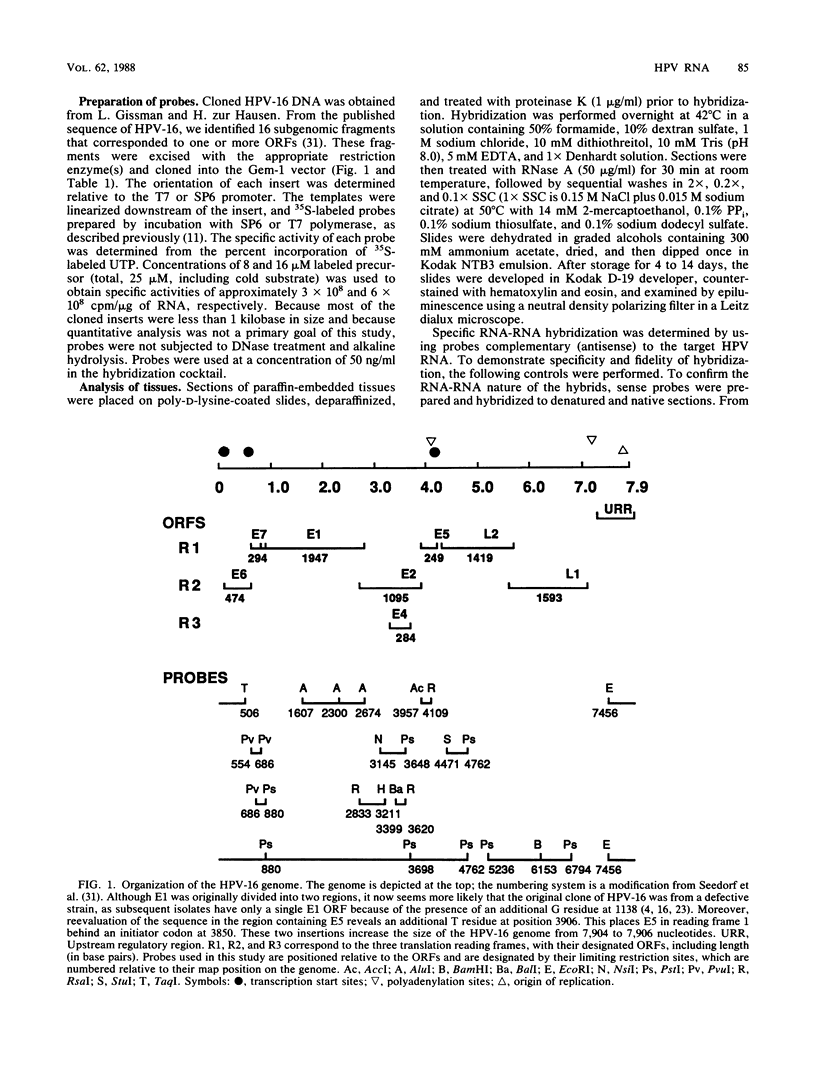
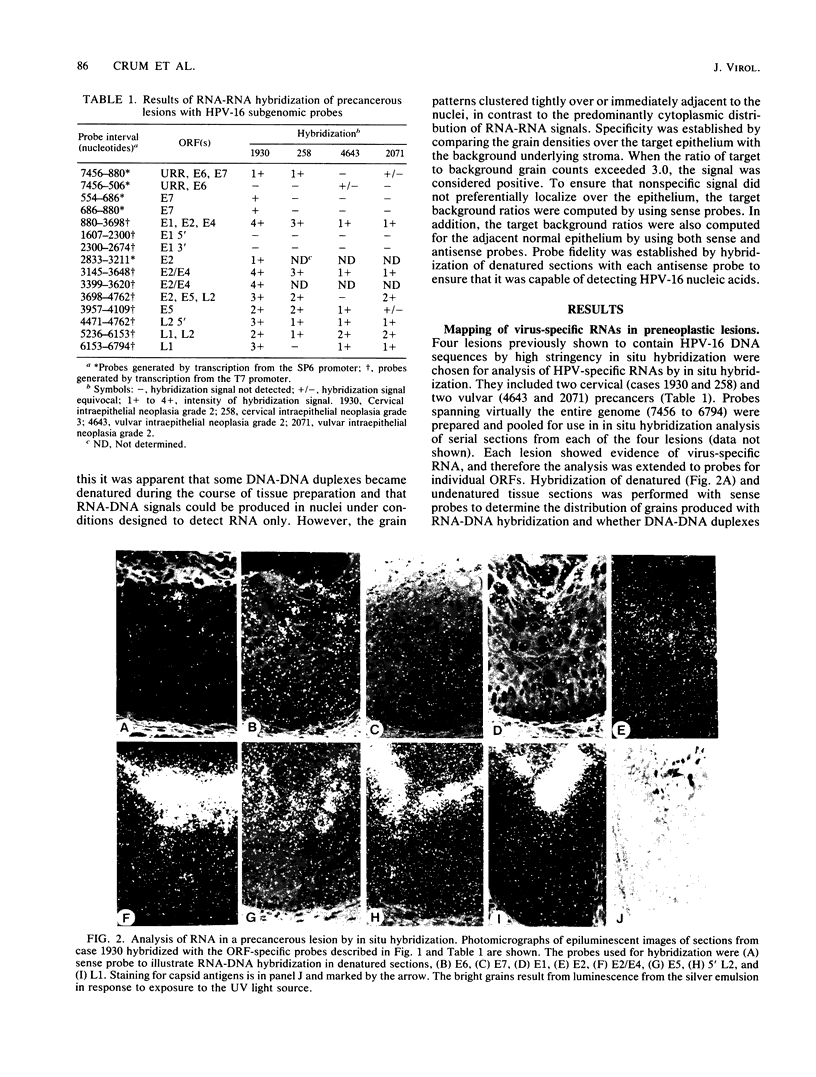
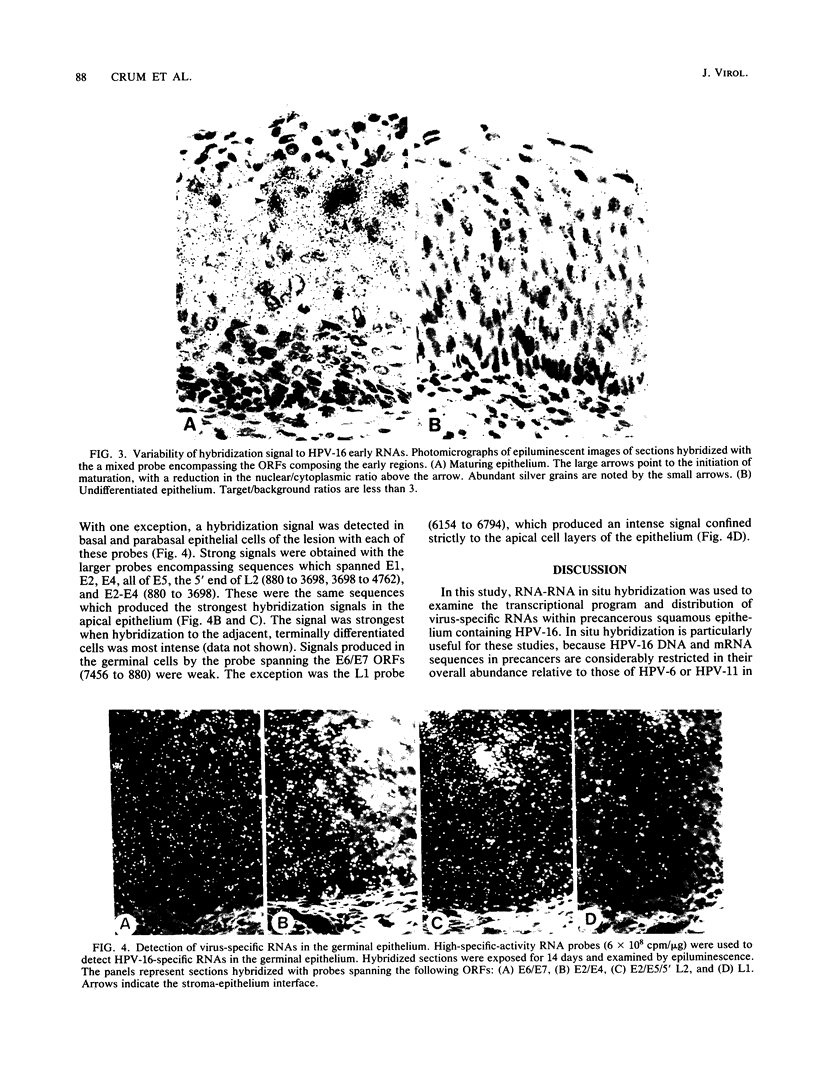
The accumulation of human papillomavirus type 16 (HPV-16)-specific RNAs in tissue sections from biopsies of patients with genital precancers was studied by in situ hybridization with single-stranded 35S-labeled RNA. These analyses revealed that the most abundant early-region RNAs were derived from the E4 and E5 open reading frames (ORFs). RNAs homologous to the E6/E7 ORFs were also detected, whereas RNAs homologous to the intervening E1 ORF were not. This suggests that the E4 and E5 mRNAs are derived by splicing to the upstream E6/E7 ORFs, consistent with studies of HPV-11 in condylomata (L. T. Chow et al., Cancer Cells (Cold Spring Harbor) 5:55-72, 1987). Abundant RNAs homologous to the 5' portion of L1 were also detected. These RNAs were localized to the apical strata of the epithelium. HPV-16 RNAs accumulated in discrete regions of these lesions, and when present were most abundant in the upper cell layers of the precancerous epithelium. RNAs homologous to early ORFs were also detected in some germinal cells within the basal layer of the epithelium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahola H., Stenlund A., Moreno-López J., Pettersson U. Promoters and processing sites within the transforming region of bovine papillomavirus type 1. J Virol. 1987 Jul;61(7):2240–2244. doi: 10.1128/jvi.61.7.2240-2244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androphy E. J., Hubbert N. L., Schiller J. T., Lowy D. R. Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines. EMBO J. 1987 Apr;6(4):989–992. doi: 10.1002/j.1460-2075.1987.tb04849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Howley P. M. Differential promoter utilization by the bovine papillomavirus in transformed cells and productively infected wart tissues. EMBO J. 1987 Apr;6(4):1027–1035. doi: 10.1002/j.1460-2075.1987.tb04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudenon S., Kremsdorf D., Croissant O., Jablonska S., Wain-Hobson S., Orth G. A novel type of human papillomavirus associated with genital neoplasias. Nature. 1986 May 15;321(6067):246–249. doi: 10.1038/321246a0. [DOI] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Ikenberg H., Richart R. M., Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984 Apr 5;310(14):880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Mitao M., Levine R. U., Silverstein S. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985 Jun;54(3):675–681. doi: 10.1128/jvi.54.3.675-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Nagai N., Levine R. U., Silverstein S. In situ hybridization analysis of HPV 16 DNA sequences in early cervical neoplasia. Am J Pathol. 1986 Apr;123(1):174–182. [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Guralski D., Schiller J. T. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1797–1801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Kleinheinz A., Hotz M., Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985 Jul;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- Fu Y. S., Reagan J. W., Richart R. M. Definition of precursors. Gynecol Oncol. 1981 Oct;12(2 Pt 2):S220–S231. doi: 10.1016/0090-8258(81)90076-7. [DOI] [PubMed] [Google Scholar]
- Kanda T., Watanabe S., Yoshiike K. Human papillomavirus type 16 transformation of rat 3Y1 cells. Jpn J Cancer Res. 1987 Feb;78(2):103–108. [PubMed] [Google Scholar]
- Kirkland J. A., Stanley M. A., Cellier K. M. Comparative study of histologic and chromosomal abnormalities in cervical neoplasia. Cancer. 1967 Nov;20(11):1934–1952. doi: 10.1002/1097-0142(196711)20:11<1934::aid-cncr2820201122>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lorincz A. T., Lancaster W. D., Temple G. F. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. J Virol. 1986 Apr;58(1):225–229. doi: 10.1128/jvi.58.1.225-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Matsukura T., Kanda T., Furuno A., Yoshikawa H., Kawana T., Yoshiike K. Cloning of monomeric human papillomavirus type 16 DNA integrated within cell DNA from a cervical carcinoma. J Virol. 1986 Jun;58(3):979–982. doi: 10.1128/jvi.58.3.979-982.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitao M., Nagai N., Levine R. U., Silverstein S. J., Crum C. P. Human papillomavirus type 16 infection: a morphological spectrum with evidence for late gene expression. Int J Gynecol Pathol. 1986;5(4):287–296. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richart R. M. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301–328. [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Lowy D. R. Identification of a second transforming region in bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7880–7884. doi: 10.1073/pnas.81.24.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. The major human papillomavirus protein in cervical cancers is a cytoplasmic phosphoprotein. J Virol. 1987 May;61(5):1686–1689. doi: 10.1128/jvi.61.5.1686-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler M. H., Broker T. R. In situ hybridization detection of human papillomavirus DNAs and messenger RNAs in genital condylomas and a cervical carcinoma. Hum Pathol. 1986 Dec;17(12):1250–1258. doi: 10.1016/s0046-8177(86)80569-x. [DOI] [PubMed] [Google Scholar]
- Taichman L. B., Reilly S. S., LaPorta R. F. The role of keratinocyte differentiation in the expression of epitheliotropic viruses. J Invest Dermatol. 1983 Jul;81(1 Suppl):137s–140s. doi: 10.1111/1523-1747.ep12540909. [DOI] [PubMed] [Google Scholar]
- Tsunokawa Y., Takebe N., Nozawa S., Kasamatsu T., Gissmann L., zur Hausen H., Terada M., Sugimura T. Presence of human papillomavirus type-16 and type-18 DNA sequences and their expression in cervical cancers and cell lines from Japanese patients. Int J Cancer. 1986 Apr 15;37(4):499–503. doi: 10.1002/ijc.2910370405. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Spalholz B. A., Rabson M. S., Howley P. M. Dissociation of transforming and trans-activation functions for bovine papillomavirus type 1. Nature. 1985 Dec 12;318(6046):575–577. doi: 10.1038/318575a0. [DOI] [PubMed] [Google Scholar]