Abstract
The contribution of C-N bond-breaking/making steps to the rate of the free-radical-mediated deamination of vicinal amino alcohols by adenosylcobalamin-dependent ethanolamine ammonia-lyase has been investigated by 15N isotope effects (IE's) and by electron paramagnetic resonance (EPR) spectroscopy. 15N IE's were determined for three substrates, ethanolamine, (R)-2-aminopropanol, and (S)-2-aminopropanol using isotope ratio mass spectrometry analysis of the product ammonia. Measurements with all three substrates gave measurable, normal 15N IE's; however, the IE of (S)-2-aminopropanol was ∼ 5-fold greater than the other two. Reaction mixtures frozen during the steady-state show that the 2-aminopropanols give EPR spectra characteristic of the initial substrate radical whereas ethanolamine gives spectra consistent with a product-related radical [Warncke, K.; Schmidt, J. C.; Kee, S.-C., J. Am. Chem. Soc. 1999, 121, 10522-10528]. The steady-state concentration of the radical with (R)-2-aminopropanol is ∼ half that observed with the S isomer, and with (R)-2-aminopropanol the steady-state level of radical is further reduced upon deuteration at C1. The results show that relative heights of kinetic barriers differ among the three substrates such that levels or identities of steady-state intermediates differ. 15N-Sensitive steps are significant contributors to V/K with (S)-2-aminopropanol.
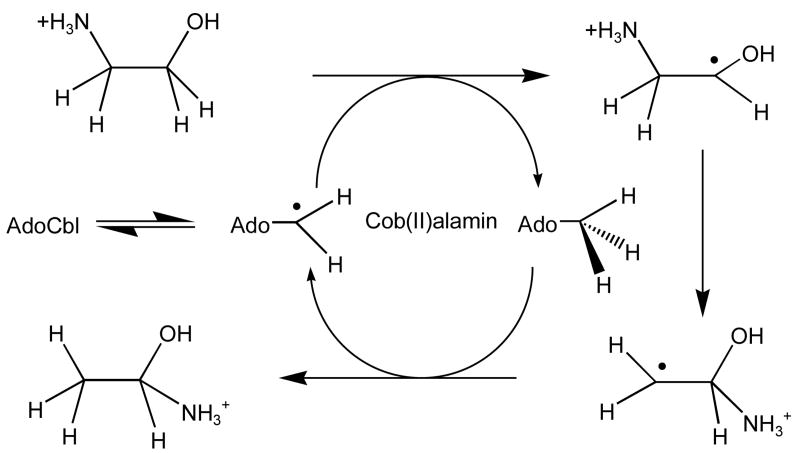
The bacterial enzyme, ethanolamine ammonia-lyase (EAL, EC 4.3.1.7) catalyzes the adenosylcobalamin (AdoCbl)-dependent deamination of ethanolamine or 2-aminopropanols to ammonia and the corresponding aldehyde.1 The reaction is one of several 1-2 radical-rearrangements/eliminations catalyzed by AdoCbl-dependent enzymes.2-4 The paradigm for these reactions is an interchange of a group at C2 and a hydrogen atom at C1. With the exception of glutamate mutase,5 mechanistic details of the migration steps in the AdoCbl-dependent enzymes are not well understood.3 The EAL reaction can be considered as a C2 → C1 migration of ammonia, followed by decomposition of the product carbinolamine (Scheme 1).1
Scheme I.
EAL Reaction–Carbinolamine intermediate.
Migration of ammonia to C1 is not strictly required to form acetaldehyde.6 The migration mechanism has been demonstrated in the related enzyme dioldehydrase, and derives some support from stereochemistry.7 Computational studies have indicated that both an internal migration of ammonia or direct elimination are energetically feasible.6, 8
The mechanism includes two H atom abstraction steps that are energetically demanding. Isotopes of H have been used to determine the contributions of these H atom transfers to the overall rate.1 Transfer of 3H from 5′ position of AdoCbl to acetaldehyde during turnover shows an isotope effect (IE) of ∼100.9 Deuteration of carbon 1 of ethanolamine results in a IE on cob(II)alamin formation (carbon-cobalt bond cleavage) of >10 in the pre-steady state.10 The observation of a deuterium KIE on carbon-cobalt bond cleavage is evidence that this step is kinetically coupled to H atom transfer.11 The large hydrogen IE observed with EAL and other AdoCbl-dependent enzymes have been attributed to hydrogen tunneling.4, 12 The steady-state IE on Vmax for [1,1-2H2]-ethanolamine is ∼ 6.9, 13 For (S)- and (R)-2-aminopropanol, DV's are both ∼5.14 Attenuation of the 2H IE in the Vmax suggests that H-insensitive steps in the reaction contribute to limiting of the rate. For example, V/K for the EAL reaction is sensitive to external magnetic fields, suggesting that radical pair formation or recombination steps are reflected in V/K.15 The contribution to V/K of C–N bond breaking and making steps in the mechanism has not been measured. In order to assess the contribution of C-N bond breaking and making to V/K of the EAL reaction, we measured 15N IE's on the deamination reaction using ethanolamine, (R) - 2 -aminopropanol and (S)-2-aminopropanol as substrates. These three substrates provide a range of 3 orders of magnitude in V/K (see Table 1).
Table 1.
EAL Kinetic Parameters
| Substrate | Km (μM) | kcat (sec-1) |
|---|---|---|
| Ethanolamine | 1.9±0.2 | 30±1 |
| (R)-2-aminopropanol | 9±2 | 0.067±0.001 |
| (S)-2-aminopropanol | 0.80±0.06 | 0.12±0.01 |
Salmonella typhimurium EAL was over expressed in E. coli and purified essentially as described previously.16 EAL reactions were run either to completion or to ∼50% completion, quenched by addition of HCl, and EAL removed by ultrafiltration (see supplementary). The ammonia generated was steam distilled17 and analyzed by isotope ratio mass spectrometry.18 Fractional reaction was determined by a coupled assay for ammonia using glutamate dehydrogenase.19 Isotope effects were calculated using equation 1:
| (1) |
where f is fractional extent of reaction, Ro is the 15N/14N ratio of the starting substrate determined by 100% conversion to product ammonia, and Rp is the 15N/14N ratio for the product after partial conversion. Km's and kcat's for the three substrates were measured in 10 cm pathlength cuvettes using a coupled assay with alcohol dehydrogenase.
All three of the substrates tested gave measurable, normal 15N IE's. For ethanolamine and (R)-2-aminopropanol, the effects are on the order of 0.1%. For (S)-2-aminopropanol, the effect is nearly 5 times larger at 0.5% (Table 2).
Table 2.
15N Isotope Effects on EAL
| Substrate | 15(V/K) | na |
|---|---|---|
| Ethanolamine | 1.0017±0.0004 | 7 |
| (R)-2-aminopropanol | 1.0012±0.0002 | 3 |
| (S)-2-aminopropanol | 1.0055±0.0002 | 3 |
Number of determinations.
Cleland and coworkers have determined intrinsic 15N IE's in excess of 3% for enzyme catalyzed polar reactions.20 Assuming that intrinsic 15N IE's for radical reactions are similar to their polar equivalents, then it is clear that the 15N IE's measured here are significantly attenuated by other steps in the catalytic cycle.
There are two steps in the reaction that can give rise to 15N IE's–the rearrangement of substrate radical, and the elimination of ammonia from the carbinolamine. EPR measurements on radical intermediates can provide some insight into which steps are rate limiting. When reaction mixtures are frozen in the steady-state, the dominant radical species will be the intermediate just prior to a slow step. For (S)-2-aminopropanol the EPR spectrum is that of the initial substrate radical, and the intensity of the signal is consistent with ∼90% of the enzyme being present in the radical form.21 This steady-state build up of the substrate radical intermediate just prior to the radical rearrangement suggests that the rearrangement is a slow step in the reaction with (S)-2-aminopropanol. The 15N IE of 0.5% for (S)-2-aminopropanol is less than expected for the intrinsic IE on the C–N bond breaking. This result suggests that although the 15N-sensitive steps contribute to V/K, other steps in the catalytic cycle must also contribute. Samples frozen in the steady-state with (R)-2-aminopropanol give essentially the same EPR spectrum as (S)-2-aminopropanol. However, the signal intensity (double integral) with (R)-2-aminopropanol is ∼ half that of (S)-2-aminopropanol. Moreover, the intensity of the EPR signal drops another 5-fold when [1-2H2]-(R)-2-aminopropanol (prepared from d-alanine as described previously)21 is the substrate (Figure 1.).
Figure 1.
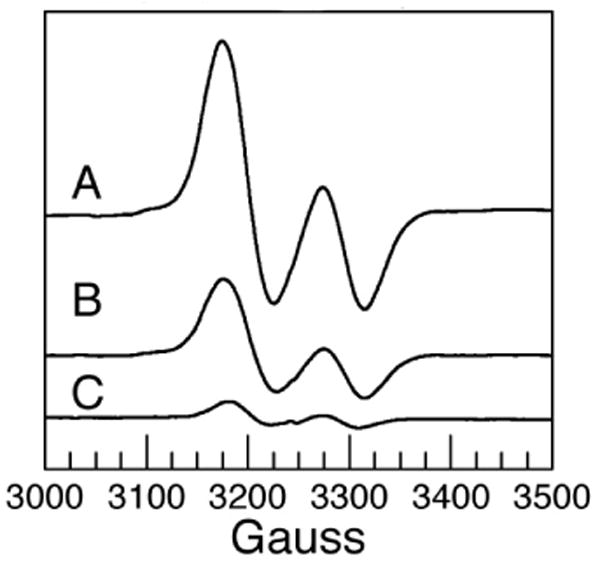
EPR spectra of steady-state 2-aminopropanol radicals at the active site of ethanolamine ammonia-lyase. Ethanolamine ammonia lyase and adenosylcobalamin were mixed with 2-aminopropanol, and frozen by dipping in liquid nitrogen. A (S)-2-aminopropanol. B (R)-2-aminopropanol. C [1-2H2]-(R)-2-aminopropanol. Spectra were recorded at 77 K. The ordinate is scaled such that amplitudes are proportional to concentration. Samples contained 0.24 mM enzyme, 0.47 mM adenosylcobalamin, 25 mM 2-aminopropanol, and 0.01 M Hepes/NaOH pH 7.5.
The intensity of EPR signals of samples made up with [1-2H2]-(S)-2-aminopropanol is the same as that of the unlabeled sample (not shown). The balance between formation, and breakdown of the radical determines its steady-state level. The lower amount of radical with (R)-2-aminopropanol, and the sensitivity of the steady-state radical concentration to deuteration at C1 suggest that the initial hydrogen atom abstraction is at least comparable in rate to the subsequent steps including 15N sensitive ones. The large D(V/K) (∼20) reported for the enzyme from Clostridium supports the notion that H atom abstraction is significantly rate limiting for (R)-2-aminopropanol.22 Rate limitation by H atom abstraction can account for the lower (compared to (S)-2-aminopropanol) 15N IE for (R)-2-aminopropanol.
For ethanolamine the spectrum is dominated, not by the substrate radical, but by a product, or product-related radical.23 The observation of a product-related radical for ethanolamine in the steady-state suggests that steps subsequent to the formation of the product radical are slower than the 15N sensitive steps in the catalytic cycle – a scenario consistent with the observation of a small 15N IE.
These results show that the relative heights of kinetic barriers encountered by the three substrates differ such that intermediates corresponding to different stages of the reaction or their steady-state levels differ (see Supporting Information). 15N-sensitive steps make a significant contribution to V/K with (S)-2-aminopropanol.
Supplementary Material
Sample preparation for isotope ratio mass spectrometry, further discussion of barrier heights. This material is available free of charge via the Internet at http://pubs.acs.org
Acknowledgments
This work was supported by NIH Grants GM35752 (G.H.R.) and GM18938 (W.W.C.). The authors acknowledge helpful discussions with Steven Mansoorabadi.
References
- 1.Bandarian V, Reed GH. Ethanolamine Ammonia-Lyase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. Wiley-Interscience; New York: 1999. pp. 811–833. [Google Scholar]
- 2.Banerjee R, Ragsdale SW. Annual Review of Biochemistry. 2003;72:209–47. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]; Toraya T. Chem Rev. 2003;103:2095–2128. doi: 10.1021/cr020428b. [DOI] [PubMed] [Google Scholar]; Reed GH. Curr Opin Chem Biol. 2004;8:477–83. doi: 10.1016/j.cbpa.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee R. Chem Rev. 2003;103:2083–2094. doi: 10.1021/cr0204395. [DOI] [PubMed] [Google Scholar]
- 4.Marsh ENG, Drennan CL. Curr Opin Chem Biol. 2001;5:499–505. doi: 10.1016/s1367-5931(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 5.Chih HW, Marsh ENG. J Am Chem Soc. 2000;122:10732–10733. [Google Scholar]
- 6.Semialjac M, Schwartz H. J Am Chem Soc. 2002;124:8974–8983. doi: 10.1021/ja020101s. [DOI] [PubMed] [Google Scholar]
- 7.Retey J, Umani-Ronchi A, Seibl J, Arigoni D. Experientia. 1966;22:502–503. doi: 10.1007/BF01898652. [DOI] [PubMed] [Google Scholar]; Rétey J, Suckling CJ, Arigoni D, Babior BM. J Biol Chem. 1974;249:6359–6360. [PubMed] [Google Scholar]
- 8.Wetmore SD, Smith DM, Bennett JT, Radom L. J Am Chem Soc. 2002;124:14054–14065. doi: 10.1021/ja027579g. [DOI] [PubMed] [Google Scholar]
- 9.Weisblat DA, Babior BM. J Biol Chem. 1971;246:6064–6071. [PubMed] [Google Scholar]
- 10.Bandarian V, Reed GH. Biochemistry. 2000;39:12069–12075. doi: 10.1021/bi001014k. [DOI] [PubMed] [Google Scholar]
- 11.Marsh ENG, Ballou DP. Biochemistry. 1998;37:11864–11872. doi: 10.1021/bi980512e. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee R. Biochemistry. 2001;40:6191–6198. doi: 10.1021/bi0104423. [DOI] [PubMed] [Google Scholar]
- 13.Babior BM. J Biol Chem. 1969;244:449–456. [PubMed] [Google Scholar]; Graves SW, Fox JA, Babior BM. Biochemistry. 1980;19:3630–3. doi: 10.1021/bi00556a032. [DOI] [PubMed] [Google Scholar]
- 14.Bandarian V. Ph D Thesis. University of Wisconsin; Madison: 1998. Radical Enzymology of Coenzyme B12-dependent Ethanolamine Ammonia-Lyase. [Google Scholar]
- 15.Harkins TT, Grissom CB. Science. 1994;263:958–960. doi: 10.1126/science.8310292. [DOI] [PubMed] [Google Scholar]; Taoka S, Padmakumar R, Grissom CB, Banerjee R. Bioelectromagnetics. 1997;18:506–513. doi: 10.1002/(sici)1521-186x(1997)18:7<506::aid-bem6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Bandarian V, Reed GH. Biochemistry. 1999;38:12394–402. doi: 10.1021/bi990620g. [DOI] [PubMed] [Google Scholar]
- 17.Hermes JD, Weiss PM, Cleland WW. Biochemistry. 1985;24:2959–67. doi: 10.1021/bi00333a023. [DOI] [PubMed] [Google Scholar]
- 18.Rishavy MA, Cleland WW, Lusty CJ. Biochemistry. 2000;39:7309–15. doi: 10.1021/bi000435z. [DOI] [PubMed] [Google Scholar]; Snider MJ, Reinhardt L, Wolfenden R, Cleland WW. Biochemistry. 2002;41:415–21. doi: 10.1021/bi011410i. [DOI] [PubMed] [Google Scholar]
- 19.Kaltwasser H, Schlegel HG. Anal Biochem. 1966;16:132–138. doi: 10.1016/0003-2697(66)90088-1. [DOI] [PubMed] [Google Scholar]
- 20.Wright SK, Rishavy MA, Cleland WW. Biochemistry. 2003;42:8369–76. doi: 10.1021/bi030092f. [DOI] [PubMed] [Google Scholar]
- 21.Bandarian V, Reed GH. Biochemistry. 2002;41:8580–8. doi: 10.1021/bi0201217. [DOI] [PubMed] [Google Scholar]
- 22.Babior BM. Ethanolamine Ammonia-Lyase. In: Dolphin D, editor. B12. Vol. 2 John Wiley & Sons, Inc.; New York: 1982. [Google Scholar]
- 23.Warncke K, Schmidt JC, Ke SC. J Am Chem Soc. 1999;121:10522–10528. [Google Scholar]; Warncke K, Canfield JM. J Am Chem Soc. 2004;126:5930–5931. doi: 10.1021/ja031569d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample preparation for isotope ratio mass spectrometry, further discussion of barrier heights. This material is available free of charge via the Internet at http://pubs.acs.org