Abstract
We asked whether coagulopathy worsened during femoral intramedullary nailing in the presence of lung contusion and hemorrhagic shock and whether reamed or unreamed nailing influenced these results. In 30 Merino sheep, we induced hemorrhagic shock and/or standardized lung contusion followed by femoral nailing. Six groups of five each were assigned as follows: thoracotomy control groups treated with reamed or unreamed nailing, lung contusion groups treated with reamed or unreamed nailing, and shock and lung contusion groups treated with reamed or unreamed nailing. After lung contusion alone (first hit), the serum values of antithrombin III, factor V, and fibrinogen were considerably altered after reamed and unreamed femoral nailing (second hit) 4 hours postoperatively. In the lung contusion and shock groups, we found a substantial reduction for all serum coagulative parameters between baseline and fixation after reamed and unreamed nailing. The magnitude of the first hit is increased if hemorrhagic shock is added to a lung contusion determined by hemostatic reactions. The magnitude of the injury appears equally important as the type of subsequent surgery and should be considered in planning for fracture fixation in patients at high risk for complications.
Introduction
Intramedullary nailing is the preferred treatment for femoral diaphyseal fractures [4, 7–9, 17, 27, 41, 42, 73, 74]. Some of the known side effects of reaming the femoral canal in preparation for the nailing process are fat embolization and secondary activation of coagulatory and inflammatory pathways [38, 45]. These mechanisms may become clinically apparent in patients who are at high risk to have pulmonary complications develop from lung contusion, severe shock states, or both [1, 11, 15, 30, 33–35, 52–59, 63, 65–67]. It appears a synergistic effect among fat embolization, hemorrhagic shock, and lung contusion may exist [5, 19, 21, 22, 24–26, 48, 54–56, 72, 75, 76]. In a previous clinical study, one of the authors (PVG) documented a difference in the inflammatory response exists according to the type of surgery selected, namely reamed versus unreamed nailing [26].
Unreamed femoral nailing causes less increase in intramedullary canal pressures and less systemic intravasation of bone marrow fat than reamed nailing [32, 44, 55, 72]. However, previous clinical studies have failed to show a reduction in pulmonary complications [10, 26]. A large study supported by the Canadian Orthopaedic Trauma Society in which 322 patients were randomized to either reamed or unreamed femoral nailing reported no difference in the incidence of acute respiratory distress syndrome [10].
However, these studies did show trends for worse outcome after reamed nailing or transient lung dysfunction. Therefore, the effects induced by reamed and unreamed nailing may be subtle, and a clinical study might be unable to prove a difference using conventional parameters, as it was estimated more than 39,000 patients would be needed to detect a difference between groups [10]. Nonetheless, in controlled experiments, differences in pulmonary embolization induced by reamed and unreamed femoral nailing were confirmed [53–55, 59].
Constituents of bone marrow are thrombogenic, and coagulopathic states have been observed after intramedullary nailing [11, 12, 54, 64, 72]. Hemorrhagic shock also activates the inflammatory system and is a well-known cofactor of lung injuries [3, 16, 28, 29, 47, 60, 68, 77, 79]. The effect of additional hemorrhage on the fat embolism-induced changes in the presence of a pulmonary contusion has not been investigated before.
We therefore asked whether intramedullary femoral nailing (reamed and unreamed technique) negatively influenced the coagulation system after lung contusion with and without shock. We then asked whether (1) trauma-induced coagulopathy was altered by a greater degree of trauma (ie, lung contusion and/or lung contusion and shock) and (2) surgical procedures (femoral nailing) had a further sustained impact on hemostasis.
Materials and Methods
Thirty adult skeletally mature Merino sheep were divided into six groups with five sheep randomly assigned to each group. The six groups included thoracotomy control groups treated with (1) reamed or (2) unreamed nailing, lung contusion groups treated with (3) reamed or (4) unreamed nailing, and shock and lung contusion groups treated with (5) reamed or (6) unreamed nailing. We used the same sample size that provided reliable data in the past based on previous pilot studies [54–56, 58]. A post hoc power analysis showed, with a sample size of five animals per group, the study had 80% power to detect a significant difference in terms of the coagulation parameters evaluated. In the control groups, we performed a sham thoracotomy followed by either reamed or unreamed femoral nailing. In the study groups, the animals received a lung contusion or lung contusion and shock followed by either reamed or unreamed femoral nailing. All animals were female and had an average age of 1.4 ± 0.2 years. The average weight was 26.3 kg (range, 24.9–27.3 kg). The treatment protocol for animal subjects was approved by our institutional animal care and use committee (Number 504–42502–02/512). All animal care and surgical treatment were performed according to the German Council’s “Guide for the Use of Laboratory Animals.” All animals had free access to water but were denied access to food 1 day before the experiments.
We used a large animal model because the cardiovascular system in sheep is more comparable to that of humans than that of smaller animals, eg, rabbits [24]. We used an unfractured model to augment the degree of fat embolization. This is important if a sheep is used as an experimental model because the ovine femur is short in relationship to body size. The length of the femur in sheep is only 23% of the vertebral column length (as compared with 60% in humans). The pulmonary effects of femoral reaming are therefore only comparable to the human situation of a midshaft fracture if an intact sheep femur is used [8, 39, 43]. We relied on the same coagulatory parameters used in our previous studies. Although in this kind of clinical scenario, the use of the platelet count has been a good alternative providing rapid information, this is not true for the experiments with sheep. We found the hemostatic parameters used in this and previous experiments provided more accurate and sensitive data. Shock and trauma are recognized as additional important variables in shock models [43, 67, 76]. Moreover, in unanesthetized animals, a higher degree of shock is applicable. In our model, an anesthetized fixed-pressure model was necessary because lung contusion had to be performed at the same time. Also, the severity of shock must be adapted to the animal model; in rodents, a higher relative volume is necessary to induce systemic damage. Blood loss to 50 mm Hg pressure, as performed in the current model, reduces renal blood flow to 25%. We experienced two fatalities during the performance of the study but to magnify the effects caused by the surgical procedures, we believe the degree of shock and lung contusion in the current model has been adequate despite the drawbacks of an anesthetized shock model. Therefore, this experiment enhances our ability to identify changes, which may not be differentiated in a clinical study.
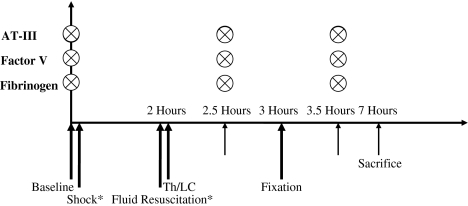
We anesthetized the sheep using injectable Propofol® (AstraZeneca, Wedel, Germany) through the internal jugular vein and Isoflurane® (Abbott, Wiesbaden, Germany) through an 8-mm orotracheal tube. We used an arterial catheter placed into the right carotid artery and a venous line placed into the right internal jugular vein for blood pressure monitoring and blood sampling, respectively. We performed blood sampling in all investigated groups for antithrombin III, factor V, and fibrinogen at set points perioperatively: baseline, 30 minutes after lung contusion or sham thoracotomy, femoral fixation (reamed or unreamed femoral nailing), and 4 hours after beginning the femoral fixation (4 hours postoperatively) (Fig. 1). During the experimental process, two sheep died before the end of the observation and sampling period and no values could be recorded after that time. These deaths occurred in the group submitted to shock and lung contusion and the results of these were excluded from the study. During pilot studies performed in 1989, we used a shock state of 2 hours and 40 mm Hg mean arterial pressure. However, with the combination of lung contusion, the intraoperative mortality had been greater than 60%. Because the animals had to survive until Day 3, less sustained shock could be applied. That later setup was used subsequently for all additional studies.
Fig. 1.
A diagram illustrates the methodology and timing of blood sampling. * = timing in study groups in which hemorrhagic shock was associated with lung contusion; ⊗ = blood sampling; AT-III = antithrombin III; Th = thoracotomy; LC = lung contusion.
Blood samples were centrifuged and stored at −80° C for additional analysis. All animals were sacrificed 4 hours after intramedullary nailing by injecting 25 mL 10% KCl while the animals were deeply anesthetized and ventilated.
For reamed nailings, an intact femur using a third-generation AO-ASIF reaming system (Synthes, Bochum, Germany) was used as previously described [53–55, 58]. We performed sequential reaming with 0.5-mm steps and an implant 0.5 mm smaller than the size of the largest reamer was inserted in each case. In the groups submitted to unreamed femoral nailing, a small size (9-mm) AO-ASIF solid nail was inserted without reaming the medullary canal [27].
We performed thoracotomy of the right chest wall in all control group animals. In the lung contusion groups, a standardized unilateral contusion of the right middle and lower lobes was inflicted as previously described [55, 56]. The pneumothorax was avoided by inserting a chest tube intraoperatively.
Hemorrhagic shock was induced by withdrawing 50-mL fractions of blood through the femoral venous line to a mean arterial pressure of 50 mm Hg. The mean arterial pressure was maintained at this level for 2 hours. At the conclusion of the shock period, we rapidly administered isotonic crystalloid solution (Ringer’s lactate) to restore mean arterial pressure and central filling pressures to baseline levels. In all animals, the temperatures were kept at normal levels throughout the experiment by using a heating pad and a heating lamp. We determined coagulative parameters with EDTA plasma. We measured fibrinogen levels according to the method described by Clauss [12], which is used to measure hemostatically active fibrin. We added thrombin to the plasma samples to induce coagulation. The coagulation time was inversely proportional to the fibrinogen concentration. A 1:40 dilution was set as the 100% value. The test kit and hooklet coagulator were Diagnostica Stago® (Roche Diagnostics, Mannheim, Germany) and Schnitger and Gros® (Amelung GmbH, Lemgo, Germany), respectively.
We determined factor V concentrations using the previously mentioned kits based on the principle that the coagulation time is exclusively dependent on factor V activity and a surplus of all other coagulation factors. A 1:20 dilution was set as the 100% value. Adding heparin and thrombin to the sample resulted in transfer of antithrombin III to an inactive complex. The remaining amount of thrombin was in inverse proportion to the antithrombin III concentration. We determined antithrombin III activity using the AT-III test kit® (Roche Diagnostics) measuring the increase of extinction at 405 nm (Hitachi 912®; Roche Diagnostics).
We compared group differences (factor V, fibrinogen, antithrombin III) at a given time (Fig. 1) by two-way analysis of variance with repeated measurement design. We performed paired t tests to compare data before and after instrumentation in each subgroup. We performed multiple range tests of Tukey and Bonferroni to compare the data of the postoperative period. We tested for statistical differences in the serum values at baseline, thoracotomy/lung contusion, fixation, and 4 hours postoperatively (ie, baseline versus thoracotomy/lung contusion, baseline versus fixation, baseline versus 4 hours postoperatively, thoracotomy/lung contusion versus fixation, thoracotomy/lung contusion versus 4 hours postoperatively, fixation versus 4 hours postoperatively). Significance was set at p < 0.05.
Results
Intramedullary nailing negatively influenced the coagulation system after lung contusion with and without shock. Antithrombin III levels were reduced (p = 0.02) in the two control groups after reamed and unreamed nailing. Larger reductions in antithrombin III were noted in the four study groups, with even greater reductions in the two groups submitted to lung contusion and shock (p = 0.0009) than in those submitted to lung contusion alone (p = 0.003) (Table 1). Factor V levels were reduced (p = 0.04 and p = 0.014) from baseline to 4 hours postoperation in the two control groups with reamed and unreamed nailing, respectively. There was an additional reduction (p = 0.01) in the lung contusion groups and this decrease was more severe (p = 0.001) in the lung contusion and shock groups (Table 2). In the control groups, we found no effect of the surgical procedure on the fibrinogen levels. In the groups submitted to lung contusion, there were reductions (p = 0.005) at the 4-hour postoperation time only. In the shock and lung contusion groups, we detected decreased levels (p = 0.001) of fibrinogen at the time of surgery (Table 3).
Table 1.
Perioperative antithrombin III levels in the central venous blood of control and study groups
| Type of nailing | Times | Thoracotomy | Lung contusion | Shock and lung contusion |
|---|---|---|---|---|
| Reamed | Baseline | 81.02 (6.866) | 83.96 (5.123) | 85.08 (3.196) |
| Thoracotomy/ lung contusion | 76.14 (6.790) | 77.54 (2.278) | 66.9 (9.165) | |
| Fixation | 75.16 (8.562) | 73.96 (4.755) | 53.76 (11.936) | |
| 4 hours postoperatively | 70.7 (5.629) | 67.22 (6.330) | 52.65 (0.55) | |
| p values | Baseline vs fixation, p = 0.027; baseline vs 4 hours postoperatively, p = 0.03 | Baseline vs fixation, p = 0.021; baseline vs 4 hours postoperatively, p = 0.003 | Baseline vs fixation, p = 0.0009 | |
| Unreamed | Baseline | 84.5 (6.914) | 84.08 (8.500) | 82.62 (8.873) |
| Thoracotomy/ lung contusion | 74.52 (2.524) | 77.03 (4.541) | 55.7 (7.161) | |
| Fixation | 74.1 (3.481) | 71.96 (4.600) | 44.68 (5.018) | |
| 4 hours postoperatively | 75.96 (4.172) | 68.56 (4.952) | 41.35 (11.738) | |
| p values | Baseline vs fixation, p = 0.037; baseline vs 4 hours postoperatively, p = 0.045 | Baseline vs fixation, p = 0.0186; baseline vs 4 hours postoperatively, p = 0.0034 | Baseline vs fixation, p = 7.31 × 10−5 |
Values are expressed as means (% of normal levels), with standard deviations in parentheses.
Table 2.
Perioperative factor V values in central venous blood of control and study groups
| Type of nailing | Times | Thoracotomy | Lung contusion | Shock and lung contusion |
|---|---|---|---|---|
| Reamed | Baseline | 101 (12.930) | 116.6 (35.051) | 138.2 (19.722) |
| Thoracotomy/ lung contusion | 85.8 (11.923) | 103.6 (20.421) | 93.6 (24.686) | |
| Fixation | 89.2 (11.956) | 90.2 (19.538) | 62.8 (22.569) | |
| 4 hours postoperatively | 84.6 (15.160) | 72.6 (21.029) | 52 (3) | |
| p values | Baseline vs 4 hours postoperatively, p = 0.04 | Baseline vs 4 hours postoperatively, p = 0.011 | Baseline vs lung contusion, p = 0.022; baseline vs 4 hours postoperatively fixation, p = 0.001 | |
| Unreamed | Baseline | 100.6 (23.277) | 94.666 (19.703) | 120.8 (45.827) |
| Thoracotomy/ lung contusion | 78.2 (13.962) | 81.833 (10.023) | 77.8 (52.954) | |
| Fixation | 80.8 (11.634) | 75.166 (14.993) | 46.2 (23.6) | |
| 4 hours postoperatively | 63.4 (4.923) | 58.166 (15.015) | 41.25 (22.708) | |
| p values | Baseline vs 4 hours postoperatively, p = 0.014 | Baseline vs 4 hours postoperatively, p = 0.012 | Baseline vs fixation, p = 0.00; baseline vs 4 hours postoperatively, p = 0.001 |
Values are expressed as means (% of normal levels), with standard deviations in parentheses.
Table 3.
Perioperative fibrinogen values in central venous blood of control and study groups
| Type of nailing | Times | Thoracotomy | Lung contusion | Shock and lung contusion |
|---|---|---|---|---|
| Reamed | Baseline | 108 (22.432) | 95.4 (10.762) | 89.4 (14.122) |
| Thoracotomy/ lung contusion | 98.6 (14.263) | 88 (12.521) | 64.4 (9.645) | |
| Fixation | 97.6 (14.263) | 81.6 (12.862) | 45.8 (10.4) | |
| 4 hours postoperatively | 91.4 (13.139) | 61.6 (14.263) | 38.2 (4.664) | |
| p values | Baseline vs 4 hours postoperatively, p = 0.1 | Baseline vs 4 hours postoperatively, p = 0.005 | Baseline vs lung contusion, p = 0.019; baseline vs fixation, p = 0.001 | |
| Unreamed | Baseline | 104.6 (20.115) | 90 (12.583) | 104.8 (16.460) |
| Thoracotomy/ lung contusion | 96.8 (21.414) | 82.5 (10.657) | 66.6 (26.620) | |
| Fixation | 97.8 (22.139) | 74.8 (8.818) | 45.4 (19.126) | |
| 4 hours postoperatively | 88.2 (19.156) | 63.5 (9.5) | 42.66 (16.519) | |
| p values | Baseline vs 4 hours postoperatively, p = 0.14 | Baseline vs 4 hours postoperatively, p = 0.003 | Baseline vs lung contusion, p = 0.04; baseline vs fixation, p = 0.001 |
All values in table are expressed as means (% of normal levels), with standard deviations in parentheses.
We found no differences in levels of antithrombin II, factor V, and fibrinogen between the groups regarding the type of nailing (Tables 1–3).
Discussion
Many surgeons consider intramedullary nailing the preferred treatment for femoral diaphyseal fractures. However, side effects of reaming the femoral canal include fat embolization and secondary activation of coagulatory and inflammatory pathways [26]. Although these mechanisms usually are not apparent clinically, they may become important in patients at high risk of having pulmonary complications from lung contusion, severe shock states, or both. In this study, we wished to determine whether intramedullary nailing (inserted either with the reamed or the unreamed technique) induced alternations of the coagulation system after lung contusion with and without shock. Furthermore, we examined whether trauma-induced coagulopathy was affected by a greater degree of trauma (lung contusion and/or lung contusion and shock) and whether femoral nailing had an additional consequence on hemostasis.
The main limitations of this study are as follows. First, we only reamed the femur without having previously created a fracture. Having a fracture may reduce the intramedullary pressure, as contents may exist at the fracture site. Second, the hemorrhagic shock was induced by withdrawing blood from the systemic circulation. However, in patients with an isolated femoral fracture, diffuse bleeding without gross blood loss to the exterior occurs. Third, we only observed the animals for 7 hours. In contrast, patients are treated in the intensive care unit with a large regime of technical measures and drugs. Thus, we only observed the initial immunologic sequelae. Nevertheless, our study is valid for understanding the underlying disorders on lung contusion with concomitant hemorrhagic shock with subsequent reamed or unreamed nailing.
We used a standardized sheep model to explore the impact of intramedullary nailing on the coagulation system in the presence of lung contusion with and without shock. Based on the current understanding of the immunoinflammatory response to injury and the acceptance of the principles of the first- and second-hit phenomena, shock and lung contusion were perceived as the first hit and femoral nailing as the second hit [23, 26]. To evaluate the severity of the hits, we measured hemostatic parameters that have been described as sensitive indicators of the severity of trauma and the magnitude of surgery [6, 11, 14, 60–62]. Furthermore, the relationship between coagulopathy and subsequent alterations of inflammatory factors has been reported [24, 26]. These have important clinical implications because the inflammatory response has been shown to correlate with posttraumatic complications [24, 31].
We found after lung contusion alone the serum values of antithrombin III, factor V, and fibrinogen were reduced 4 hours postoperatively after reamed and unreamed femoral nailing. Furthermore, lung contusion and shock further reduced all serum coagulative parameters between baseline and fixation after reamed and unreamed nailing. This finding supports the view that the magnitude of the first hit is increased if hemorrhagic shock is added to a lung contusion determined by hemostatic reactions.
Hemorrhage in a pig model showed reduction in blood clotting time (92.7% ± 1.6%), acceleration of the fibrinogen breakdown rate, and unchanged absolute fibrinogen rate synthesis [49]. Studies in a rat model showed activated partial thromboplastin time and prothrombin time were gradually prolonged during shock and were considerably low within 4 to 6 hours after shock induction [78]. The levels of fibrinogen, tissue factor, von Willebrand’s factor, and factor VIII considerably increased in the initial stage of shock but decreased to their minimum 7 hours after shock [78].
The use of D-dimers (breakdown products of fibrin) for detection of coagulopathy must be differentiated from the other parameters; the serum concentration of D-dimers is believed to be a very sensitive indicator of the fibrinolytic cascade function [37] and the severity of osseous and soft tissue injury [13, 46]. D-dimer concentrations have been used to detect patients at high risk of postoperative complications after femoral nailing [64] and have been proposed for the prognosis of patients experiencing shock [78]. However, their peak levels appear to occur only 8 hours after shock induction [78]. Because there was severe predamage (first hit) leading to two fatalities in the study groups in our study, we believed the parameters used in the current setup were adequate.
After hypovolemic shock, nonetheless, one has to consider the potential impact of other important parameters in the coagulation system. To begin with, trauma-related hypothermia is known to cause a considerable increase in coagulopathy, especially if the core temperature decreases below 34° C [51, 70]. Clotting time prolongation appears to be proportional to the number of enzymatic steps involved, and the coagulopathy observed during hypothermia is, in part, independent of the serum levels of the clotting factors [43, 61, 62, 70]. Experimental studies showed acidosis associated with hypothermia (in swine) inhibits the thrombin generation and prolongs the prothrombin time and partial thromboplastin time [50]. In our study, the additional effect from hypothermia can be excluded because the core temperature was monitored and kept stable using heating lights throughout the duration of the study.
In addition, the presence of thoracic trauma is another important factor. In the clinical setting, it represents the most frequent associated injury in polytraumatized patients with femoral fractures [2] and it adds vastly to the morbidity and mortality [71]. This is based on the fact that direct and indirect disturbances to the pulmonary system by systemic inflammatory reactions represent a promoter for posttraumatic acute respiratory distress syndrome [53, 69]. Acute respiratory distress syndrome and multiple organ failure are associated with hypercoagulable states [18, 20, 31, 39]. Long bone canal instrumentation can induce bone marrow embolism (bone marrow contents activate coagulation and fibrinolysis) [55, 63] and can increase pulmonary permeability, inflammatory cell invasion of the lungs [1, 36, 40, 41], and the coagulopathic states in them [64].
In another sheep model, the concentrations of factor V and fibrinogen were reduced after reamed nailing [64]. Femoral instrumentation in sheep with induced shock and lung contusion increased the pulmonary permeability and the consumption of coagulation factors [54]. The data from that study showed there was reduction but not abolition of pulmonary sequelae when unreamed femoral nailing was used [54]. We could not confirm this effect by unreamed nailing. Only one parameter and in one subanalysis only (antithrombin III in control animals) showed a trend toward a difference between reamed and unreamed nailing, with less severe alterations in the unreamed group. Several aspects may be responsible for the fact that this finding was different from all other studies published by our group so far [26, 54–56, 59]. First, the magnitude of trauma may have been worse than in the other studies. The fact that two animals died in the shock and lung contusion group appears to confirm this. However, the experiments all were performed by the same main investigators (FH, MVG) involved in the previous experiments. In addition, we found similar amounts of fatal outcomes in previous experiments and in most trauma models; this is regarded as normal. Second, the choice of the parameters used may have played a role. In previous experiments, we used mainly D-dimers—which are more sensitive indicators of trauma-induced coagulopathy—to assess the hemostatic reaction. Therefore, the parameters currently used may not have been able to assess the differences between both procedures. Third, we have not performed a comparison between different magnitudes of the first-hit damage as yet. In all previous investigations, the predamage was unchanged and usually consisted of hemorrhagic shock and lung contusion. It is possible the influence of the initial trauma plays a more important role than previously appreciated. Moreover, our findings confirm those of other reports [23, 25, 34, 35, 41, 52–54, 57, 58, 64, 69, 71], suggesting the degree of shock and lung contusion should be considered when deciding on fracture management.
The magnitude of the traumatic event appears to be an important determinant of the additional damage (hemostatic response) caused by the second hit. Therefore, we believe it is justified to evaluate the influence of the first hit on the ability of the second hit to alter the incidence of posttraumatic complications. Clinical randomized, controlled studies that differentiate between a stable and an unstable clinical condition may clarify these aspects.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Balk RA, Jacobs RF, Tryka AF, Townsend JW, Walls RC, Bone RC. Effects of ibuprofen on neutrophil function and acute lung injury in canine endotoxin shock. Crit Care Med. 1988;16:1121–1127. [DOI] [PubMed]
- 2.Bardenheuer M, Obertacke U, Waydhas C, Nast-Kolb D. [Epidemiology of the severely injured patient: a prospective assessment of preclinical and clinical management. AG Polytrauma of DGU.] Unfallchirurg. 2000;103:355–363. [DOI] [PubMed]
- 3.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. [DOI] [PubMed]
- 4.Bone LB, Johnson KD, Weigelt J, Scheinberg R. Early versus delayed stabilization of femoral fractures: a prospective randomized study. J Bone Joint Surg Am. 1989;71:336–340. [PubMed]
- 5.Brill SA, Stewart TR, Brundage SI, Schreiber MA. Base deficit does not predict mortality when secondary to hyperchloremic acidosis. Shock. 2002;17:459–462. [DOI] [PubMed]
- 6.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. [DOI] [PubMed]
- 7.Brumback RJ, Ellison PS Jr, Poka A, Lakatos R, Bathon GH, Burgess AR. Intramedullary nailing of open fractures of the femoral shaft. J Bone Joint Surg Am. 1989;71:1324–1331. [PubMed]
- 8.Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part I: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70:1441–1452. [PubMed]
- 9.Brumback RJ, Uwagie-Ero S, Lakatos RP, Poka A, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70:1453–1462. [PubMed]
- 10.Canadian Orthopaedic Trauma Society. Reamed versus unreamed intramedullary nailing of the femur: comparison of the rate of ARDS in multiple injured patients. J Orthop Trauma. 2006;20:384–387. [DOI] [PubMed]
- 11.Christie J. The coagulatory effects of fat embolization during intramedullary manipulative procedures. Tech Orthop. 1996;11:14–21. [DOI]
- 12.Clauss A. [Rapid physiological coagulation method in determination of fibrinogen.] Acta Haematol. 1957;17:237–246. [DOI] [PubMed]
- 13.Dahl OE, Aspelin T, Lyberg T. The role of bone traumatization in the initiation of proximal deep vein thrombosis during cemented hip replacement surgery in pigs. Blood Coagul Fibrinolysis. 1995;6:709–717. [DOI] [PubMed]
- 14.DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin. 2004;20:13–24. [DOI] [PubMed]
- 15.Duwelius PJ, Huckfeldt R, Mullins RJ, Shiota T, Woll TS, Lindsey KH, Wheeler D. The effects of femoral intramedullary reaming on pulmonary function in a sheep lung model. J Bone Joint Surg Am. 1997;79:194–202. [DOI] [PubMed]
- 16.Fan J, Li Y, Vodovotz Y, Billiar TR, Wilson MA. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage primed lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;290:L738-L746. [DOI] [PubMed]
- 17.Folleras G, Ahlo A, Stromsoe K, Ekeland E, Thoresen BO. Locked intramedullary nailing of fractures of femur and tibia. Injury. 1990;21:385–388. [DOI] [PubMed]
- 18.Gando S, Kameue T, Nanzaki S, Hayakawa T, Nakanishi Y. Participation of tissue factor and thrombin in posttraumatic systemic inflammatory syndrome. Crit Care Med. 1997;25:1820–1826. [DOI] [PubMed]
- 19.Gando S, Nakanishi Y, Tedo I. Cytokines and plasminogen activator inhibitor-1 in posttrauma disseminated intravascular coagulation: relationship to multiple organ dysfunction syndrome. Crit Care Med. 1995;23:1835–1842. [DOI] [PubMed]
- 20.Gando S, Nanzaki S, Morimoto Y, Kobayashi S, Kemmotsu O. Systemic activation of tissue-factor dependent coagulation pathway in evolving acute respiratory distress syndrome in patients with trauma and sepsis. J Trauma. 1999;47:719–723. [DOI] [PubMed]
- 21.Gando S, Tedo I, Kubota M. Posttrauma coagulation and fibrinolysis. Crit Care Med. 1992;20:594–600. [DOI] [PubMed]
- 22.Garcia-Barreno P, Balibrea JL, Aparicio P. Blood coagulation changes in shock. Surg Gynecol Obstet. 1978;147:6–12. [PubMed]
- 23.Giannoudis PV. Surgical priorities in damage control in polytrauma. J Bone Joint Surg Br. 2003;85:478–483. [DOI] [PubMed]
- 24.Giannoudis PV, Abbott C, Stone M, Bellamy MC, Smith RM. Fatal systemic inflammatory response syndrome following early bilateral femoral nailing. Intensive Care Med. 1998;24:641–642. [DOI] [PubMed]
- 25.Giannoudis PV, Pape HC. Damage control orthopaedics in unstable pelvic ring injuries. Injury. 2004;35:671–677. [DOI] [PubMed]
- 26.Giannoudis PV, Smith RM, Bellamy MC, Morrison JF, Dickson RA, Guillou PJ. Stimulation of the inflammatory system by reamed and unreamed nailing of femoral fractures: an analysis of the second hit. J Bone Joint Surg Br. 1999;81:356–361. [DOI] [PubMed]
- 27.Haas N, Schutz M, Sudkamp N, Hoffmann R. The new unreamed AO nails for the tibia and the femur. Acta Orthop Belg. 1995;61(suppl 1):204–206. [PubMed]
- 28.Hamilton SM. The use of blood in resuscitation of the trauma patient. Can J Surg. 1993;36:21–27. [PubMed]
- 29.Harkin DW, Marron CD, Rother RP, Romaschin A, Rubin BB, Lindsay TF. C5 complement inhibition attenuates shock and acute lung injury in an experimental model of ruptured abdominal aortic aneurysm. Br J Surg. 2005;92:1227–1234. [DOI] [PubMed]
- 30.Harris I, Hatfield A, Donald G, Walton J. Outcome after intramedullary nailing of femoral shaft fractures. ANZ J Surg. 2003;73:387–389. [DOI] [PubMed]
- 31.Hasegawa N, Husari AW, Hart WT, Kandra TG, Raffin TA. Role of the coagulation system in ARDS. Chest. 1994;105:268–277. [DOI] [PubMed]
- 32.Heim D, Regazzoni P, Tsakiris DA, Aebi T, Schlegel U, Marbet GA, Perren SM. Intramedullary nailing and pulmonary embolism: does unreamed nailing prevent embolization? An in vivo study in rabbits. J Trauma. 1995;38:899–906. [DOI] [PubMed]
- 33.Hiippala S. Replacement of massive blood loss. Vox Sang. 1998;74(suppl 2):399–407. [DOI] [PubMed]
- 34.Hildebrand F, Giannoudis P, Kretteck C, Pape HC. Damage control: extremities. Injury. 2004;35:678–689. [DOI] [PubMed]
- 35.Hildebrand F, Giannoudis P, van Griensven M, Chawda M, Probst C, Harms O, Harwood P, Otto K, Fehr M, Krettek C, Pape HC. Secondary effects of femoral instrumentation on pulmonary physiology in a standardised sheep model: what is the effect of lung contusion and reaming? Injury. 2005;36:544–555. [DOI] [PubMed]
- 36.Hofmann S, Huemer G, Kratochwill C, Koller-Strametz J, Hopf R, Schlag G, Salzer M. [Pathophysiology of fat embolisms in orthopedics and traumatology.] Orthopade. 1995;24:84–93. [PubMed]
- 37.Hogevold HE, Lyberg T, Kierulf P, Reikeras O. Generation of procoagulant (thromboplastin) and plasminogen activator activities in peripheral blood monocytes after total hip replacement surgery: effects of high doses of corticosteroids. Thromb Res. 1991;62:449–457. [DOI] [PubMed]
- 38.Hughes SP, Reichert IL, McCarthy ID. Biological effects of intramedullary reaming. J Bone Joint Surg Br. 1993;75:845–847. [DOI] [PubMed]
- 39.Idell S. Extravascular coagulation and fibrin deposition in acute lung injury. New Horiz. 1994;2:566–574. [PubMed]
- 40.Jacobovitz-Derks D, Derks CM. Pulmonary neutral fat embolism in dogs. Am J Pathol. 1979;95:29–42. [PMC free article] [PubMed]
- 41.Johnson KD, Cadambi A, Seibert GB. Incidence of adult respiratory distress syndrome in patients with multiple musculoskeletal injuries: effect of early operative stabilization of fractures. J Trauma. 1985;25:375–384. [DOI] [PubMed]
- 42.Kempf I, Grosse A, Beck G. Closed locked intramedullary nailing: its application to comminuted fractures of the femur. J Bone Joint Surg Am. 1985;67:709–720. [PubMed]
- 43.Krause KR, Howells GA, Buhs CL, Hernandez DA, Bair H, Schuster M, Bendick PJ. Hypothermia-induced coagulopathy during hemorrhagic shock. Am Surg. 2000;66:348–354. [PubMed]
- 44.Kropfl A, Berger U, Neureiter H, Hertz H, Schlag G. Intramedullary pressure and bone marrow fat intravasation in unreamed femoral nailing. J Trauma. 1997;42:946–954. [DOI] [PubMed]
- 45.Kropfl A, Davies J, Berger U, Hertz H, Schlag G. Intramedullary pressure and bone marrow fat extravasation in reamed and unreamed femoral nailing. J Orthop Res. 1999;17:261–268. [DOI] [PubMed]
- 46.Kwan HC. Role of fibrinolysis in disease processes. Semin Thromb Hemost. 1984;10:71–79. [DOI] [PubMed]
- 47.Levy SE, Shapiro BJ, Simmons DH. Pulmonary hemodynamics after autologous in vivo pulmonary thromboembolism. J Appl Physiol. 1969;27:53–60. [DOI] [PubMed]
- 48.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. [DOI] [PubMed]
- 49.Martini WZ, Chinkes DL, Pusateri AE, Holcomb JB, Yu YM, Zhang XJ, Wolfe RR. Acute changes in fibrinogen metabolism and coagulation after hemorrhage in pigs. Am J Physiol Endocrinol Metab. 2005;289:E930–E934. [DOI] [PubMed]
- 50.Martini WZ, Pusateri AE, Uscilowicz JM, Delgado AV, Holcomb JB. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58:1002–1009. [DOI] [PubMed]
- 51.McInerney JJ, Breakell A, Madira W, Davies TG, Evans PA. Accidental hypothermia and active rewarming: the metabolic and inflammatory changes observed above and below 32 degrees C. Emerg Med J. 2002;19:219–223. [DOI] [PMC free article] [PubMed]
- 52.Pape H, Stalp M, van Griensven M, Weinberg A, Dahlweit M, Tscherne H. [Optimal timing for secondary surgery in polytrauma patients: an evaluation of 4,314 serious-injury cases.] Chirurg. 1999;70:1287–1293. [DOI] [PubMed]
- 53.Pape HC, Auf’m’Kolk M, Paffrath T, Regel G, Sturm JA, Tscherne H. Primary intramedullary femur fixation in multiple trauma patients with associated lung contusion—a cause of posttraumatic ARDS? J Trauma. 1993;34:540–547. [DOI] [PubMed]
- 54.Pape HC, Bartels M, Pohlemann T, Werner T, von Glinski S, Baur H, Tscherne H. Coagulatory response after femoral instrumentation after severe trauma in sheep. J Trauma. 1998;45:720–728. [DOI] [PubMed]
- 55.Pape HC, Dwenger A, Grotz M, Kaever V, Negatsch R, Kleemann W, Regel G, Sturm JA, Tscherne H. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8:300–309. [DOI] [PubMed]
- 56.Pape HC, Dwenger A, Regel G, Schweitzer G, Jonas M, Remmers D, Krumm K, Neumann C, Sturm JA, Tscherne H. Pulmonary damage after intramedullary femoral nailing in traumatized sheep: is there an effect from different nailing methods? J Trauma. 1992;33:574–581. [DOI] [PubMed]
- 57.Pape HC, Giannoudis P, Krettek C. The timing of fracture treatment in polytrauma patients: relevance of damage control orthopedic surgery. Am J Surg. 2002;183:622–629. [DOI] [PubMed]
- 58.Pape HC, van Griensven M., Rice J, Gansslen A, Hildebrand F, Zech S, Winny M, Lichtinghagen R, Krettek C. Major secondary surgery in blunt trauma patients and perioperative cytokine liberation: determination of the clinical relevance of biochemical markers. J Trauma. 2001;50:989–1000. [DOI] [PubMed]
- 59.Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87:2515–2522. [DOI] [PubMed]
- 60.Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg. 1995;32:925–1002. [DOI] [PubMed]
- 61.Reed RL, Bracey AW Jr, Hudson JD, Miller TA, Fischer RP. Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141–152. [PubMed]
- 62.Reed RL, Johnson TD, Hudson JD, Fischer RP. The disparity between hypothermic coagulopathy and clotting studies. J Trauma. 1992;33:465–470. [DOI] [PubMed]
- 63.Regel G, Nerlich ML, Dwenger A, Seidel J, Schmidt C, Sturm JA. Induction of pulmonary injury by polymorphonuclear leucocytes after bone marrow fat injection and endotoxemia: a sheep model. Theor Surg. 1989;4:22–30.
- 64.Robinson CM, Ludlam CA, Ray DC, Swann DG, Christie J. The coagulative and cardiorespiratory responses to reamed intramedullary nailing of isolated fractures. J Bone Joint Surg Br. 2001;83:963–973. [DOI] [PubMed]
- 65.Scalea TM, Boswell SA, Scott JD, Mitchell KA, Kramer ME, Pollak AN. External fixation as a bridge to intramedullary nailing for patients with multiple injuries and with femur fractures: damage control orthopedics. J Trauma. 2000;48:613–621. [DOI] [PubMed]
- 66.Schemitsch EH, Jain R, Turchin DC, Mullen JB, Byrick RJ, Anderson GI, Richards RR. Pulmonary effects of fixation of a fracture with a plate compared with intramedullary nailing: a canine model of fat embolism and fracture fixation. J Bone Joint Surg Am. 1997;79:984–996. [DOI] [PubMed]
- 67.Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005;11:590–597. [DOI] [PubMed]
- 68.Spivey M, Parr MJ. Therapeutic approaches in trauma-induced coagulopathy. Minerva Anestesiol. 2005;71:281–289. [PubMed]
- 69.Stellin G. Survival in trauma victims with pulmonary contusion. Am Surg. 1991;57:780–784. [PubMed]
- 70.Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C. Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998;44:846–854. [DOI] [PubMed]
- 71.Waydhas C, Nast-Kolb D, Trupka A. Die Bedeutung des traumatisch-haemorrhagischen Schocks und der Thoraxverletzung fur die Prognose nach Polytrauma. Hefte Unfallheilkd. 1990;212:104–105.
- 72.Wenda K, Ritter G, Ahlers J, von Issendorff WD. [Detection and effects of bone marrow intravasations in operations in the area of the femoral marrow cavity.] Unfallchirurg. 1990;93:56–61. [PubMed]
- 73.Winquist RA, Hansen ST Jr, Clawson DK. Closed intramedullary nailing of femoral fractures: a report of five hundred and twenty cases. 1984. J Bone Joint Surg Am. 2001;83:1912. [PubMed]
- 74.Wiss DA, Brien WW, Stetson WB. Interlocked nailing for treatment of segmental fractures of the femur. J Bone Joint Surg Am. 1990;72:724–728. [PubMed]
- 75.Wolinsky PR, McCarty E, Shyr Y, Johnson K. Reamed intramedullary nailing of the femur: 551 cases. J Trauma. 1999;46:392–399. [DOI] [PubMed]
- 76.Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36:202–207. [DOI] [PubMed]
- 77.Younger JG, Sasaki N, Waite MD, Murray HN, Saleh EF, Ravage ZB, Hirschl RB, Ward PA, Till GO. Detrimental effects of complement activation in hemorrhagic shock. J Appl Physiol. 2001;90:441–446. [DOI] [PubMed]
- 78.Zhang YJ, Pan JY, Wang MS. [Study on changes of blood coagulation factors in rats with hemorrhagic shock.] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13:110–113. [PubMed]
- 79.Zimmermann T, Laszik Z, Nagy S, Kaszaki J, Joo F. The role of the complement system in the pathogenesis of multiple organ failure in shock. Prog Clin Biol Res. 1989;308:291–297. [PubMed]