Abstract
Using A10B single-chain fragment variable (scFv) as a model system, we demonstrated that the flexibility of scFv linker engineering can be combined with the inherent quick and adaptable characters of surface coupling chemistry (e.g., electrostatic, hydrogen bonding, or covalent attachment) to attach scFv to preformed functionalized self-assembled monolayers (SAMs). Six arginines, which were separated by glycine or serine as spacer, were incorporated in the peptide linker to form a 15-mer peptide linker (RGRGRGRGRSRGGGS). The polycationic arginine peptide was engineered into the A10B scFv-RG3 to favor its adsorption at anionic charged template surface (11-mercaptoundecanoic acid (MUA) and poly(sodium 4-styrenesulfonate (PSS))). This new approach was compared with the other engineered scFv constructs. Our results demonstrated that the anionic charged SAM template facilitated the oriented immobilization of scFvs on the SAM template surface as well as reduced the possibility of protein denaturation when directly immobilized on the solid surface. A 42-fold improvement of detection limits using MUA/A10B scFv-RG3 (less than 0.2 nM experimentally determined) was achieved compared to A10B Fab antibody and a 5-fold improvement was observed compared to A10B scFv that was engineered with a cysteine in the linker sequence. Using protein A-coated gold nanoparticles, a picomolar experimental detection limit was achieved. With 20 amino acids to choose from, engineered recombinant scFv in combination with SAM technology and nanoparticle mass amplification provide an emerging strategy for the development of highly sensitive and specific scFv immunosensors.
Biosensors are of increasing interest in the field of biology and medicine. The key component of most biosensors is the recognition element. The recognition element interacts with the analyte to be detected, thereby encountering a characteristic change in one of its physical properties, such as mass, refractive index, light absorbance, reduction potential, etc. Among all available recognition elements, (e.g., antibody, aptamer, enzyme, and nucleic acid), antibodies are by far the most widely used due to their excellent selectivity and sensitivity. The intensity of a specific signal produced on an antigen–antibody recognition is related to the amount of analyte captured from the biological mixture by the immobilized antibody (the “capture agent”). This in turn is a function of the surface density and fractional activity of the antibody capture agent. Current trends in the development of antibody-based immunoassay include the use of smaller antibody fragments such as recombinant single-chain fragment variable (scFv) antibodies, assay miniaturization, multimode detection, and automation. Unlike traditional polyclonal and monoclonal antibodies, recombinant antibodies selected from phage display libraries or produced using recombinant DNA technology are maintained in bacteria and offer a stable antibody source and the accessibility to the antibody DNA for further genetic manipulations. One of the smallest recombinant antibody fragments is the scFv. The scFvs are small heterodimers that are composed of the antibody variable heavy (VH) and light (VL) chains connected by a peptide linker that is used to stabilize the molecule.1,2 Recombinant scFvs represent the smallest functional VH–VL domains of an antibody necessary for high-affinity binding of antigen.3 The key advantage of using scFv antibody fragment immunoassays is that the recognition element can be inexpensively produced and genetically engineered into a ready to use form by expressing the scFv inexpensively in bacteria. Apart from reductions in costs, scFv can be produced without the use of animals and can be readily selected for antigen specificity through the use of phage display antibody libraries.4–7
The peptide linker connecting scFv VH and VL domains joins the carboxyl terminus of one variable region domain to the amino terminus of the another variable domain without compromising the fidelity of the VH–VL paring and antigen-binding sites. Peptide linkers can vary from 10 to 25 amino acids in length and are typically, but not always, composed of hydrophilic amino acids such as glycine (G) and serine (S). Peptide linkers of shorter lengths (0–4 amino acids) have also been used. However, scFv bearing shorter linkers can form multimers. Generally, the (GGGGS)3 peptide is used as an scFv peptide linker.8–10 This 15-amino acid linker sequence [designated as the (GGGGS)3 linker] is used in the Recombinant Phage Antibody System (RPAS kit) commercially available from Amersham. Our previous study demonstrated that scFvs (MW ~27 000) containing metal-binding amino acids (i.e., cysteine or histidine) in the scFv peptide linker can be directly immobilized onto a gold surface in a favorable antigen-binding orientation at high density that significantly increased assay sensitivity by 3–5-fold over whole IgG or Fab antibody fragments, respectively.11,12 The successful implementation of scFv for immunosensing requires that the scFv not only be engineered for direct and functional immobilization onto transducer surfaces but also be economically produced. Certain amino acids in the linker sequence can affect the yield of functional scFvs. For example, an unpaired cysteine residue was genetically engineered near the C-terminus of four scFv constructs. Results demonstrated that the cysteine reduced the yield of bacterial protein expression.13
The A10B scFv recombinant antibody was used as part of a model system to develop a streamlined approach for immunosensor development. The A10B scFv is a recombinant antibody that binds specifically to its antigen, rabbit IgG. Rabbit IgG is inexpensive and commercially available. The A10B scFv was expressed using the pCANTAB5E expression vector (G. E. Healthcare). A10B scFv expressed in Escherichia coli using the pCANTAB5E expression vector displays a 12-amino acid (GAPVPYPDPLEPR) tag (the E-Tag) that is recognized by the anti-E tag monoclonal antibody conjugated to peroxidase (anti-E/HRP). The anti-E/HRP is used in immunoassays to detect E-tagged A10B scFv bound to antigens. The A10B scFv/rabbit IgG system has proven useful in developing immunosensors.12 Unlike other scFv, A10B scFv can be readily expressed in E. coli and can be mutated or engineered to contain a variety of amino acids, without losing its antigen-binding activity. The A10B scFv can also be biotinylated through free amines, present on A10B scFv, using commercially available biotinylation reagents (e.g., biotinamidocaproate-N-hydroxysuccinimide). As such, the A10B scFv/rabbit IgG system is flexible and can be used to identify chemical modifications to sensor surfaces that will be compatible with specific amino acids, engineered into scFv, for developing novel methodology for scFv immobilization.
We report here alternative methods to develop flexible, inexpensive, label free immunosensors by combining the flexibility of scFv linker engineering with the inherent quick and adaptable characters of surface coupling chemistry (e.g., electrostatic, hydrogen bonding, or covalent attachment) to attach A10B scFv-RG3 to preformed, functionalized, self-assembled monolayers and compared it with the other scFv constructs. The Arg-tag, first reported in 1984, usually contains five or six arginines.14,15 The positively charged Arg-tag can be used to immobilize functional proteins on the surface by electrostatic interaction with a negative charged template.15–18 Six arginines, which were separated by a glycine or serine as spacer, were incorporated in the linker to form a 15-mer peptide linker (RGRGRGRGRSRGGGS). The polycationic peptide was engineered into the A10B scFv-RG3 to favor adsorption onto an anionic charged template surface. Two functional template surfaces, poly(sodium 4-styrenesulfonate) (PSS) and 11-mercaptoundecanoic acid (MUA), were used for the specific adsorption of hexaarginine linked scFv (scFv-RG3). Our results demonstrated that the anionic charged, self-assembled monolayer (SAM) template facilitated the oriented immobilization of scFvs on the SAM template surface as well as reduced the possibility of protein denaturation when directly immobilized on the solid surface.19,20 A 42-fold improvement of detection limits using MUA/A10B scFv-RG3 (less than 0.2 nM experimentally determined) was achieved compared to A10B Fab antibody and a 5-fold improvement was observed compared to A10B scFv that was engineered to contain a cysteine within the linker sequence. Using protein A-coated gold nanoparticle, a picomolar experimental detection limit was achieved. With 20 amino acids to choose from, engineered recombinant scFv in combination with SAM technology and nanoparticle mass amplification provides an emerging strategy for the development of highly sensitive and specific scFv immunosensors.
MATERIALS AND METHODS
Chemicals and Materials
Rabbit IgG (Catalog No. I-5006), bovine serum albumin (BSA; Catalog No. A-4503), goat anti-rabbit IgG (Catalog No. R-2004), and goat anti-human IgG (Catalog No. I-3382), protein A (Catalog No. P-6031), human serum albumin (Catalog No. A9511), PSS (Catalog No. 527483, MW70 000), MUA (Catalog No. 450561), 1-dodecanethiol (Catalog No. 471364), and cysteamine (Catalog No. M9768) were purchased from Sigma Inc. Goat anti-rabbit IgG Fab fragment (Catalog No. 111-007003) was purchased from Jackson Immunolabs. Mouse IgG and PTEN mouse IgG1 were purchased from Santa Cruz Biotechnology. Peptides RGRGRGRGRSRGGGS and RGRG were purchased from Openbiosystems Inc. The peroxidase conjugated anti-E tag mono-clonal antibody (Catalog No. 27941301) was obtained from Amersham. Gold nanoparticles (15 nm) were purchased from Ted Pella, Inc. Phosphate-buffered saline (PBS), pH 7.2 (BRL 20012–027) and fetal bovine serum (FBS) (BRL 16000-044) were from Gibco. All other chemicals (Aldrich) are reagent grade and used as received.
Preparation and Purification of Engineered Anti-Rabbit IgG ScFv RG3 from Clone A10B
Modified anti-rabbit IgG scFv from clone A10B RG3 (RGRGRGRGRSRGGGS linker) was tested by ELISA. Data confirmed these scFvs are highly specific, efficient, and effective against rabbit IgG. The bacterial clone producing A10B RG3 scFv reacted with rabbit IgG was inoculated and cultured in 500 mL of 2 × YT + AG medium, which contains Bacto Tryptone 17 g, Bacto Yeast extract 10 g, sodium chloride 5.0 g, ampicillin 100 mg, and glucose 20 g per liter. E. coli bacteria were incubated at 30 °C overnight with shaking at 125 rpm and then centrifuged to pellet bacterial cells. The cell pellet was then resuspended in 500 mL of 2 × YT + AI (ampicillin and IPTG 1 mM) medium and then incubated at 30 °C with shaking at 125 rpm overnight. The periplasmic extract was made by incubating the bacterial cell pellet in 25 mL of 1 × TES [0.2 M Tris (pH 8.0), 0.5 mM EDTA, 0.5 M sucrose] and 46 mL of 1/5 TES. The resuspended cells were placed on ice for at least 1 h by vigorous shaking. The cell lysate was centrifuged at 5000 rpm. An anti-E tag affinity column (G. E. Healthcare RPAS Purification Module) was used to purify soluble E-tagged scFv from periplasmic extract. The concentration of purified scFv was tested by spectrophotometer at OD 280 nm. The specificity and binding activity of this purified scFv antibody to rabbit IgG was determined by enzyme-linked immunosorbent assay (ELISA).
Recombinant scFv Characterization by ELISA
An ELISA was used to characterize the soluble recombinant scFv antibody A10B-RG3. Wells of a 384-well microtiter plate (Nunc Catalog No. 242757) were coated with serial-diluted rabbit IgG. Diluted soluble scFv antibody A10B-RG3 with RGRGRGRGRSRGGGS linker at a concentration of 1.25 µg/mL was applied to wells of the rabbit IgG-coated microtiter plate. The Anti-E/HRP antibody, hydrogen peroxide and ABTS as a color developer were used to detect E-tagged scFv bound to rabbit IgG. The scFv bound to antigen was detected with the horseradish peroxidase (HRP)-conjugated anti-E tag monoclonal antibody (GE Healthcare); hydrogen peroxide and ABTS as a color indicator were then finally added to the wells. A microtiter plate reader, operating at 405 nm, was used to determine the absorbance readings for each well in the ELISA.
A10B ScFv Immobilization
The nonpolished gold quartz surface was cleaned sequentially with mixed concentrated acid solution (H2SO4/HNO3, 1:1 v/v), biograde water, and ethanol three times. Then, the surface was rinsed with biograde water and dried with nitrogen. The freshly washed gold surface was first immersed either into 4 mM MUA solution overnight or in 2 mg/mL PSS solution for 2 h to form an anionic charged layer. The anionic charged sensor surface was then immersed into A10B scFv-RG3 solution for 8 h, followed by the treatment of blocking reagent, 0.1% BSA, for 0.5 h. After the incubation, the excess scFvs on the surface of the quartz crystal microbalance (QCM) was washed away with PBS buffer and biograde water. The MUA/scFv-RG3 or PSS/scFv-RG3 modified surface was dried under nitrogen and used for the detection.
QCM Measurement
The gold quartz crystal electrode used throughout this study was AT cut quartz coated with 1000-Å gold in ~0.23 cm2 geometric area (International Crystal Co.). It was cleaned as above and modified with scFvs. Then it was mounted in a Kel-F cell sealed by two O-rings and filled with 1 mL of PBS buffer. The cell was continuously stirred during the measurement and was placed in a Faraday cage to reduce the potential electromagnetic noise.21 The frequency change and the damping resistance change caused by the analyte addition were monitored by the Network/Spectrum/Impedance Analyzer (Agilent 4395A).
Electrochemical Characterization
The gold QCM electrode was used as the working electrode. A platinum wire and a saturated calomel electrode (SCE) were used as counter and reference electrodes, respectively. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out in a solution of 0.1 M NaClO4 containing 1 mM K3Fe(CN)6/K4-Fe(CN)6 and performed using a Parstat 2263 advanced electrochemical system (Princeton Applied Research).
RESULTS AND DISCUSSION
HRP ELISA Quantification and Characterization of A10B scFvs
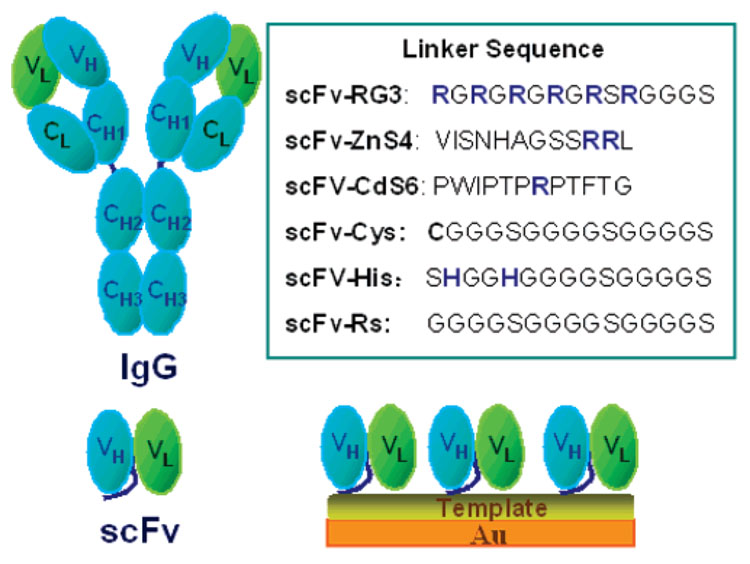
Chart 1 is an illustration of an IgG, an scFv, and an scFv immobilized onto a template-coated gold sensor surface. The linker sequences depicted in Chart 1 summarize the six different amino acid sequences engineered into the A10B scFv linker to immobilize the scFv onto the gold sensor or coupled to the template on gold.
Chart 1. Schematic Illustrationa.
a Amino acid abbreviation: A, alanine; C, cysteine; F, phenylalanine; G, glycine; H, histidines, I, isoleucine; L, leucine; N, asparagine; P, proline; R, arginine; S, serine; V, valine; and W, tryptophan)
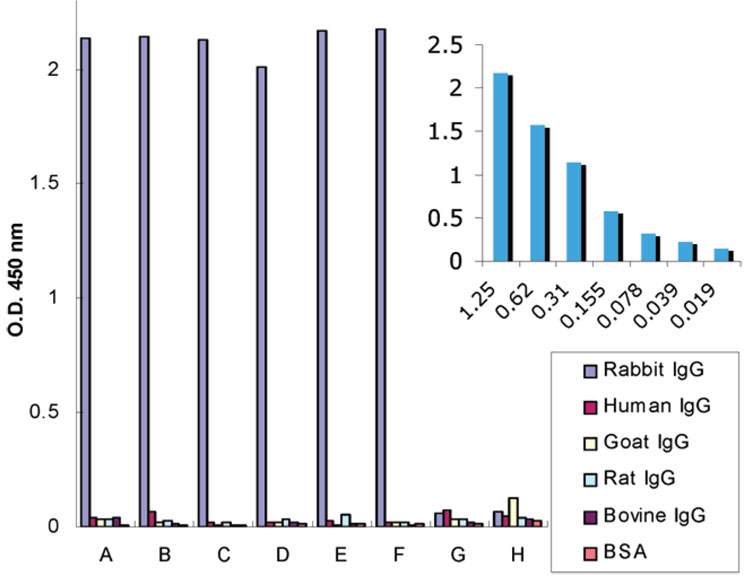
The expression levels of engineered scFvs-Cys (5.64 mg/L), scFv-His (5.20 mg/L), and scFv-RG3 (4.70 mg/L) are higher than that of other scFvs such as scFv-Rs (3.50 mg/L). By ELISA, A10B scFv engineered to contain different linker amino acid sequences retained antigen-binding specificity. As shown in Figure 1, A10B scFv engineered to contain a variety of different linker amino acids in Chart 1 specifically bound to rabbit IgG in a dose-dependent fashion (Figure 1 inset) and not negative control antigens (human, goat, rat, and bovine IgG and BSA). All A10B-scFvs have an E-tag incorporated into the amino terminus of the protein (E-tag: GAPVPYPDPLEPR) stemming from the rodent library. The A10B scFv bound to antigens immobilized onto microtiter plates were detected using anti-E tag/HRP monoclonal antibody, hydrogen peroxide, and ABTS (color developer). Negative control E-tagged scFv (designated I-20 scFv-RS and D-11 scFv-Cys) specific, respectively, for CYP1B1 and levulglandin (an isoketal) did not bind rabbit IgG or the negative control antigens and demonstrate the specificity of the ELISA used to characterize the engineered A10B scFv.
Figure 1. ELISA results for different scFvs on rabbit IgG, human IgG, rat IgG, goat IgG, bovine IgG, and BSA.
(A) scFv-RG3, (B) scFv-Cys, (C) scFv-His, (D) scFv-ZnS4, (E) scFv-CDS6, (F) scFv-RS, and negative controls: (G) I-20 scFv-RS specific for P450 CYP1B1, (H) D-11 scFv-Cys specific for isoketal protein adduct. Insertion depicts ELISA results for varying concentrations of rabbit IgG (1.25 to 0.019 µg/mL) binding with scFv-RG3.
Electrochemical Characterization of the Adsorption of A10B scFv-RG3
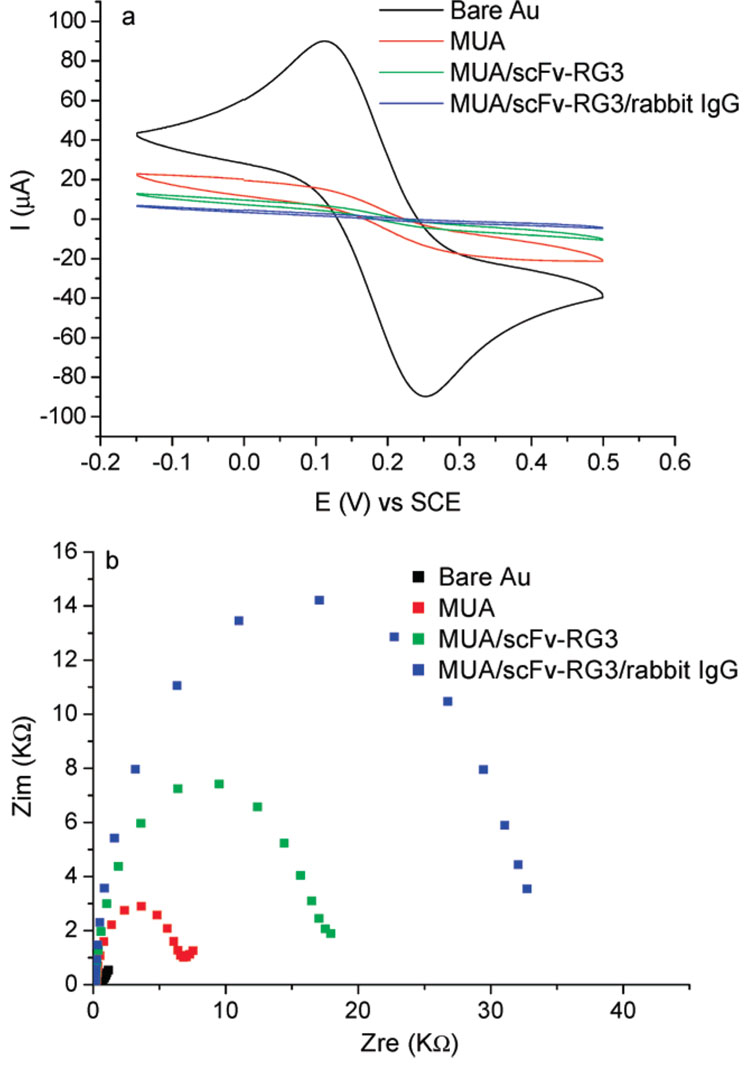
Electrochemistry methods such as CV and EIS have been used to study the growth mechanism and defects or porosity of surface-modified layers.22,23 Typically, a redox probe Fe(CN)63−/Fe(CN)64− was added. The passivation of the electron-transfer process of the redox probe Fe(CN)63−/Fe(CN)64− gives information on ligand–receptor binding and the integrity of surface-immobilized biological recognition elements. As shown in Figure 2a, CV of K4Fe(CN)6/K3Fe(CN)6 solution on the bare gold surface gave reversible redox peaks. The faradic current was dramatically decreased once a SAM of MUA was formed on the Au surface and further attenuated when scFv-RG3 was coupled onto the MUA SAM and bound with rabbit IgG. The electrochemical impedance spectroscopy was also used to test the surface passivation to electron transfer. The advantage of EIS over other electrochemical techniques is that only small-amplitude perturbations from steady state are needed, and information concerning the interface can be obtained. As shown in Nyquist plots (Figure 2b), the electron-transfer resistance (Ret) of the Fe(CN)6IV/Fe(CN)6III redox reactions increased drastically after the formation of the MUA SAM and further increased when scFv-RG3 was adsorbed onto MUA surface and bound with rabbit IgG. The cyclic voltammetry and electrochemical impedance characterization clearly indicated that scFv-RG3 was successfully immobilized onto the anionic templates, and the resulting interface binds with the rabbit IgG antigen. Shown in Supporting Information (Figure S1), both EIS and CV were also used to characterize PSS/scFv-RG3 modified surfaces.
Figure 2.
(a) CVs of 1 mM K4Fe(CN)6/K3Fe(CN)6 in 0.1 M NaClO4 on bare gold electrode (black), MUA (red), MUA/scFv-RG3 (green), and MUA/scFv-RG3 binding with rabbit IgG (blue) modified electrode. Scan rate, 100 mV/s. (b) EIS Nyquist plots. Frequency range is 0.1 Hz–100 kHz. Bias potential equals to open circuit potential. ac amplitude is 10 mV.
QCM Study of scFv-RG3 Immobilization onto Negative, Positive, and Neutral Templates
The essence of QCM as an analytic method lies in its ability to directly sense mass deposited on a crystal surface. Interfacial mass changes can be related to changes in the QCM oscillation frequency by applying Sauerbrey’s equation24 (Δf=−2Δmnf02/[A(µqρq)1/2]), where n is the overtone number, µq is the shear modulus of the quartz (2.947 × 1011 g cm−1 s−2), and ρq is the density of the quartz (2.648 g cm−3). It is assumed the foreign mass is strongly coupled to the resonator. Due to additional advantages of QCM (i.e., direct, noninvasive and real time), QCM was used as our transducer to characterize scFv–template coupling and the scFv binding with the target antigen throughout this study. EIS/CV was used as an alternative technology to verify the QCM results.
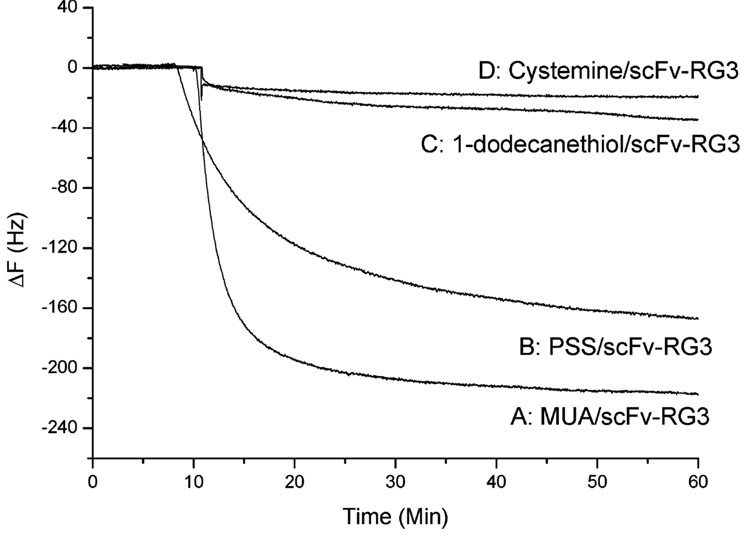
To further demonstrate that scFv-RG3 was immobilized onto an anionic template through electrostatic interaction, two other templates, a neutral template and a cationic template, were tested and compared with anionic templates (MUA, PSS) via QCM. For the neutral template, a long-chain alkanethiol, 1-dodecanethiol (C12H25SH), was used to form SAM. For the cationic template, cysteamine (NH2C2H4SH), which contained a terminal cationic group, was used. These four sensors (1-dodecanethiol-QCM cysteamine-QCM, PSS-QCM), and MUA-QCM) were first treated with scFv-RG3, followed by the addition of the same concentration rabbit IgG. As shown in Figure 3, scFv-RG3 immobilized onto the anionic template (MUA/scFv-RG3) gave the largest signal response (~224 Hz). ScFv-RG3 immobilized through neutral template and cationic template bound with rabbit IgG poorly (~35 and ~20 Hz, respectively), indicating much less efficient immobilization. These studies demonstrated that the hexaarginine-linked scFv (scFv-RG3) was efficiently adsorbed on the negative charged functional surface via electrostatic interaction to form a highly packed layer. Studies show that polyarginine can only be partially removed by acidic solution, such as acetic acid (pH 3), and there is practically no effect for the polyarginine adsorption on solid substrate until pH 11.17 The multiple ion pair interactions between polyarginine and surface carboxylates minimized or eliminated the possibility that scFv-RG3 could be displaced by the protein that has high pI value in the sample matrix.
Figure 3. Frequency change vs time curves.
When (A) MUA/scFv-RG3, (B) PSS/scFv-RG3, (C) 1-dodecanethiol /scFv-RG3, and (D) cysteamine/scFv-RG3 QCM sensors were exposed to the 132 nM rabbit IgG.
A10B scFv-RG3 QCM Sensor Sensitivity and Specificity
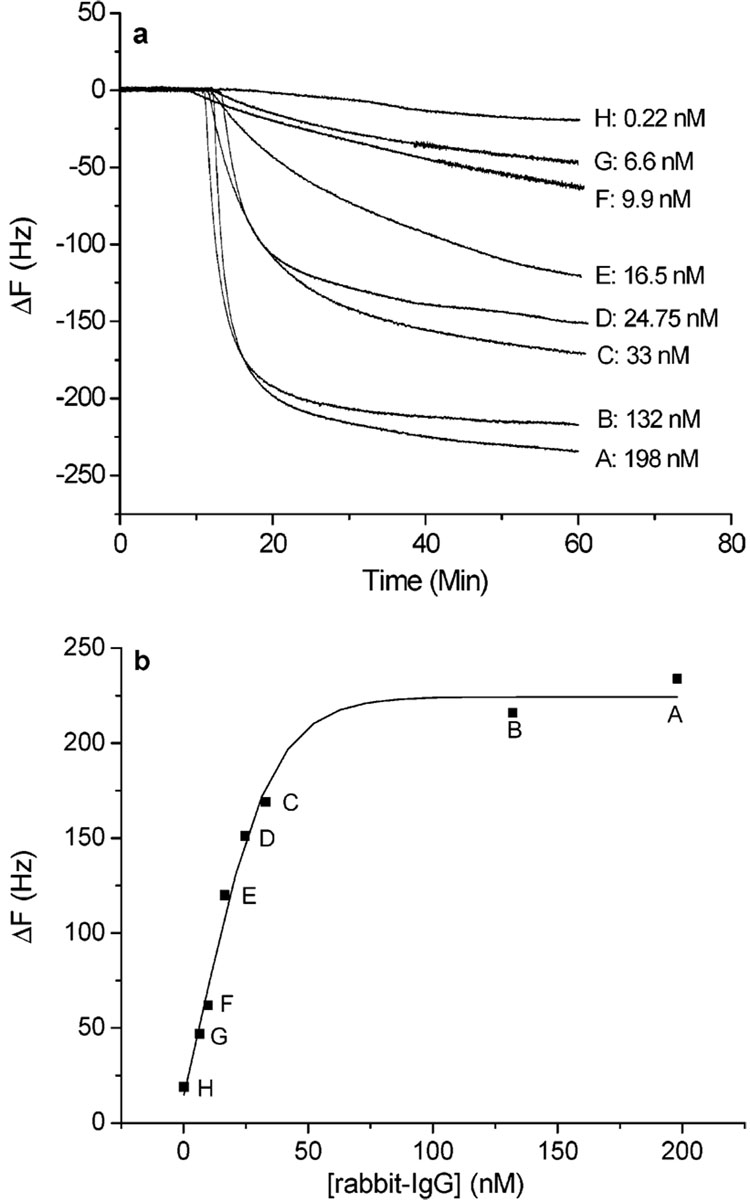
The A10B scFv-RG3 QCM sensors immobilized either through MUA or PSS template were used to detect rabbit IgG with varying concentrations. Figure 4a shows the typical time course of frequency decrease in the presence of various concentrations of rabbit IgG for the MUA/scFv-RG3 sensor. A linear relationship ranging from 0.2 to 33 nM was obtained by plotting the frequency changes versus the concentrations of rabbit IgG (Figure 4b) for MUA/scFv-RG3 sensor and 0.3–33 nM for PSS/scFv-RG3 sensors. The frequency change saturated when the concentration of rabbit IgG approached ~198 nM.
Figure 4.
(a) MUA/scFv-RG3 modified Au QCM electrodes exposed to various concentrations of rabbit IgG (0.22–198 nM). (b) Calibration curve, frequency change vs rabbit IgG concentration.
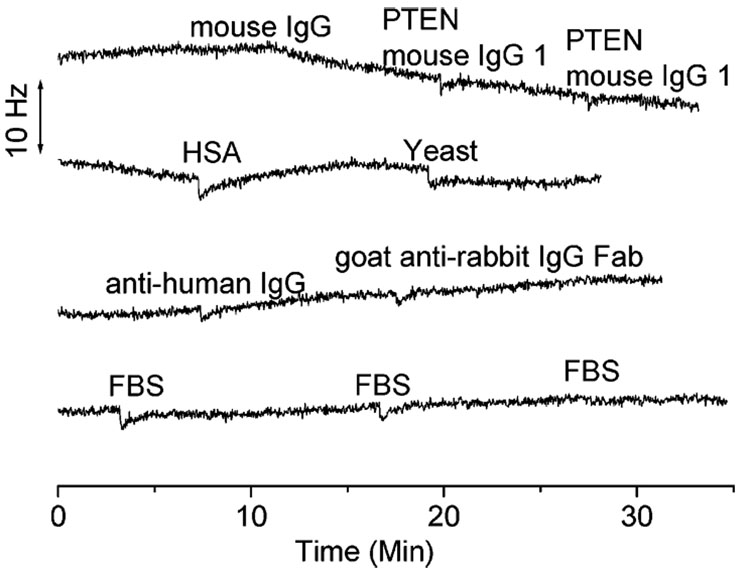
The specificity of MUA/scFv-RG3 QCM sensors was examined by adding the following negative control antigens (i.e., mouse IgG, PTEN mouse IgG1, FBS. albumin from human serum (HSA), yeast, anti-human IgG, and goat anti-rabbit IgG Fab) to the detection sensor cells. As depicted in Figure 5, only little nonspecific adsorptions were detected. These results indicate that the MUA/scFv-RG3 modified QCM sensors exhibit excellent antigen specificity.
Figure 5.
Negative controls (132 nM mouse IgG, 66 nM PTEN mouse IgG1, 10 µg/mL HSA, 20 µg/mL yeast, 66 nM anti-human IgG, 122 nM goat anti rabbit IgG Fab) and 22 µg/mL FBS.
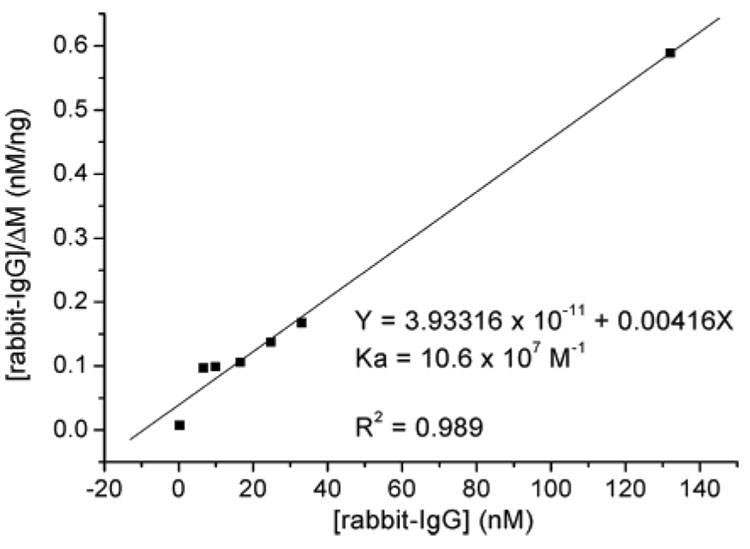
The change of damping resistances in all cases was smaller than 1.0% (Table 1). This suggests that the biofilm-immobilized A10B scFv-RG3 were exhibiting rigid, rather than viscoelastic behavior, and the Sauerbrey equation is valid. Both A10B scFv-RG3 surfaces show superior sensitivity to those developed using A10B scFv-Cys, A10B scFv-His, and A10B scFv-biotin indicating the RG3 linker design is an effective approach to immobilize scFv onto sensor surfaces. A Langmuir adsorption isotherm25,26 was used to determine association constants (Ka) for binding between rabbit IgG and scFv. The Ka for the A10B scFv-RG3/rabbit IgG interaction was highest in comparison to other engineered A10B scFvs and was 10.6 × 107 (MUA/scFv-RG3) and 9.6 × 107 M−1 (PSS/scFv-RG3), respectively (Table 2 and Figure 6).
Table 1.
| Figure 3 | |ΔRq|/Rq, % | Figure 4 | |ΔRq|/Rq, % | Figure 7 | |ΔRq|/Rq, % | Figure 8 | |ΔRq|/Rq, % |
|---|---|---|---|---|---|---|---|
| A | 0.3 | A | 0.4 | A | 0.6 | A | 0.7 |
| B | 0.3 | B | 0.7 | B | 0.4 | B | 0.5 |
| C | 0.6 | C | 0.3 | C | 0.7 | C | 0.4 |
| Figure 5 | 0.1 | D | 0.3 | D | 0.8 | D | 0.4 |
| Figure 9 A | 0.4 | E | 0.3 | E | 0.3 | E | 0.7 |
| Figure 9 B | 0.5 | F | 0.8 | F | 0.3 | ||
| Figure 9 C | 1.0 | G | 0.6 | G | 0.1 | ||
| Figure 9 D | 0.3 | H | 0.5 | H | 0.3 |
Table 2.
Comparison of Surface Coverage and the Analytical Characteristics of Different Engineered A10B scFv QCMs
| QCM sensors | surface coverage(mol /cm2) | exp detection limit (nM) | linear range (nM) | Ka (M−1) |
|---|---|---|---|---|
| MUA/scFv-RG3 | 8.8 × 10−11 | 0.2 | 0.2–33 | 10.6 × 107 |
| PSS/scFv-RG3 | 1.8 × 10−10 | 0.3 | 0.3–33 | 9.6 × 107 |
| scFv-Cys | 1.7 × 10−10 | 1.1 | 1.1–66 | 1.9 × 107 |
| scFv-His | 6.8 × 10−11 | 2.3 | 2.3–33 | 5.2 × 107 |
Figure 6.
Plot of [rabbit-IgG]0/ΔM vs [rabbit-IgG]0 for MUA/scFv-RG3-modified QCM sensor.
Comparison of Different Engineered A10B scFv QCMs
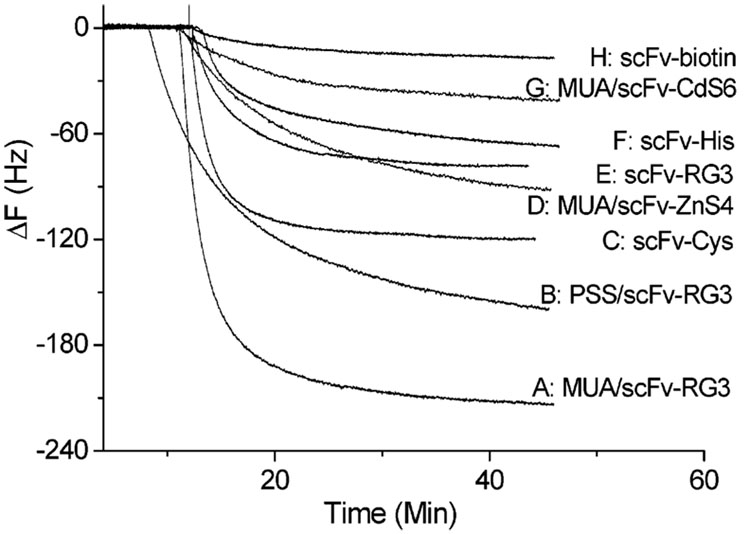
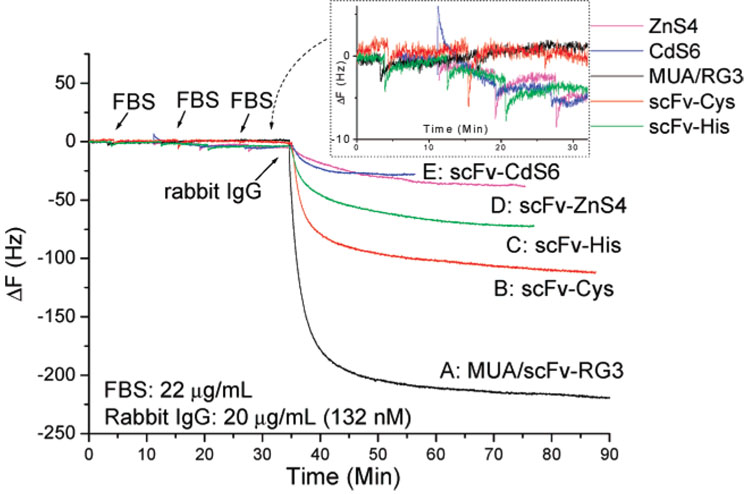
The performances of scFv-RG3 QCM sensors immobilized through electrostatic adsorption on negative charged MUA or PSS template were compared with other A10B QCM sensor formats in two conditions (with or without FBS). The A10B scFv-CdS6 and A10B scFv-ZnS4 were adsorbed onto the negative charged template surfaces and the A10B scFv-biotin was immobilized on the precoated avidin gold surface.11,12 As shown in Figure 7, all A10B scFv-modified QCM gold electrodes were exposed to the 132 nM rabbit IgG solution. MUA/scFv-RG3-modified sensors gave the largest frequency decrease and the lowest detection limit (0.2 nM). This represents a 5-fold improvement in QCM sensor detection limit compared to A10B scFv-Cys, a 11-fold improvement compared to A10B scFv-His, and a 42-fold improvement compared to A10B Fab antibody QCM sensors.11,12 Studies were also conducted to determine the effect of linker arginine number and location on scFv immobilization to anionic charged gold surfaces. A10B scFvs engineered to contain one or two arginines located at different locations within the linker sequence were generated. A10B scFv-CdS6 (linker, PWIPTPRPTFTG) and A10B scFv-ZnS4 (linker, VISNHAGSSRRL) were immobilized onto MUA and PSS template surfaces and used to detect rabbit IgG (132 nM). When assayed for rabbit IgG binding activity by QCM, the A10B scFv-RG3 gave the largest response (Figure 7 curves A, B), A10B scFv-ZnS4 (Figure 7 curve D) produced a much smaller response, and A10B scFv-CdS6 (Figure 7 curve G) produced the smallest response. This difference was presumably due to the different linker sequences, which suggests that the location and the number of arginines potentially play important roles in scFv immobilization antigen-binding activity. The sensitivity and the specificity of different engineered A10B scFv QCMs were also compared in the presence of FBS for binding with the target antigen rabbit IgG. The results obtained from the five types of scFv QCM sensors are illustrated in Figure 8. To test the specificity of these sensors, FBS was first added on three separate occasions. The MUA/scFv-RG3-and scFv-Cys-modified QCM sensors displayed almost no nonspecific binding signal (black curve and red curve), while scFv-His scFv-ZnS4 and scFv-CdS6 sensors produced small nonspecific binding (~5 Hz). After the exposure of 22 µg/mL FBS, rabbit IgG was then added (the final concentration of rabbit IgG is 20 µg/mL (132 nM)). The MUA/scFv-RG3 biosensor demonstrated significantly higher sensitivity to rabbit IgG compared to other QCM sensors even after repeated exposure to FBS.
Figure 7. Comparison of various engineered scFv-based QCM sensors’ sensitivity.
All above modified scFv QCM sensors were exposed to 132 nM rabbit IgG.
Figure 8. Comparison of sensor selectivity and sensitivity for MUA/scFv-RG3 (black), scFv-Cys (red), scFv-His (green), scFv-ZnS4 (megenta), and scFv-CdS6 (blue).
For each sensor, 20 µL of FBS was added three times to 1 mL of PBS buffer. This was followed by the addition of 20 µL of rabbit IgG to 1 mL of PBS buffer evaluating antigen detection in a complex matrix. The final concentration of FBS is 22 µg/mL; rabbit IgG is 20 µg/mL (132 nM).
Table 2 compared the surface coverage and the analytical characteristics of A10B scFv-RG3 with A10B scFv-Cys and A10B scFv-His. Even though PSS/scFv-RG3 has the highest surface coverage, A10B scFv-RG3 immobilized on a MUA template gave the highest sensitivity. Additionally, a one-decade larger dynamic range of antigen detection was obtained using A10B scFv-RG3 on anionic templates (MUA, PSS) in comparison to A10B scFv immobilized using other sensor surface coupling approaches. This result further confirmed the advantages of using sensor surface chemical modification to couple engineered scFvs. The negatively charged MUA monolayer may also reduce the possibility of protein denaturization and allow for a better oriented immobilized A10B scFv-RG3.
Signal Amplification by Protein A-Coated Gold Nanoparticles
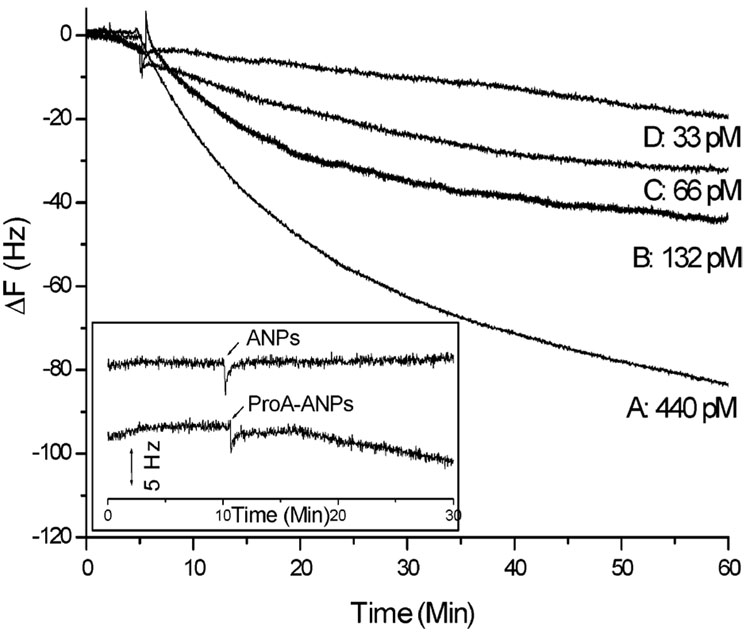
QCM is a mass sensor. Various strategies can be used to increase mass of the target analytes and further increase the detection sensitivity as long as the rigidity of the binding is preserved after mass amplification. Nanoparticle-based signal amplification strategy27–31 was demonstrated here to further increase the sensitivity of the scFv piezoimmunosensors. The mass of the target rabbit IgG can be easily increased using protein A-coated gold nanoparticles, which was prepared by treating gold nanoparticle (15 nm) solution with 0.5 mg/mL protein A aqueous solution. A series of protein A-coated gold nanoparticle solutions were mixed with different concentrations of rabbit IgG and then added into MUA/scFv-RG3 modified Au QCM sensors. Figure 9 depicts the frequency change versus time curve with respect to changes in rabbit IgG concentrations. The approximate number of gold nanoparticles was 1.4 × 1010 /mL. Negligible frequency change was observed for the addition of blank gold nanoparticle solution and ~5 Hz nonspecific binding for the addition of protein A-coated gold nanoparticles. Taking into the consideration the noise observed using protein A-coated gold nanoparticles, we achieved 33 pM experimentally determined detection limits, almost 7 times lower detection limit before the mass amplification of the target analyte. By further optimizing gold nanoparticle mass amplification strategies, even lower detection limits could be achieved.
Figure 9. Detection of rabbit IgG by MUA/scFv-RG3-modified Au QCM.
Binding signal amplified using protein A-coated Au nanoparticle. Insertion (zero dose blank): the addition of gold nanoparticle (ANP) solution and protein A-coated gold nanoparticle (ProA-ANP) solution.
CONCLUSIONS
We demonstrated that the scFv engineering and template surface coupling (e.g., MUA and PSS) chemistry reported here could be used as a flexible platform to circumvent scFv protein expression and sensor surface immobilization problems. Similar strategies in which negatively charged, hydrogen-bonding, hydrophobic, or hydrophilic amino acids are engineered into scFv linker sequences can also be used to immobilize scFv onto corresponding templates. Single-chain antibodies (scFvs) allow rapid isolation of antibodies and their in vitro manipulation at the gene level. They represent the smallest immunorecognition elements, which provide an emerging strategy in the development of new immunosensors. The scFv linker chain peptide length, amino acid composition, overall net charge, or hydrophobicity can be controlled through genetic engineering to enhance scFv-based, label-free immunosensor performance. The linker peptide design in combination with surface template coupling chemistries and gold nanoparticle mass amplification strategies provides a flexible platform that can be used to improve upon scFv-based, label-free immunosensor throughput, sensitivity, specificity, versatility, and cost per measurement.
Supplementary Material
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
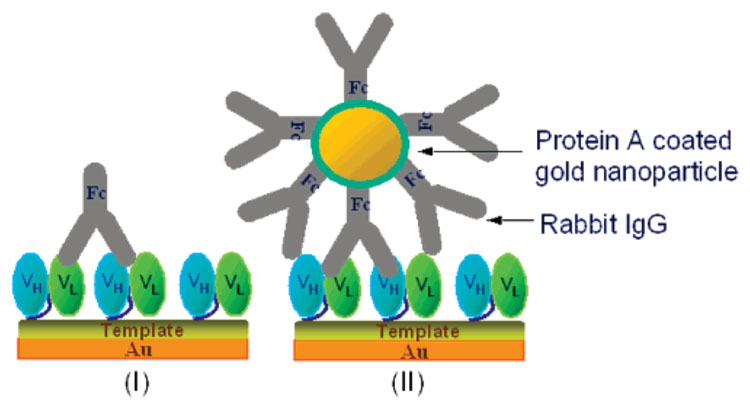
Chart 2. Schematic Illustration of Direct Detection of Rabbit IgG (I) and Gold Nanoparticle Amplification (II).
ACKNOWLEDGMENT
X.Z. thanks the support of Oakland University and the NIH (1R21EB000672-01, 4R33EB000672-02, 5P30 CA68485-07, 5P30 ES00267-36). We thank Julie Brown from the Vanderbilt University Molecular Recognition and Screening Facility for purifying scFv DNA used for sequencing and proteins used for immunoassays described in the present study.
References
- 1.Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Science. 1988;242:423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 2.Huston JS, Levinson D, Mudgett-Hunter M, Tai M-S, Novotny J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R, Oppermann H. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padlan EA. Mol. Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 4.Goodchild S, Love T, Hopkins N, Mayers C. Advances in Applied Microbiology. Vol 58. San Diego, CA: Academic Press; 2005. pp. 185–226. [DOI] [PubMed] [Google Scholar]
- 5.Kramer K, Hock B. Anal. Bioanal. Chem. 2003;377:417–426. doi: 10.1007/s00216-003-2161-1. [DOI] [PubMed] [Google Scholar]
- 6.Saerens D, Frederix F, Reekmans G, Conrath K, Jans K, Brys L, Huang L, Bosmans E, Maes G, Borghs G, Muyldermans S. Anal. Chem. 2005;77:7547–7555. doi: 10.1021/ac051092j. [DOI] [PubMed] [Google Scholar]
- 7.Wingren C, Borrebaeck CAK. Omics: J. Integr. Biol. 2006;10:411–427. doi: 10.1089/omi.2006.10.411. [DOI] [PubMed] [Google Scholar]
- 8.Leath CA, Douglas JT, Curiel DT, Alvarez RD. Int. J. Oncol. 2004;24:765–771. [PubMed] [Google Scholar]
- 9.Holliger P, Prospero T, Winter G. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iliades P, Kortt AA, Hudson PJ. FEBS Lett. 1997;409:437–441. doi: 10.1016/s0014-5793(97)00475-4. [DOI] [PubMed] [Google Scholar]
- 11.Shen Z, Mernaugh RL, Yan H, Yu L, Zhang Y, Zeng X. Anal. Chem. 2005;77:6834–6842. doi: 10.1021/ac0507690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Z, Stryker GA, Mernaugh RL, Yu L, Yan H, Zeng X. Anal. Chem. 2005;77:797–805. doi: 10.1021/ac048655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmiedl A, Breitling F, Winter CH, Queitsch I, Dubel S. J. Immunol. Methods. 2000;242:101–114. doi: 10.1016/s0022-1759(00)00243-x. [DOI] [PubMed] [Google Scholar]
- 14.Sassenfeld HM, Brewer SJ. Bio-Technology. 1984;2:76–81. [Google Scholar]
- 15.Terpe K. Appl. Microbiol. Biotechnol. 2003;60:523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- 16.Nock S, Spudich JA, Wagner P. FEBS Lett. 1997;414:233–238. doi: 10.1016/s0014-5793(97)01040-5. [DOI] [PubMed] [Google Scholar]
- 17.Barreira SVP, Silva F. Langmuir. 2003;19:10324–10331. [Google Scholar]
- 18.Ha TH, Jeong JY, Chung BH. Chem. Commun. 2005:3959–3961. doi: 10.1039/b504184h. [DOI] [PubMed] [Google Scholar]
- 19.Ros R, Schwesinger F, Anselmetti D, Kubon M, Schafer R, Pluckthun A, Tiefenauer L. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7402–7405. doi: 10.1073/pnas.95.13.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiefenauer LX, Kossek S, Padeste C, Thiebaud P. Biosens. Bioelectron. 1997;12:213–223. doi: 10.1016/s0956-5663(97)85339-0. [DOI] [PubMed] [Google Scholar]
- 21.Shen Z, Huang M, Xiao C, Zhang Y, Zeng X, Wang PG. Anal. Chem. 2007;79:2312–2319. doi: 10.1021/ac061986j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz E, Willner I. Electroanalysis (New York) 2003;15:913–947. [Google Scholar]
- 23.Fernandez-Sanchez C, McNeil CJ, Rawson K. TrAC-Trends Anal. Chem. 2005;24:37–48. [Google Scholar]
- 24.Sauerbrey GZ. Phys. 1959;155:206–222. [Google Scholar]
- 25.Ebara Y, Itakura K, Okahata Y. Langmuir. 1996;12:5165–5170. [Google Scholar]
- 26.Okahata Y, Matsuura K, Ito K, Ebara Y. Langmuir. 1996;12:1023–1025. [Google Scholar]
- 27.Weizmann Y, Patolsky F, Willner I. Analyst. 2001;126:1502–1504. doi: 10.1039/b106613g. [DOI] [PubMed] [Google Scholar]
- 28.Kalogianni DP, Koraki T, Christopoulos TK, Ioannou PC. Biosens. Bioelectron. 2006;21:1069–1076. doi: 10.1016/j.bios.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Bentzen E, Wright DW, Crowe JE. Future Virol. 2006;1:769–781. [Google Scholar]
- 30.Li F, Jiang Z. J. Intel. Mater. Syst. Struct. 2006;17:823–830. [Google Scholar]
- 31.Mao X, Yang L, Su XL, Li Y. Biosens. Bioelectron. 2006;21:1178–1185. doi: 10.1016/j.bios.2005.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.