Abstract
Background
The metabolic syndrome is a high-risk state for diabetes and cardiovascular disease. Little is known about its prevalence and prevention in those with impaired glucose tolerance.
Objective
To determine the prevalence of the metabolic syndrome at baseline in the Diabetes Prevention Program and the effect of intensive lifestyle intervention and metformin therapy on the syndrome’s incidence and resolution.
Design
Randomized, controlled clinical trial.
Setting
Research and community-based centers.
Participants
Participants had impaired glucose tolerance (World Health Organization criteria plus fasting plasma glucose level ≥5.3 mmol/L [≥95 mg/dL]) and were followed for a mean of 3.2 years after random assignment to intensive lifestyle intervention, metformin therapy, or placebo.
Interventions
Metformin, 850 mg twice daily, or intensive life-style intervention designed to achieve and maintain a 7% weight loss and 150 minutes of exercise per week.
Measurements
The metabolic syndrome was defined as having 3 or more characteristics (waist circumference; blood pressure; and levels of high-density lipoprotein cholesterol, triglycerides, and fasting plasma glucose) that met criteria from the National Cholesterol Education Program Adult Treatment Panel III.
Results
Fifty-three percent of participants (n = 1711) had the metabolic syndrome at baseline; incidence did not vary substantially by age. However, low levels of high-density lipoprotein cholesterol predominated in younger participants (age 25 to 44 years), and high blood pressure predominated in older participants (age 60 to 82 years). In life-table analyses (log-rank test), incidence of the metabolic syndrome was reduced by 41% in the lifestyle group (P < 0.001) and by 17% in the metformin group (P = 0.03) compared with placebo. Three-year cumulative incidences were 51%, 45%, and 34% in the placebo, metformin, and lifestyle groups, respectively. There was no significant heterogeneity by ethnic group.
Limitations
The study involved a volunteer group with impaired glucose tolerance, which limits generalizability.
Conclusions
The metabolic syndrome affected approximately half of the participants in the Diabetes Prevention Program at baseline. Both lifestyle intervention and metformin therapy reduced the development of the syndrome in the remaining participants.
Considerable attention has recently been paid to the metabolic syndrome, a constellation of risk factors associated with insulin resistance and increased cardiovascular and diabetes risk. The third report of the National Cholesterol Education Program’s Adult Treatment Panel now calls for the identification and treatment of this high-risk state and provides a simple set of criteria for diagnosis (1). The World Health Organization (WHO) and the American College of Endocrinology have also provided definitions (2). Although recent studies have provided estimates of the prevalence of the metabolic syndrome in the United States (3, 4), its interrelationship with impaired glucose tolerance is unclear. In particular, it is largely unknown what proportion of participants with impaired glucose tolerance have the metabolic syndrome and whether this varies by ethnicity, age, and sex. Clearly, because an elevated blood glucose level is a common criterion for all definitions, a close association is to be expected. This association may be even stronger in the subgroup of persons with both impaired glucose tolerance and impaired fasting glucose (that is, a fasting plasma glucose level 6.1 to 6.9 mmol/L [110 to 125 mg/dL]). The extent to which we may be able to reduce cardiovascular risk in patients with impaired glucose tolerance by preventing the metabolic syndrome through lifestyle or medication interventions is also unknown.
The Diabetes Prevention Program (5, 6) provides a unique opportunity to begin to address these issues. It involves a large sample of more than 3000 participants with impaired glucose tolerance who were carefully followed and randomly allocated to treatment with an intensive lifestyle intervention, metformin, or placebo. In this report, we address 2 questions: the prevalence of the metabolic syndrome at baseline in the trial population (and how this varies by age and sex) and whether the 2 interventions reduced the incidence of new cases of the metabolic syndrome or increased resolution of existing cases compared with placebo.
Context
Intensive diet and exercise or metformin can prevent the development of diabetes in individuals with impaired fasting glucose, but the effects of these interventions on development of the metabolic syndrome are unknown.
Contribution
This secondary analysis of Diabetes Prevention Program data showed that lifestyle intervention and metformin each reduced the development of the metabolic syndrome among the 45% of participants who did not have it at baseline. The impact of lifestyle intervention was much more marked than that of metformin.
Implications
Interventions that prevent diabetes will also reduce the development of the metabolic syndrome.
–The Editors
METHODS
Participants and Procedures
Full details of the protocol have been published elsewhere (5, 6). The current report includes 3234 participants seen at baseline. This number includes participants from the 3 treatment arms investigated (that is, standard lifestyle or placebo, intensive lifestyle, and metformin), but not participants from the troglitazone arm, which was discontinued. Individuals were recruited between June 1996 and May 1999 from a variety of sources, including community screenings and household mailings, on the basis of perceived risk for diabetes. Written informed consent was obtained from all participants before screening, consistent with the Declaration of Helsinki and the guidelines of each center’s institutional review board.
The initial screening step consisted of a fasting glucose measurement. If the participant was eligible, this was followed by a 75-g oral glucose tolerance test. Inclusion criteria were as follows: a fasting plasma glucose level of 5.3 to 7.0 mmol/L (95 to 125 mg/dL) (≥7.0 mmol/L [≥125 mg/dL] for Native Americans); a 2-hour plasma glucose level of 7.8 to 11.1 mmol/L (140 to 199 mg/dL) following the glucose load; age of at least 25 years; and body mass index of at least 24 kg/m2 (≥22 kg/m2 for Asian Americans because of differences in body size in this group). Main exclusion criteria were recent myocardial infarction, symptoms of coronary heart disease, major illness, previous diagnosis of diabetes, use of medications known to impair glucose tolerance, or triglyceride level of at least 6.8 mmol/L (≥600 mg/dL), as previously detailed (5). Standardized interviewer-administered questionnaires were used to obtain self-reported data on personal medical history, medications, and diet. Self-reported race or ethnicity was classified according to the question used in the 1990 U.S. Census questionnaire (7). Overall, adiposity was assessed by body mass index. Waist circumference was assessed in the standing position midway between the highest point of the iliac crest and the lowest point of the costal margin in the mid-axillary line. All anthropometric measures reflected the average of 2 measurements. Blood pressure was measured twice at 30-second intervals by using a standard mercury manometer. The participant was seated in a chair for 5 minutes before the first measurement was taken, and the mean of the 2 readings was used in the analyses.
The metabolic syndrome was defined according to criteria from the National Cholesterol Education Program’s Adult Treatment Panel III (1), namely 3 or more of the following conditions: waist circumference greater than 102 cm in men and greater than 88 cm in women; serum triglyceride level of at least 1.7 mmol/L (≥150 mg/dL); high-density lipoprotein (HDL) cholesterol level less than 1.03 mmol/L (<40 mg/dL) in men and less than 1.3 mmol/L (<50 mg/dL) in women; blood pressure of 130/85 mm Hg or greater; and fasting plasma glucose level of 6.2 mmol/L (110 mg/dL).
Participants who were being treated with blood pressure–lowering or triglyceride-lowering medications (niacin or fibric acid derivatives) were classified as positive for the respective criterion. We chose the Adult Treatment Panel III (1) criteria because they are commonly used in the United States and are simpler to apply in clinical practice than, for example, the WHO criteria (2).
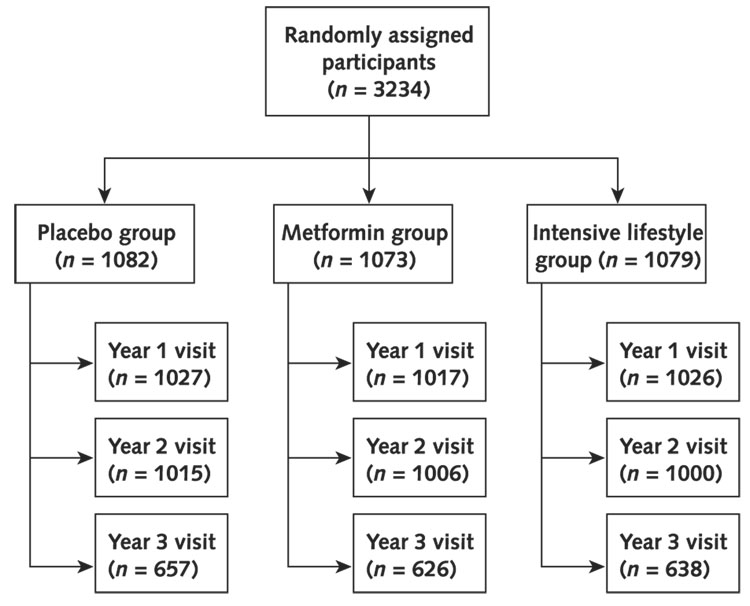
Participants were randomly assigned to receive 1 of 3 interventions: standard lifestyle recommendations plus metformin, 850 mg twice per day; standard lifestyle recommendations plus placebo; or an intensive program of lifestyle intervention. The randomization was done centrally by computer; assignments to the lifestyle group were blinded until randomization, while assignments to the medication groups were blinded until the end of the study. The goals of the lifestyle program were to achieve and maintain a weight reduction of at least 7% of clinical body weight through a healthy low-calorie, low-fat diet and to engage in physical activity of moderate intensity, such as brisk walking, for at least 150 minutes per week. Participants were seen quarterly, when blood pressure was assessed. Fasting glucose levels were determined at the 6-month visits, and fasting lipid levels and waist circumference were measured annually. Further details have been published elsewhere (5, 6). Figure 1 shows the number of participants observed at each annual examination by treatment group.
Figure 1.
Randomly assigned participants by treatment group and annual visit.
Laboratory Methods
All of the analytic measurements were performed at the central biochemistry laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle, Washington). Fasting plasma glucose level was measured on a chemistry autoanalyzer by the glucokinase method. Insulin measurements were performed by using a polyethylene glycol–accelerated double antibody radioimmunoassay method developed in the Diabetes Endocrinology Research Center Immunoassay Core Laboratory (University of Washington, Seattle, Washington). This method is based on the use of an anti–human insulin guinea pig antibody and measures total immunoreactive insulin. The homeostasis model assessment for insulin resistance was calculated as follows (8):
Measurements of total plasma cholesterol and triglycerides were performed enzymatically on a chemistry autoanalyzer by using methods standardized to the Centers for Disease Control and Prevention reference methods (9). We obtained HDL fractions for cholesterol analysis by treating whole plasma with dextran sulfate magnesium chloride to precipitate all of the apolipoprotein B–containing lipoproteins (10). We calculated low-density lipoprotein cholesterol by using the Friedewald equation (11). In participants with triglyceride levels higher than 4.5 mmol/L (>400 mg/dL), the lipoprotein fractions were separated by using preparative ultracentrifugation of plasma by beta quantification (12).
Statistical Analyses
Participants were followed for an average of 3.2 years (range, 0.04 to 5.0 years) from the start of the study in June 1996 through 31 July 2001, a period 4 months longer than that reported previously (5). This period was chosen to maximize the available data that were collected during the masked phase of the Diabetes Prevention Program, since unmasking occurred in early August 2001.
Random treatment assignments were stratified according to clinical center and were generated by the coordinating center through computer linkup to the field center at time of randomization. Therefore, assignment was unknown until randomization. Assignments to metformin and placebo were double-blinded. The study design and analysis followed the intention-to-treat principle. Nominal (unadjusted) P values and confidence intervals are reported. Logistic regression was used to compare the prevalence of the metabolic syndrome and its components at baseline among the demographic variables. The time to the outcome was assessed by using life-table methods (13). Modified product-limit curves for the cumulative incidence of the metabolic syndrome and for its resolution were compared by using the log-rank test. The estimated cumulative incidence, or resolution, at 3 years and the risk reduction, heterogeneity among strata, and interactions between treatment assignments and covariates were assessed by using proportional-hazards regression. Fixed-effects models with the assumption of normally distributed errors (14) were used to assess differences over time in body weight and in plasma glucose and glycosylated hemoglobin values among the 3 groups.
Role of the Funding Sources
The Diabetes Prevention Program was principally supported by the National Institute for Diabetes and Digestive and Kidney Diseases and other components of the National Institutes of Health and the Centers for Disease Control and Prevention. Representatives of the National Institutes of Health and the Centers for Disease Control and Prevention participated in the design of the study and in the reporting of the results. Although additional funding was provided by a variety of other sources, as listed in the acknowledgments, these sources had no role in the design or conduct of the study or in the reporting of results.
RESULTS
Baseline Prevalence
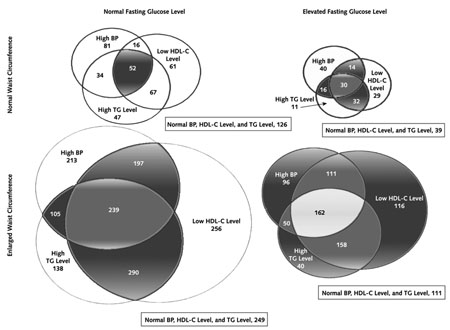
Table 1 shows the prevalence of the metabolic syndrome and each of its components in the 3234 participants examined at baseline. Fifty-three percent of the participants (n = 1711) fulfilled the criteria for the metabolic syndrome; this proportion was relatively constant by age group. However, significant differences by age group (<45 years, 45 to 59 years, and ≥60 years) emerged for the separate components of the syndrome. Prevalence of low HDL cholesterol level ranged from 70% in those 25 to 44 years of age to only 40% in those age 60 years or older. Increased waist circumference, the most common component in all age groups, was present in 82% of those younger than 45 years of age, 78% of those 45 to 59 years of age, and 73% of those at least 60 years of age. High triglyceride levels (present in 46% of all participants) varied little by age, while high fasting plasma glucose level, whose prevalence ranged from 31% to 35%, was more common in the older age groups. High blood pressure was also more prevalent in older participants, doubling from 31% in those younger than age 45 years to more than 60% in those 60 to 82 years of age. Prevalence of the components also varied by sex: Women more frequently had increased waist circumference and low HDL cholesterol levels, while men more frequently had high triglyceride levels, high fasting blood glucose levels, and hypertension. (It should be noted that the National Cholesterol Education Program has different criteria for waist circumference and HDL level for each sex.) Although significant ethnic variation was also seen, it could not be clearly separated from the differing entry criteria used for different ethnic groups. For example, the metabolic syndrome was least prevalent in Asian Americans (41%) and in Native Americans (46%), groups who had lower thresholds for BMI and fasting glucose level, respectively, at study entry. Finally, if the criterion of more than 5.6 mmol/L (>100 mg/dL) were used for fasting glucose level instead of 6.2 mmol/L (110 mg/dL), the overall prevalence at baseline would have been 69% (2223 of 3234) rather than the 53% noted earlier. The interrelationship of the components of the metabolic syndrome is shown in the Appendix Figure (available at www.annals.org).
Table 1.
Prevalence of the Metabolic Syndrome and Its Components by Age and Sex in the Diabetes Prevention Program*
| Variable | All Participants (n= 3234) | Age |
Sex |
|||
|---|---|---|---|---|---|---|
| <45 y (n= 1000) | 45–59 y (n= 1586) | ≥60 y (n = 648) | Men (n = 1043) | Women (n = 2191) | ||
| Metabolic syndrome, n (%) | 1711 (53) | 521 (52) | 868 (55) | 322 (50) | 550 (53) | 1161 (53) |
| Waist circumference, n (%)†‡ | 2532 (78) | 818 (82) | 1240 (78) | 474 (73) | 656 (63) | 1876 (86) |
| Low level of HDL cholesterol, n (%)†‡ | 1838 (57) | 698 (70) | 883 (56) | 257 (40) | 529 (51) | 1309 (60) |
| High triglyceride levels or receiving treatment for high triglyceride levels, n (%)‡ | 1472 (46) | 423 (42) | 764 (48) | 285 (44) | 522 (50) | 950 (43) |
| High fasting plasma glucose level, n (%)†‡ | 1060 (33) | 307 (31) | 526 (33) | 227 (35) | 435 (42) | 625 (28) |
| High blood pressure or receiving treatment for high blood pressure, n (%)† | 1460 (45) | 310 (31) | 740 (47) | 410 (63) | 569 (55) | 891 (41) |
The metabolic syndrome is defined by using the National Cholesterol Education Program’s Adult Treatment Panel III criteria. HDL = high-density lipoprotein.
P < 0.05 for comparisons across all age groups.
P < 0.05 across all sex groups.
The severity of the metabolic syndrome, in terms of the number of positive criteria (mean, 2.6), did not differ by treatment group, sex, or age. No differences were seen among the treatment groups in the prevalence of the metabolic syndrome or of its components at baseline, except for low HDL cholesterol level (P = 0.01), which was more prevalent in the placebo group than in the metformin and lifestyle groups (60.4%, 54.3%, and 55.8%, respectively). The prevalence of high triglyceride levels was also marginally higher (P = 0.06) in the placebo group than in the metformin and lifestyle groups (48.6%, 44.0%, and 44.2%, respectively).
Baseline Correlations
Table 2 shows the correlations (Spearman rank) among the different components of the metabolic syndrome at baseline after adjustment for age, sex, and ethnicity. Also shown are the correlations with the homeostasis model assessment score for insulin resistance. Apart from the anticipated high correlation between systolic and diastolic blood pressure (r = 0.60) and between triglyceride level and HDL cholesterol level (r = −0.39), other major correlations were those between homeostasis model assessment for insulin resistance and 1) waist circumference (r = 0.44), 2) HDL cholesterol level (r = −0.31), and 3) fasting plasma glucose level (r = 0.31).
Table 2.
Baseline Spearman Partial Correlations of Metabolic Syndrome Risk Factors and Homeostasis Model Assessment Score for Insulin Resistance Adjusted for Age, Sex, and Ethnicity*
| Variable | Triglyceride Level | HDL Cholesterol Level | Systolic Blood Pressure | Diastolic Blood Pressure | Fasting Plasma Glucose Level | Homeostasis Model Assessment Score for Insulin Resistance |
|---|---|---|---|---|---|---|
| Waist circumference | 0.07 | −0.25 | 0.16 | 0.19 | 0.19 | 0.44 |
| Triglyceride level | – | −0.39 | 0.04 | 0.05 | 0.05 | 0.19 |
| HDL cholesterol level | – | – | 0.01 | −0.09 | −0.18 | −0.31 |
| Systolic blood pressure | – | – | – | 0.60 | 0.13 | 0.09 |
| Diastolic blood pressure | – | – | – | – | 0.07 | 0.14 |
| Fasting plasma glucose level | – | – | – | – | – | 0.31 |
Correlations with absolute values of 0.04 or greater, 0.05 or greater, and 0.06 or greater are statistically significant at P < 0.05, P < 0.01, and P < 0.001, respectively. HDL = high-density lipoprotein.
Incidence of the Metabolic Syndrome among Participants without the Syndrome at Baseline
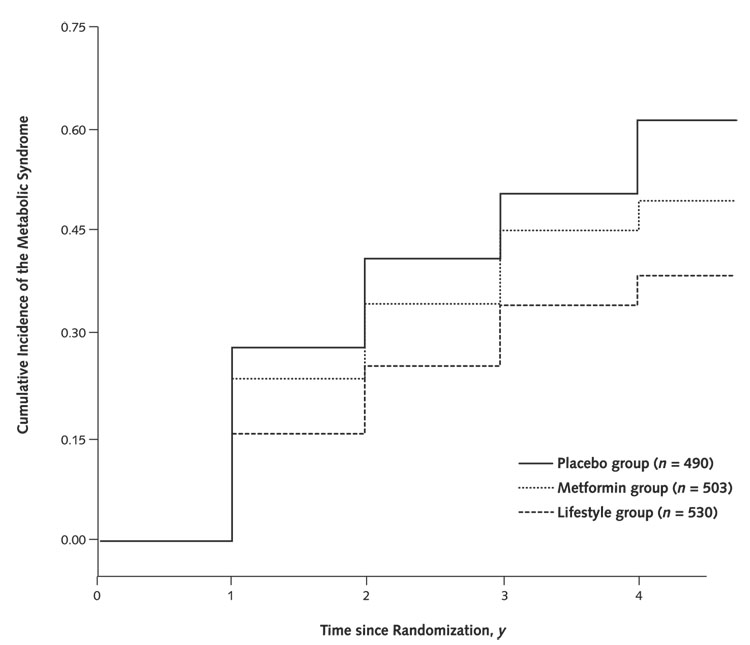
Figure 2 separately shows the cumulative incidence of the metabolic syndrome by time since randomization for the 3 intervention groups after exclusion of baseline cases. The incidence is highest in the placebo group. By 3 years, 53% of the participants in the placebo group (260 of 490) had acquired the metabolic syndrome compared with 47% (236 of 503) in the metformin group and 38% (201 of 530) in the lifestyle group. The cumulative incidence overall (per 100 person-years) was 61% for the placebo group, 50% for the metformin group, and 38% for the lifestyle group. In a proportional hazards analysis, lifestyle intervention yielded a reduction of 41% (95% CI, 28% to 52%) in incidence of the metabolic syndrome compared with placebo (P < 0.001) and a significant 29% (CI, 13% to 42%) reduction compared with metformin (P < 0.001), which itself yielded a 17% (CI, 0% to 31%) lower incidence than placebo (P = 0.03). Redefining the metabolic syndrome by using the criterion of a glucose level greater than 110 mg/dL (>6.2 mmol/L) yielded very similar results. Three-year incidences were 40%, 33%, and 27% for the placebo, metformin, and lifestyle groups, respectively. No major adverse events were noted, but musculoskeletal problems were more common in the lifestyle group and gastrointestinal symptoms were more common in the metformin group and less common in the lifestyle group than in the group receiving placebo.
Figure 2.
Development of the metabolic syndrome by intervention group in the Diabetes Prevention Program.
We also examined whether the efficacy of the 2 interventions differed by age, sex, ethnicity, or baseline fasting insulin level. Lifestyle intervention was least effective in those 25 to 44 years of age. Lifestyle intervention was effective compared with placebo in both men and women (P < 0.001), but more so in men (64% vs. 37%) (P = 0.02 for heterogeneity). Metformin was not effective compared with placebo in women (P > 0.2) but was effective in men (P = 0.002), again demonstrating significant heterogeneity (P = 0.02). Although numbers became too small for definitive results when divided according to ethnicity, it appears that risk reduction compared with placebo was greater for the lifestyle group than for the metformin group in both white persons (50% vs. 12%, respectively) and Hispanic persons (57% vs. 2%, respectively). In African Americans (42% vs. 29%) and Native Americans (43% vs. 42%), efficacy for the lifestyle group and the metformin group appeared more similar, while for Asian Americans, metformin showed a nonsignificantly greater reduction than intensive lifestyle intervention (62% vs. 30%). The efficacy of neither lifestyle nor metformin showed significant heterogeneity across the 5 ethnic groups. Finally, although lifestyle was effective compared with placebo regardless of baseline fasting insulin level, metformin showed significant heterogeneity (P = 0.03). Metformin was most effective in participants with a fasting insulin level of 136 pmol/L or less and had no effect in those with fasting insulin levels of 144 to 215 pmol/L or greater than 125 pmol/L compared with placebo.
Incidence of Metabolic Syndrome Components among Participants Not Meeting Criteria at Baseline
To examine the effect of the interventions on individual components, life-table analyses were performed for those who did not met the criteria at baseline. The incidence of specific components over time compared with placebo suggests that lifestyle reduces the incidence of all components except HDL cholesterol level, while metformin is effective only in reducing the incidence of waist circumference and fasting glucose level (Table 3).
Table 3.
Three-Year Incidence and Prevalence of Metabolic Syndrome Components among Diabetes Prevention Program Participants by Treatment Group*
| Metabolic Syndrome Component | Cumulative Incidence among Participants Not Meeting Criterion at Baseline, % (n) |
Prevalence among Participants Meeting Criterion at Baseline, % (n) |
||||
|---|---|---|---|---|---|---|
| Placebo Group | Metformin Group | Lifestyle Group | Placebo Group | Metformin Group | Lifestyle Group | |
| Waist circumference | 33 (241) | 15 (222)† | 8 (236)† | 93 (515) | 89 (492)† | 81 (492)‡ |
| Low HDL cholesterol level | 70 (429) | 67 (490) | 68 (477) | 87 (398) | 79 (333)‡ | 78 (377)‡ |
| High triglyceride level | 27 (555) | 30 (601) | 18 (601)† | 73 (305) | 72 (289) | 60 (286)† |
| High fasting plasma glucose level | 40 (726) | 29 (714)† | 28 (734)† | 74 (221) | 60 (210)† | 55 (210)† |
| High blood pressure | 41 (613) | 44 (571) | 35 (590)† | 81 (297) | 80 (307) | 68 (262)† |
Numbers in parentheses are numbers at baseline. HDL = high-density lipoprotein.
P < 0.001 versus placebo.
P < 0.05 versus placebo.
Resolution of the Metabolic Syndrome among Participants Who Had the Syndrome at Baseline
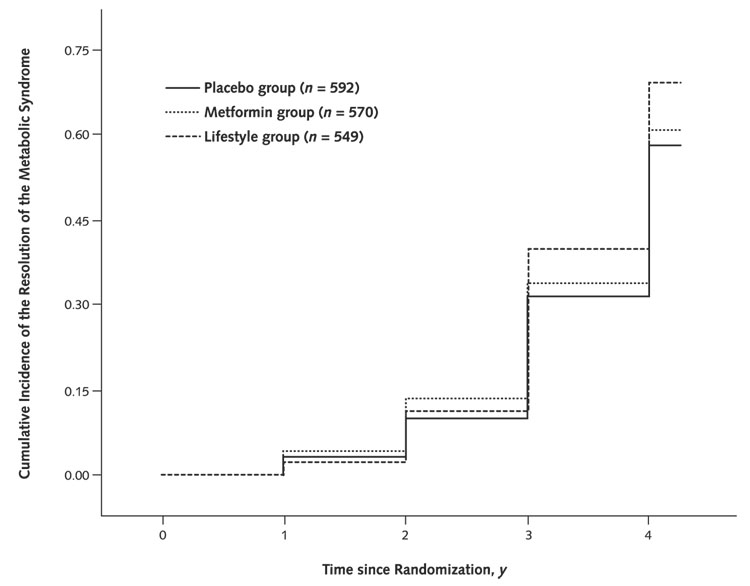
Figure 3 shows the cumulative incidence of resolution of the metabolic syndrome (that is, no longer meeting the criteria) by treatment group. Although the pattern is somewhat similar to that seen for incidence of the metabolic syndrome, with the best result at 3 years occurring in the lifestyle group and an intermediary effect noted for metformin, the differences are less striking. By the log-rank test, only lifestyle showed a significant effect compared with placebo (P = 0.002). Nevertheless, prevalence at 3 years did vary significantly by treatment group (P < 0.001): Eighteen percent of the placebo group, 23% of the metformin group, and 38% of the lifestyle group no longer had the syndrome.
Figure 3.
Resolution of the metabolic syndrome by intervention group in the Diabetes Prevention Program.
Prevalence of Metabolic Syndrome Components among Participants Meeting Criteria at Baseline
Also shown in Table 3 is the effect of the interventions on each component among participants who met the specific component criterion at baseline. A slightly different pattern emerges, that is, both interventions decreased the prevalence of low levels of HDL cholesterol, increased waist circumference, and fasting glucose level, while intensive lifestyle intervention also lowered the prevalence of increased blood pressure and triglyceride levels.
Prevalence of the Metabolic Syndrome in All Participants at 3 Years
The prevalence of the metabolic syndrome in all participants increased from 55% at baseline to 61% after 3 years in the placebo group (P = 0.003) and from 54% to 55% in the metformin group (P > 0.2). In the lifestyle group, overall prevalence decreased from 51% to 43% (P < 0.001).
DISCUSSION
These data provide further evidence of the potential benefit of the Diabetes Prevention Program interventions beyond diabetes prevention alone and raise the additional possibility of cardiovascular prevention. Although we are unaware of any publications specifically examining the prevalence of the metabolic syndrome in patients with impaired glucose tolerance, a recent analysis of the data set from the Third National Health and Nutrition Examination Survey (NHANES III) suggests that 33% of U.S. adults who are 50 years of age or older and have impaired glucose tolerance also have the metabolic syndrome (4). Our overall prevalence of 53% is probably higher, even allowing for our additional requirement of a fasting plasma glucose level of at least 5.3 mmol/L (≥95 mg/dL). Several reports have also documented the general prevalence of this syndrome by using either the Adult Treatment Panel III criteria (1) used in the current report or the WHO definitions (2). Ford and colleagues (3) recently estimated from NHANES III that the metabolic syndrome was present in approximately 22% of all U.S. adults age 20 years and older. Of interest, unlike our data, which showed a relatively constant prevalence by age group, the age-specific prevalence of the metabolic syndrome in the general population increased dramatically, from just over 12% among individuals in their thirties to 20% among those in their forties, 35% among those in their fifties, and 45% thereafter. Again, it would seem that our population, as expected, has a higher prevalence of the metabolic syndrome and presumably higher cardiovascular and diabetes risk than the general population. However, it should be noted that our population is highly selected for having impaired glucose tolerance but not diabetes. One might thus anticipate a less marked prevalence relationship with age in the Diabetes Prevention Program, since we would be increasingly eliminating participants with the metabolic syndrome who have also developed diabetes as they age. The NHANES data from Ford and colleagues (3) also examined prevalence by sex and, as in our population, showed little difference overall. The American Diabetes Association recently redefined prediabetes by using a fasting plasma glucose criterion (15) of greater than 5.6 mmol/L (>100 mg/dL). As anticipated, this increased the prevalence of the condition but had little effect on the relative impact of the interventions on incidence.
Prevalence studies that have examined the association of the metabolic syndrome with cardiovascular disease generally support the syndrome’s identification and prevention. The Bruneck study (16), for example, reported that participants with the WHO definition of the metabolic syndrome had an increased risk for both carotid and coronary disease after 5 years of follow-up. Alexander and associates’ analysis of the NHANES III data (4) also suggests that participants with the metabolic syndrome (with or without diabetes) have an increased prevalence of coronary artery disease, thus further underscoring the potential benefit of preventing or delaying the syndrome. However, these findings should be interpreted in the context of other related states, for example, impaired glucose tolerance itself. The Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe (DECODE) study (17, 18) recently firmly established a strong predictive role for all-cause and cardiovascular mortality. Nonetheless, these interventions, especially the lifestyle intervention, should yield cardiovascular benefit by improving glucose tolerance and the metabolic syndrome, no matter how the risk is attributed between the two.
Our data, although derived from a volunteer population with impaired glucose tolerance but not diabetes, provide potentially important clinical information concerning the metabolic syndrome and its prevention. Although the population studied had moderately severe impaired glucose tolerance (fasting plasma glucose level ≥5.3 mmol/L [≥95 mg/dL]), only 53% overall had the metabolic syndrome, suggesting that this high-risk state is far from omnipresent even in impaired glucose tolerance. Of particular note is the variation by age group of the components contributing to the diagnosis. Waist circumference and low HDL cholesterol level appear to be particular features of the younger participants with the metabolic syndrome, while blood pressure is particularly important for the older participants. Triglyceride levels, on the other hand, appear fairly constant for all ages. It is interesting to speculate whether this age difference relates to the increasing conversion to diabetes (and thus study ineligibility) with age or differences in perceived diabetes risk leading to Diabetes Prevention Program screening. Similarly, the difference between the sexes is interesting: Waist circumference and low HDL cholesterol level predominate in women and high fasting glucose level and high blood pressure are more noticeable in men. Because the criteria for entry varied by ethnic group to some degree, meaningful interpretation of ethnicity-specific data is limited, although it is important to note that no heterogeneity was seen for treatment effects by ethnic group.
Because the metabolic syndrome is not consistent over age groups, these findings could be interpreted as an argument for treating the individual risk factors themselves and not limiting intervention to the syndrome. These findings also raise the issue recently addressed by Stern and colleagues (19) that cardiovascular risk prediction is probably better addressed by multivariable models than by categorizing participants by glucose tolerance status. Of interest, waist circumference and low HDL cholesterol levels were the best predictors in the San Antonio Heart Study (12).
Particularly encouraging is the dramatic effect of life-style on both the incidence of new metabolic syndrome (and its components) and on participants who had the syndrome at baseline. The beneficial effect of lifestyle intervention on components other than glucose level is particularly encouraging and provides important evidence of the value of this approach to those with blood pressure, weight, and lipid disturbances in general. The intermediary effect of metformin, which closely mirrors the pattern of results for the overall trial in terms of diabetes prevention or delay, further underscores the preferential value of life-style as the initial approach to prevention of the metabolic syndrome and its cardiovascular complications.
The complete lack of effect of metformin in women is surprising, given its overall effect on diabetes prevention. This finding can be only partly related to the smaller sample available for prevention of the metabolic syndrome. Sex differences in the prediction of the metabolic syndrome have, however, been noted before. For example, Han and colleagues (20) reported that C-reactive protein level predicted the metabolic syndrome in women but not men. It has also been observed that metformin decreases basal testosterone levels in men (21) but not women (22). In addition, the sex-specific criteria for the syndrome may play a role. These criteria are somewhat arbitrary and may not effectively represent underlying metabolic differences between the sexes. The reduced efficacy of metformin in participants with higher baseline insulin levels also deserves further investigation, although it probably reflects a relatively weaker effect of metformin on insulin resistance than that seen for lifestyle.
The dramatic effect of lifestyle on both the prevention of incident metabolic syndrome and reduction of its overall prevalence appears to be most strongly related to a reduction in waist circumference and in blood pressure and not, as might have been anticipated, through a correction of the lipid abnormalities of triglycerides and HDL cholesterol. The incidence of abnormal HDL cholesterol level was virtually identical by treatment group, while waist circumference and frequency of hypertension were both significantly reduced by intensive lifestyle intervention. It is interesting to speculate why lifestyle should have relatively little effect on HDL cholesterol level but such a marked effect on blood pressure. Similar results have been reported by the Finnish Diabetes Prevention Study (23). One possibility may be that although total calories were reduced in the lifestyle group, the relatively greater focus on fat content may have altered the ratio of polyunsaturated to saturated fats, which is related to HDL cholesterol levels (24).
Although we were not able to determine the best way to define the metabolic syndrome as an intervention target, or even whether to define it at all, future follow-up of the cohort will enable us to determine whether this particular combination of risk factors or some other variation better predicts cardiovascular outcomes. Naturally, our intervention results apply primarily to persons with impaired glucose tolerance and cannot be immediately translated to other groups.
These data provide an extensive and detailed description of the metabolic syndrome in a high-risk population and reveal important differences in composition of the syndrome by age and sex. They also demonstrate the value of lifestyle intervention, in particular, in both the prevention and treatment of this condition, above and beyond improvement in glycemia alone. Lifestyle intervention may reduce cardiovascular risk in persons with impaired glucose tolerance.
Acknowledgments
Grant Support: Funding was provided by the National Institutes of Health (5U01DK048489) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center on Minority Health and Health Disparities, the National Institute of Child Health and Human Development, the Office of Women’s Health, and the National Institute on Aging. In addition, the Indian Health Service, the Centers for Disease Control and Prevention, the American Diabetes Association, and 2 pharmaceutical companies—Bristol-Myers Squibb and Parke-Davis—contributed support. The General Clinical Research Center Program, National Center for Research Resources, supported many of the clinical centers. Support to the clinical centers and the Coordinating Center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases through a cooperative agreement, except for the Southwestern American Indian Centers, which were supported directly by the National Institute of Diabetes and Digestive and Kidney Diseases and the Indian Health Service.
Appendix Figure
Venn diagram of the components of the metabolic syndrome
Numbers are numbers of participants at baseline. The shaded sections represent participants meeting the metabolic syndrome criteria as defined by the National Cholesterol Education Program Adult Panel II. BP = blood pressure; HDL-C = high-density lipoprotein cholesterol; TG = triglyceride.
Footnotes
Potential Financial Conflicts of Interest: Consultancies: T.J. Orchard (Metabolic Syndrome Alliance, Sanofi-Aventis); Grants received: R. Ratner (Bristol-Myers Squibb).
Permission to reproduce material from Annals of Internal Medicine: To purchase a single copy of Annals, contact the American College of Physicians Customer Service Department at 800-523-1546. For permissions information, contact Stacey Gallo, Permissions Coordinator, at 800-523-1546, extension 2678. For bulk reprint information, contact Tracy Taylor, Reprints Coordinator, at 973-808-1329.
References
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [PMID: 11368702] [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [PMID: 9686693] [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [PMID: 11790215] [DOI] [PubMed] [Google Scholar]
- 4.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [PMID: 12716754] [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [PMID: 11832527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [PMID: 10189543] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bureau of the Census. Washington, DC: U.S. Government Printing Office; Census of the Population, 1990. 1990
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [PMID: 3899825] [DOI] [PubMed] [Google Scholar]
- 9.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [PMID: 3724535] [DOI] [PubMed] [Google Scholar]
- 10.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PMID: 7074948] [PubMed] [Google Scholar]
- 11.Svejgaard A, Platz P, Ryder LP. HLA and disease 1982—a survey. Immunol Rev. 1983;70:193–218. doi: 10.1111/j.1600-065x.1983.tb00715.x. [PMID: 6339368] [DOI] [PubMed] [Google Scholar]
- 12.Hokanson JE, Austin MA, Brunzell JD. Measurement and clinical significance of low density lipoprotein subclasses. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of Lipoprotein Testing. Washington, DC: AACC Pr; 1997. pp. 267–282. [Google Scholar]
- 13.Lachin JM. The Assessment of Relative Risks. New York: J Wiley; 2000. Biostatistical Methods. [Google Scholar]
- 14.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York: Oxford Univ Pr; 1994. [Google Scholar]
- 15.Screening for type 2 diabetes. Diabetes Care. 2004;27 Suppl 1:S11–S14. doi: 10.2337/diacare.27.2007.s11. [PMID: 14693922] [DOI] [PubMed] [Google Scholar]
- 16.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, et al. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care. 2003;26:1251–1257. doi: 10.2337/diacare.26.4.1251. [PMID: 12663606] [DOI] [PubMed] [Google Scholar]
- 17.Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [PMID: 11176766] [DOI] [PubMed] [Google Scholar]
- 18.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617–621. [PMID: 10466661] [PubMed] [Google Scholar]
- 19.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med. 2002;136:575–581. doi: 10.7326/0003-4819-136-8-200204160-00006. [PMID: 11955025] [DOI] [PubMed] [Google Scholar]
- 20.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016–2021. doi: 10.2337/diacare.25.11.2016. [PMID: 12401749] [DOI] [PubMed] [Google Scholar]
- 21.Ozata M, Oktenli C, Bingol N, Ozdemir IC. The effects of metformin and diet on plasma testosterone and leptin levels in obese men. Obes Res. 2001;9:662–667. doi: 10.1038/oby.2001.90. [PMID: 11707532] [DOI] [PubMed] [Google Scholar]
- 22.Vrbikova J, Hill M, Starka L, Cibula D, Bendlova B, Vondra K, et al. The effects of long-term metformin treatment on adrenal and ovarian steroidogenesis in women with polycystic ovary syndrome. Eur J Endocrinol. 2001;144:619–628. doi: 10.1530/eje.0.1440619. [PMID: 11375796] [DOI] [PubMed] [Google Scholar]
- 23.Crouse JR., 3rd Gender, lipoproteins, diet, and cardiovascular risk. Sauce for the goose may not be sauce for the gander. Lancet. 1989;1:318–320. doi: 10.1016/s0140-6736(89)91320-2. [PMID: 2563468] [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, et al. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–3236. doi: 10.2337/diacare.26.12.3230. [PMID: 14633807] [DOI] [PubMed] [Google Scholar]