Abstract
We consider an allograft-prosthesis composite in the proximal tibia one of the better reconstructive options in this site because it combines the mechanical stability of a prosthesis with the biologic reconstruction of the extensor mechanism. We retrospectively reviewed 62 patients who had proximal tibia reconstructions with allograft-prosthesis composites to ascertain the complications and functional outcomes. By combining an allograft with a prosthesis, placing cement in the graft, and press-fitting the prosthesis in the tibial diaphysis, we obtained satisfactory Musculoskeletal Tumor Society scores in 90.4% of patients, with a 5-year survival rate (73.4%) comparable to that of reconstruction with a modular prosthesis. However, we observed high infection rates (24.2%) and rotation of the medial gastrocnemius seemed not to reduce this complication. For this reason, we do not recommend using this reconstructive technique in patients who will receive postoperative chemotherapy or in patients in whom a previous reconstructive method failed. We believe the ideal candidate is the young patient with a benign aggressive or malignant low-grade tumor who has not undergone previous surgery.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Reconstruction of the proximal tibia after tumor resection can be achieved in numerous ways [23]: an osteoarticular allograft, a modular or custom-made prosthesis, fusion with an autogenous or allogeneic graft, or an allograft-prosthesis composite (APC). In comparison to a prosthetic reconstruction, reconstruction with an osteoarticular graft has the advantage of enabling better reinsertion of the extensor mechanism and capsuloligamentous structures of the knee, thus preserving articular function with more similar dynamics to the original anatomy of the knee [2, 4, 6, 9, 12]. However, using a graft involves a long period of immobilization for union to occur among the capsulotendinous structures [12]. In our experience, osteoarticular allografts in the proximal tibia have been associated with a high percentage of subchondral fractures. A prosthetic reconstruction is technically easier and allows faster rehabilitation, and patients can bear weight soon after surgery. However, it is limited by the reconstruction of the extensor and capsuloligamentous structures of the knee that require fixation directly onto the metal parts [12, 20, 33, 35]. Knee fusion, in its various reconstructive forms, allows a simpler and faster operation. If autoplastic grafts or vascularized transplantations are used, it will be stable and permanent but at the direct expense of the loss of knee movement [5, 11, 15, 30]. The use of an APC might be the best available solution because it combines the advantages of a homoplastic osteoarticular graft, biologic insertion of the soft tissues, with those of a prosthesis, articular stability and absence of fractures, while at the same time preserving good range of movement in the knee [12, 18]. For this reason, in 1994, we began to use this reconstructive technique in patients with bone tumors located in the proximal tibia.
Given the uncertainty of outcomes using multiple techniques, we raised five questions: (1) whether prosthetic survivorship and functional outcomes would compare with published results for other techniques; (2) whether the use of muscle flap rotation decreased the risk of infection; (3) whether postoperative chemotherapy increased the risk of infection; (4) whether the extensor mechanism of the knee would be effective; and (5) which surgical technique, in terms of prosthetic design and type of prosthetic fixation, achieved the best functional scores.
Materials and Methods
We retrospectively reviewed 62 patients with 62 proximal tibia reconstructions with APC performed from 1994 to 2002. In 56 patients, the operation was performed after the resection of a primary malignant tumor, in four patients after the failure of a previous reconstructive operation, and in two after the resection of a benign tumor (giant cell tumor). The 56 malignant tumors were 39 osteosarcomas, seven Ewing’s sarcomas, four spindle cell sarcomas, three chondrosarcomas, one fibrosarcoma, one malignant fibrous histiocytoma, and one leiomyosarcoma. Twenty patients were female and 42 were male, ranging in age from 11 to 77 years (mean, 24 years; median, 18 years). No patient was lost to followup. The minimum followup was 13 months (mean, 72 months; range, 13–149 months). For the functional results, we excluded 17 patients with some sort of failure and three more patients who died of disease within 24 months, leaving 42 patients with a minimum followup of 24 months.
The grafts were harvested from cadavers under sterile conditions and maintained in a freezer at −80° C. The standard technique of reconstruction with a composite prosthesis involves the use of a rotating hinged revision modular prosthesis (Endo-Model®; Waldemar LINK GmbH and Co KG, Hamburg, Germany) cemented in the graft and implanted without cement in the residual tibial diaphysis (Fig. 1). The femoral component also is inserted without cement. In the first patient of our series, a classic Endo-Model® prosthesis was cemented in the graft and in the residual tibia and femur. In four other patients, because of the length of the resection, a tibial stem was cemented into the graft, which was fixed in turn with a plate to the diaphysis of the tibia. The kneecap was never replaced, but of the 62 composite prostheses, nine (14.5%) had a femoral component with a trochlea and 53 (85.5%) did not have a trochlear shield (Fig. 2). The patellar tendon was always reinserted by direct suture overlapping the autologous proximal part onto the distal one provided by the graft (Fig. 3). In 13 patients (21%), a medial gastrocnemius rotation combined with a split-thickness skin graft [7, 34], harvested from the homolateral thigh, was performed with to improve the biology of the patellar insertion and the muscular coverage of the implant [25, 26, 29]. The capsule, the collateral ligaments, and the pes anserinus tendons were never sutured to the graft. The mean length of the surgical resection was 13.2 cm (range, 8.5–28 cm; median, 12.5 cm).
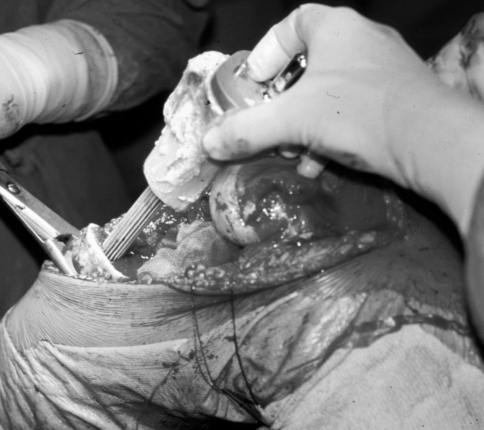
Fig. 1.
An intraoperative image of the reconstruction technique shows the prosthesis cemented in the graft and implanted press-fit in the residual tibial diaphysis using a long revision stem.
Fig. 2A–B.
(A) Anteroposterior and (B) lateral views of an APC implanted without the trochlear shield. In (B), the absence of the trochlear shield is clearly evident. In this type of prosthesis, the patellar-femoral joint is not involved in the reconstruction, whereas in prostheses with the trochlear shield, the design of the femoral component is prolonged anteriorly to completely substitute the trochlear groove of the femur (trochlear shield).
Fig. 3.
An intraoperative image shows reinsertion of the patellar tendon by direct suture overlapping the autologous proximal part of the tendon onto the distal one provided by the graft.
Antibiotic prophylaxis in all cases consisted of intravenous amikacin (Migracin®; Max Farma srl, Castel San Giorgio, Italy) on the day of the operation combined with teicoplanin (Targocid®; Gruppo Lepetit SpA, Milan, Italy), which was administered until the patient was discharged. Additional preventive antibiotics were administered orally for 2 more months. Postoperative treatment consisted of a cast for 1 month followed by functional recovery with rehabilitation therapy, and weightbearing was permitted in all cases 3 months after surgery. Forty-eight patients (77%) had postoperative chemotherapy. Postoperative radiotherapy was not performed in any patient.
We (MC, SC, CDB) assessed the functional results of the patients according to the functional rating system of the Musculoskeletal Tumor Society (MSTS) [14].
For analysis of the MSTS scores, we excluded 20 of the 62 patients with implant survival or followup less than 24 months. Prosthetic survivorship was assessed with Kaplan-Meier survival analysis excluding and including recurrences and metastases. We performed Fisher’s exact test to assess the relationship between infection and gastrocnemius rotation or chemotherapy and to assess the effectiveness of the extensor mechanism reconstruction depending on the prosthetic design.
Results
The survivorship rate of the implant including recurrences and metastases was 73% at 5 years after surgery (Fig. 4), whereas the survivorship rate of the implant excluding these failures was 78% at 5 years surgery (Fig. 5). Forty-nine of the 62 patients (79%) were disease-free at last followup and 13 (21%) died of disease. Twenty-five of the 42 patients who were evaluated for functional results had an average MSTS score of 86 and 13 had an average MSTS score of 67; therefore a score higher than 65 was obtained in 90.4% of the patients. The remaining four patients had an average MSTS score of 46, mainly attributable to stiffness and pain. Graft failure occurred in 17 cases (27.4%): 12 for infection, three for local recurrence, and two for aseptic loosening of the implant. Local recurrence caused amputation of the limb in all cases.
Fig. 4.
Kaplan-Meier analysis of the prosthetic general survivorship, including local recurrence, shows a survivorship rate at 60 months of 73.4% (95% confidence interval, 61.8%–85.0%). After 72 months, it is stable at 68% (95% confidence interval, 54.9%–81.1%).
Fig. 5.
Kaplan-Meier analysis of the prosthesis survivorship rate, excluding local recurrence, shows a survivorship rate at 60 months of 78.8% (95% confidence interval, 68.0%–89.6%). After 72 months, it is stable at 73% (95% confidence interval, 60.0%–85.5%).
Rotation of the medial gastrocnemius did not influence the rate of infection. Infection occurred in four of 13 patients (30.7%) with rotation of the medial gastrocnemius compared with 11 of 49 patients (22.4%) with no flap rotation.
Infection occurred in 13 of 48 patients (27%) treated with postoperative chemotherapy versus two of 14 (14%) with no chemotherapy in the postoperative period. Both of these patients received an APC after failure of a previous operation.
We observed failure of the extensor mechanism in nine of the 62 patients (14.5%) after a mean of 29 months (range, 5–76 months); all the other patients were able to walk using the quadriceps during the stance phase. In this group, no patient had lag of the active extension from the sitting position greater than 5°. Therefore, they all scored 5 in active movement of the leg in the function criteria. Revision surgery of the tendon was performed in seven patients, with a satisfactory result in six, whereas in the remaining two, one refused surgery and the other patient sustained a local recurrence and thus had an amputation. Patellar tendon ruptures occurred more frequently (p = 0.333) in patients who received a prosthesis without a trochlear shield (nine of 53 [17%]) than in patients who received a prosthesis with a trochlear shield (none of nine patients).
Immediate postoperative complications were seen in 14 patients (22.5%). Seven patients had temporary palsy of the peroneal nerve. In six patients, a partial dehiscence of the surgical wound occurred; in two of these patients, we performed simple revision of the wound, and in the other four, a split-thickness skin graft was performed. In the last patient, there was stiffness in the knee treated by arthroscopy. Infection occurred in 15 patients (24.2%) after a mean of 21 months (range, 1–86 months). Three of these patients had a deep infection after implant revision: one after implanting autoplastic grafts for nonunion, another after reconstruction surgery of the patellar tendon, and the last one after rotation of the gastrocnemius and a split-thickness skin graft for partial necrosis of the surgical wound. Of the 15 patients, only three recovered with only surgical débridement; in the remaining 12, more demanding surgery was necessary to achieve healing. Two patients recovered after removing the graft, leaving the prosthesis on site and adding cement loaded with antibiotic around the stem. In eight patients, the whole implant was removed and a provisional reconstruction with a cement spacer was performed followed by the implantation of a Howmedica Modular Reconstruction System prosthesis (Stryker Orthopaedics, Mahway, NJ). In the remaining two patients, the limb was amputated.
The appropriate choice of prosthetic design was confirmed by evaluation of the loosening rate at the femoral and tibial components. We observed loosening in no patients with a long uncemented stem press-fit in the tibial diaphysis. However, eight patients (12.9%) had delayed unions of the graft and underwent additional surgery to promote union of the tibial osteotomy. The first nonunion occurred in the patient with cement in the allograft and in the tibia; he also had loosening of the tibial component, which resulted in revision of the stem. Three other patients with nonunion belong to the group of four in which the tibial stem was cemented in the graft and then the graft was fixed to the residual tibial diaphysis with a plate. In all three, nonunion was associated with partial graft fracture. One was revised with a new plate and autograft, whereas in the other two, failure occurred. In one of these patients, the APC was replaced with a Global Modular Replacement System prosthesis (Stryker Orthopaedics) and in the second with a new APC. The remaining four patients, all treated with our current surgical technique (long tibial stem cemented in the allograft and press-fit in the tibia), healed by simple autografting of the osteotomy line.
Finally, at index followup, polyethylene wear occurred in five patients treated by replacement surgery after a mean of 67 months.
Discussion
The proximal tibia is the second most common site involved by sarcoma [3, 13]. The clinical outcome after reconstruction of this site is frequently poor because of the high rate of complications that may occur during the postoperative period. The main problem is related to the onset of infection and its relation with adequate soft tissue coverage (gastrocnemius rotation flap) and multiple revision surgeries [2, 3]. Given the uncertainty of outcomes using multiple techniques, we considered five questions: (1) whether prosthetic survivorship and functional outcomes would compare with published results for other techniques; (2) whether the use of muscle flap rotation decreased the risk of infection; (3) whether postoperative chemotherapy increased the risk of infection; (4) whether the extensor mechanism of the knee would be effective; and (5) which surgical technique, in terms of prosthetic design and type of prosthetic fixation, achieved the best functional results.
We acknowledge some limitations of this study. This is a retrospective study performed on a group of patients receiving proximal tibia reconstruction after bone tumor resection. We had too few patients to stratify patients by age, postoperative chemotherapy, length of resection, pathology, or type of reconstruction. Thus, some of our questions could not be definitely addressed by the study. Given the relatively rarity of this problem and the unique treatment for each individual, it would be difficult to obtain a large series with sufficient power to address these important questions. However, even retrospectively we could see some trends we believe are meaningful.
The general survivorship rate of the implant in our series was comparable to that of prosthetic reconstructions (approximately 73% at 5 years’ followup) [1, 2, 35].
Satisfactory functional results have been obtained with APC in the proximal tibia because of the good active range of motion that allows a rather normal pattern of gait; it is particularly important for flexion control during the stance phase. Although not formally measured, we believe another advantage is patient satisfaction related to the cosmetic appearance; despite modular resection prostheses, APCs replace the normal anatomic shape of the proximal tibia.
As reported in the literature, the infection rate after reconstructive surgery of proximal tibia defects is high [1, 19, 35]. In our series, it also represesented the main cause of failure (70.5%). We would have expected a reduction in comparison to the general rate for this site (12%–36% [1, 19, 35]), whereas despite the rotation of the medial gastrocnemius in numerous cases, this complication occurred in 24% of the series. Medial gastrocnemius rotation is problematic because APCs are bulkier than the common modular prostheses: the space between the prosthesis and the overlying skin is reduced. However, infection does not seem correlated with the use of an allograft; the same approach in the proximal femur does not increase the infection rate [10, 16]. Nevertheless, during surgical cleaning of the infection, it is common to observe pus or necrotic material inside the joint in the presence of an integral graft with the soft tissues well attached to it. This confirms the well-known problem of colonization of the prosthetic components, particularly in the polyethylene parts, by methicillin-resistant Staphylococcus epidermidis [27]. Also, patients receiving postoperative chemotherapy and those undergoing revision surgery resulting from previous complications are at higher risk for infection [2, 12, 19, 28].
Among different types of reconstruction, APCs promise a better outcome in terms of patellar tendon insertion, thus resulting in better walking ability. However, another key factor is related to the choice of prosthetic design and surgical technique to achieve the best bond between the allograft and the prosthesis and between the prosthesis and the host.
The problem of reconstructing the extensor mechanism is particularly present in prosthetic reconstructions because there is still no particularly effective method for reinserting the patellar tendon; therefore, biologic reconstruction of such structures is impossible [1, 3, 21, 24]. Even in the most recent studies, the possibility of failure in the reconstruction of the extensor mechanism occurred in as much as 26% of patients [1, 17, 31], although one might believe this percentage would be reduced with rotation of the medial gastrocnemius and its suture to the patellar tendon [32]. However, this technique carries some risks, such as necrosis of the fasciocutaneous flap used [3]. The use of an APC allowed reduction in the rate of this complication in our series; even after breakage had occurred, we were able to restore the efficiency of the extensor mechanism in most cases. The fact that 90.4% of our patients had MSTS scores higher than 65 indicated good articular function of the knee was achieved in the patients. However, to improve this outcome, certain important technical steps should be followed carefully, such as correctly placing the femoral component. Furthermore, the use of a prosthesis with a trochlear shield allowed precise centering of the prosthesis, thus allowing more normal subsequent knee extension forces. Also, the role of physical therapy in restoring active and passive flexion and extension is important.. In this type of reconstruction, gradual but intense recovery is needed to achieve correct use of the knee and therefore good integration between the prosthesis and the surrounding tissues.
We believe delayed union can be minimized with the use of long intramedullary stems cemented in the graft but not cemented in the host bone [12, 22]. When the prosthesis is cemented in the graft and the graft is fixed by a plate to the tibia of the host, the rate of delayed union increases. We observed fracture of the graft only in patients with the latter technique (4.8%). However, this percentage is lower than that reported in another study [35], and we believe the result also is related to the use of nonirradiated bone graft [8].
Finally, there is the problem concerning the intrinsic mechanics of the prosthesis, particularly its articular design. Considering the relative lack of periarticular tissue, despite being a particularly constrained design, there are considerable forces exerted on the polyethylene parts, especially inside the intercondylar groove and the meniscus. Component wear leads to revision surgery, which, if repeated, might result in the onset of infection with the aforementioned consequences.
Our review suggests some important technical points, such the use of deep-frozen nonirradiated allograft and the selection of a femoral prosthetic component provided with a trochlear shield. However, we emphasize the importance of cementing in the graft and press-fit insertion in the host tibial diaphysis, as has been observed for the proximal femur [16, 36]. There is still room for improvement on the prosthetic design, most of all in the articular mechanics reducing the wear of the moving polyethylene components. The problem of the high number of cases of infection remains. The rotation of the medial gastrocnemius did not reduce the risk for this type of proximal tibia reconstruction. We recommend avoiding this reconstructive technique in patients needing postoperative chemotherapy and in patients in whom a previous reconstructive method has failed. The ideal candidates are young patients with an aggressive benign or a low-grade malignant tumor.
Acknowledgments
We thank Keith Smith for help with the English translation and Elettra Pignotti for help with statistical analysis.
Footnotes
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock, ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293. [DOI] [PubMed]
- 2.Biau DJ, Dumaine V, Babinet A, Tomeno B, Anract P. Allograft-prosthesis composites after bone tumor resection at the proximal tibia. Clin Orthop Relat Res. 2007;456:211–217. [DOI] [PubMed]
- 3.Bickels J, Wittig JC, Kollender Y, Neff RS, Kellar-Graney K, Meller I, Malawer M. Reconstruction of the extensor mechanism after proximal tibia endoprosthetic replacement. J Arthroplasty. 2001;16:856–862. [DOI] [PubMed]
- 4.Brien EW, Terek RM, Healey JH, Lane JM. Allograft reconstruction after proximal tibial resection for bone tumors: an analysis of function and outcome comparing allograft and prosthetic reconstructions. Clin Orthop Relat Res. 1994;303:116–127. [PubMed]
- 5.Campanacci M, Costa P. Total resection of distal femur or proximal tibia for bone tumours: autogenous bone grafts and arthrodesis in twenty-six cases. J Bone Joint Surg Br. 1979;61:455–464. [DOI] [PubMed]
- 6.Clatworthy MG, Gross AE. The allograft prosthetic composite: when and how. Orthopedics. 2001;24:897–898. [DOI] [PubMed]
- 7.Coleman W 3rd. Complicated surgical techniques: I. Flaps and grafts. Clin Dermatol. 1987;5:94–109. [DOI] [PubMed]
- 8.Currey JD, Foreman J, Laketic I, Mitchell J, Pegg DE, Reilly GC. Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res. 1997;15:111–117. [DOI] [PubMed]
- 9.Dennis DA. The structural allograft composite in revision total knee arthroplasty. J Arthroplasty. 2002;17(4 suppl 1):90–93. [DOI] [PubMed]
- 10.Donati D, Giacomini S, Gozzi E, Mercuri M. Proximal femur reconstruction by an allograft prosthesis composite. Clin Orthop Relat Res. 2002;394:192–200. [DOI] [PubMed]
- 11.Donati D, Giacomini S, Gozzi E, Salphale Y, Mercuri M, Mankin HJ, Springfield DS, Gebhardt MC. Allograft arthrodesis treatment of bone tumors: a two-center study. Clin Orthop Relat Res. 2002;400:217–224. [DOI] [PubMed]
- 12.Donati D, Tella G, Gozzi E, Giacomini S, Mercuri M. The use of allograft prosthetic composite (APC) in the proximal tibia after bone tumor resection. In: Phillips GO, Strong DM, von Versen R, Nather A eds. Advances in Tissue Banking. Vol 4. Singapore: World Scientific Publishing Co Pte Ltd; 2000:313–324.
- 13.Dorfman HD, Czerniak B. General considerations. In: Dorfman HD, Czerniak B, eds. Bone Tumors. St Louis, MO: CV Mosby; 1998:1.
- 14.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed]
- 15.Enneking WF, Eady JL, Burchardt H. Autogenous cortical bone grafts in the reconstruction of skeletal defects. J Bone Joint Surg Am. 1980;62:1039–1058. [PubMed]
- 16.Farid Y, Lin PP, Lewis V, Yasko AW. Endoprosthetic and allograft prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. [DOI] [PubMed]
- 17.Gerdesmeyer L, Gollwitzer H, Diehl P, Burgkart R, Steinhauser E. [Reconstruction of the extensor tendons in revision total knee arthroplasty and tumor surgery.][in German]. Orthopade. 2006;35:169–175. [DOI] [PubMed]
- 18.Gitelis S, Piasecki P. Allograft prosthetic composite arthroplasty for osteosarcoma and other aggressive bone tumors. Clin Orthop Relat Res. 1991;270:197–201. [PubMed]
- 19.Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 1999;81:488–494. [DOI] [PubMed]
- 20.Harris AI, Poddar S, Gitelis S, Sheinkop MB, Rosenberg AG. Arthroplasty with a composite of an allograft and a prosthesis for knees with severe deficiency of bone. J Bone Joint Surg Am. 1995;77:373–386. [DOI] [PubMed]
- 21.Healy WL, Wasilewski SA, Takei R, Oberlander M. Patellofemoral complications following total knee arthroplasty: correlation with implant design and patient risk factors. J Arthroplasty. 1995;10:197–201. [DOI] [PubMed]
- 22.Hirn M. Long intramedullary stems of prosthetic components reduce complications, when allograft-prosthesis composite is used in tumor reconstruction. J Surg Oncol. 2002;79:201–203. [DOI] [PubMed]
- 23.Jeon DG, Kawai A, Boland P, Healey JH. Algorithm for the surgical treatment of malignant lesions of the proximal tibia. Clin Orthop Relat Res. 1999;358:15–26. [DOI] [PubMed]
- 24.Kelly MA. Patellofemoral complications following total knee arthroplasty. Instr Course Lect. 2001;50:403–407. [PubMed]
- 25.Kroll SS, Marcadis A. Aesthetic considerations of the medial gastrocnemius myocutaneous flap. Plast Reconstr Surg. 1987;79:67–71. [DOI] [PubMed]
- 26.Le Nen D, Fabre A, Yaacoub C, Lefevre C. Flaps of the gastrocnemius muscles. Rev Chir Orthop Reparatrice Appar Mot. 1995;81:66–73. [PubMed]
- 27.Merritt K, Gaind A, Anderson JM. Detection of bacterial adherence on biomedical polymer. J Biomed Mater Res. 1998;39:415–422. [DOI] [PubMed]
- 28.Natarajan MV, Sivaseelam A, Rajkumar G, Hussain SH. Custom megaprosthetic replacement for proximal tibial tumors. Int Orthop. 2003;27:334–337. [DOI] [PMC free article] [PubMed]
- 29.Podlewski J. Medial gastrocnemius myocutaneous flap. Plast Reconstr Surg. 1989;83:578–579. [DOI] [PubMed]
- 30.Rasmussen MR, Bishop AT, Wood MB. Arthrodesis of the knee with a vascularized fibular rotatory graft. J Bone Joint Surg Am. 1995;77:751–759. [DOI] [PubMed]
- 31.Sanjay BK, Moreau PG. Limb salvage surgery in bone tumour with modular endoprosthesis. Int Orthop. 1999;23:41–46. [DOI] [PMC free article] [PubMed]
- 32.Shimose S, Sugita T, Kubo T, Matsuo T, Ochi M. Reconstructed patellar tendon length after proximal tibia prosthetic replacement. Clin Orthop Relat Res. 2005;439:176–180. [DOI] [PubMed]
- 33.Sim FH, Beauchamp CP, Chao EY. Reconstruction of musculoskeletal defects about the knee for tumor. Clin Orthop Relat Res. 1987;221:188–201. [PubMed]
- 34.Snyder RJ, Doyle H, Delbridge T. Applying split-thickness skin grafts: a step-by-step clinical guide and nursing implications. Ostomy Wound Manage. 2001;47:20–26. [PubMed]
- 35.Wunder JS, Leitch K, Griffin AM, Davis AM, Bell RS. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99. [DOI] [PubMed]
- 36.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. [DOI] [PubMed]