Abstract
In vitro studies demonstrating excessive wear in polyethylene cups sterilized using gamma irradiation and stored in air led to the abandonment of this sterilization technique. We evaluated the clinical wear performance of a metal femoral component on a polyethylene cup in a hip prosthesis from a selected subset of implants in a group of patients followed for at least 20 years and assessed the time dependency of variation in penetration rates. We measured penetration in 33 polyethylene cups in 25 patients who had a Charnley low-friction arthroplasty between 1982 and 1984. All patients had Charnley Ogee® cups implanted for more than 20 years and sterilized using the gamma irradiation in air technique. If degradation occurred over time in vivo, it was not reflected by an increased penetration rate with increasing time in vivo; even after 20 years of implantation, the degree of wear remained low. This suggests gamma irradiation affects wear on ultra-high-molecular-weight polyethylene by reducing wear secondary to the crosslinking, by increasing wear as shown through in vitro studies of heavily oxidized samples, or by oxidation resulting from prolonged shelf life. The effect of progressive oxidation in vivo does not appear to affect wear in vivo.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Total hip arthroplasty is a very successful procedure that provides pain relief and restoration of mobility. It is estimated approximately one million such procedures are carried out per year worldwide. A critical component of a hip arthroplasty is the bearing surface, and good wear performance is now seen as necessary for the long-term survivorship and overall performance of the joint. The concern with hard-on-polyethylene bearings is the generation of polyethylene wear debris, which has been associated with osteolysis and aseptic loosening [11].
Furthermore, time-dependent degradation in the form of oxidation occurs in ultra-high-molecular-weight polyethylene (UHMWPE) and is accelerated by the gamma irradiation process generally used to sterilize components. This can have a substantial effect on the mechanical properties of UHMWPE [15]. Previous studies suggest UHMWPE wear is increased in hip simulator tests when the cups are sterilized by gamma irradiation and stored for an extended period before implantation [2]. Some evidence suggests the long-term in vivo and in vitro degradation of UHMWPE may differ [14]. What is not understood is the effect of the level of degradation associated with such components that have been implanted for very long periods of time, specifically how any degradation may bring about variations in the long-term wear rate of components. Interestingly, there are no reports of catastrophic increases in wear rates with similar followup studies, which predominantly used gamma irradiation followed by storage in air as the method of sterilization [12].
To answer these questions requires studies with long-term regular followup of patients with high-quality radiographs and the foresight to believe such data would be worth collecting over a 25-year period.
The low-friction arthroplasty implants and surgical technique described by Charnley [3] used an all-polyethylene cemented acetabular cup in combination with a stainless steel monoblock cemented stem with a 22.225-mm femoral head. This procedure was prevalent in the United Kingdom until the mid-1990s when its use began to decline in favor of more contemporary components. The style of cup used in our study is the Charnley Ogee® cup (DePuy International Ltd, Leeds, UK), an all-polyethylene cup with a pressure injection flange to improve the interdigitation of the acrylic cement into the prepared acetabulum. It was named ogee because the double curvature of the plane of the face of the flange passing from concave to convex resembles in cross-section the type of curve best illustrated in the ogee arch of gothic architecture. From 1969 to 1998, all components of this type were sterilized using gamma irradiation in air. This technique, although standard practice at the time, has fallen into disuse as a result of concerns over the long-term stability of polyethylene after undergoing this process [5].
We asked whether polyethylene degradation continues in vivo over time, thereby accelerating wear. We further asked if there was any relationship of patient demographics (gender, weight, height, body mass index) to penetration rates and creep.
Materials and Methods
We retrospectively reviewed 232 consecutive patients who underwent primary Charnley low-friction arthroplasties performed by the senior author (JO) between June 1982 and December 1984. Thirty-six patients had bilateral arthroplasties (28 simultaneous, eight interval), providing 268 Charnley Ogee® cups for review. Two hundred twenty-seven patients (98%) had the arthroplasty for osteoarthrosis. The mean age at operation was 67 years (range, 30–88 years). Women dominated the original cohort with a ratio 3:1. At 20 years, 151 patients had died from causes unrelated to the implant, and five (2%) were lost to followup. Five patients in the original cohort of 232 patients underwent revision for aseptic loosening; three were revised at 12 years and the remaining two at 20 years (representing a 1% revision rate at 16 years and 2% at 22 years). Therefore, 76 patients (91 hips) were alive and of these, 95% had their original Charnley Ogee® cup. Within this group, 25 patients (33 hips) had serial, but not necessarily current, radiographs available for study. These patients were arbitrarily selected from a group with some selection bias; we were limited to patients being able and willing to travel for radiographic assessment. In this group, the mean age at the time of operation was 59.6 ± 2.8 years (± 95% confidence interval; range, 43–72 years); 33% were women and 67% men. The minimum time in vivo was 20 years (mean, 20.6 years; range, 20–22.4 years). The weight, height, and body mass index was greater (p < 0.05) for male than female patients (Table 1). Eighteen were left hips and 15 were right. The patients were invited for review at approximately 1, 5, 10, 15, and 20 years; not all patients attended at each interval and some radiographs were not of sufficient quality for inclusion in the study. We used the clinical data and serial radiographs (three to 12 for each patient) to evaluate the performance of the Charnley Ogee® polyethylene cups (Fig. 1A–B). We analyzed 217 radiographs in total. The mean postoperative time for the last radiograph was 20 ± 0.6 years (range, 11.8–22.4 years).
Table 1.
Demographics of male, female, and the overall patient groups
| Group | Age (years) | Weight (kg) | Height (m) | Body Mass Index (kg/m2) |
|---|---|---|---|---|
| Male | 59.0 ± 3.9 | 74.7 ± 4.4 | 1.7 ± 0.04 | 26.1 ± 1.1 |
| Female | 60.7 ± 4.3 | 62.1 ± 6.0* | 1.6 ± 0.04* | 24.2 ± 1.7* |
| Overall | 59.6 ± 2.8 | 70.5 ± 4.0 | 1.7 ± 0.03 | 25.5 ± 1.0 |
Values are expressed as means ± 95% confidence limits; *weight, height, and body mass index for female patients were less (p < 0.05) than those for male patients.
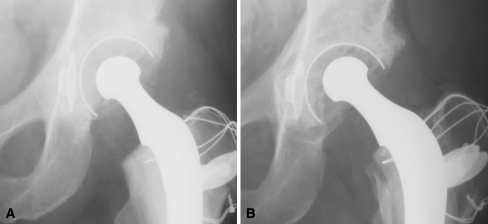
Fig. 1A–B.
Wear was measured radiographically. (A) Postoperative and (B) late (15 years) radiographs from the same patient show a small amount of polyethylene linear penetration.
The components comprised a Charnley cemented femoral stem of monoblock design (22.225-mm femoral head) made from Ortron 90® cold-worked high-nitrogen stainless steel. This was combined with a Charnley Ogee® all-polyethylene flanged cemented acetabular cup. The latter were machined from compression-molded sheets of Chirulen® UHMWPE (Hoechst, Frankfurt, Germany). The flange was molded from silane-crosslinked medium-density polyethylene and two sizes were available: 40-mm or 43-mm outer diameter. Interpretation of radiographic appearance was aided by a factory-assembled stainless steel marker wire that was confluent with the outer margin of the cup. The finished cups were sterilized by gamma irradiation in an air environment using a dose range of 25 to 40 kGy. The cement used was CMW 1™ polymethylmethacrylate. All components were supplied by DePuy International Ltd.
When the final radiographs were examined (JO), none showed evidence of osteolysis (however, it is acknowledged that plane radiographs are only partially reliable in the detection of osteolysis); 27 showed no demarcation at all, four had demarcation in Zone 1, and two in Zone 2. All radiographs were analyzed (SW) using software written in-house on a graphic programming package (IDL; ITT Corp, Boulder, CO) [13]. The program used an algorithm similar to that used by Shaver et al. [16] to determine the femoral head and wire marker positions. Five points on the outer surface of the femoral head and five on the inner surface of the wire marker were selected by the operator. On the wire marker, the five points included the medial and lateral end points, which were used to define the socket orientation. A circle and an ellipse were fitted to the points on the femoral head and wire marker, respectively. The center points of radii of the circle and ellipse were then used to define a set of sampling ray profiles emanating from the center of the femoral head and socket between the medial and lateral end points at 1° intervals. The gray-scale intensity along each profile was then obtained and the points of maximum gradient determined in the regions of the wire marker and femoral head. To remove spurious points of maximum gradient, the mean intensities on both sides of a profile were calculated and the point was only accepted if the intensity change was of a predetermined direction. Circles were fitted to the points on both the head and wire marker. The distance of each edge point from the corresponding point on the fitted circle was calculated and outlying points excluded if they were more than three standard deviations from the mean distance. Linear wear was defined from the displacement between the center of the circles fitted to the femoral head and the inner border of the wire marker. The wear depth is converted from pixels to millimeters using a calibration factor obtained from the known dimensions of the femoral head [5]. The penetration and penetration rate were plotted with respect to frequency for men and women. Data were also examined at intervals to assess the change in penetration (Table 2) and penetration rate (Table 3) with time in vivo.
Table 2.
Mean penetrations at different time intervals
| Time (months) | Penetration (mm) | Number of radiographs analyzed |
|---|---|---|
| 5.0 ± 0.5 (3.6–7.0) | 0.49 ± 0.22 (0.07–1.56) | 17 |
| 11.0 ± 0.6 (8.3–12.5) | 1.08 ± 0.32 (0.05–2.3) | 22 |
| 14.3 ± 0.6 (12.6–16.9) | 1.67 ± 0.47 (0.239–3.31) | 22 |
| 20 ± 0.3 (18.3–21.6) | 2.25 ± 0.54 (0.131–4.83) | 32 |
Values are expressed as means ± 95% confidence limits with ranges in parentheses.
Table 3.
Mean penetration rates at different time intervals
| Time (months) | Penetration rate (mm/year) | Number of radiographs analyzed |
|---|---|---|
| 5.0 ± 0.5 (3.6–7.0) | 0.10 ± 0.05 (0.014–0.36) | 17 |
| 11.0 ± 0.6 (8.3–12.5) | 0.10 ± 0.03 (0.004–0.23) | 22 |
| 14.3 ± 0.6 (12.6–16.9) | 0.11 ± 0.03 (0.017–0.24) | 22 |
| 20 ± 0.3 (18.3–21.6) | 0.11 ± 0.03 (0.007–0.24) | 32 |
Values are expressed as means ± 95% confidence limits with ranges in parentheses.
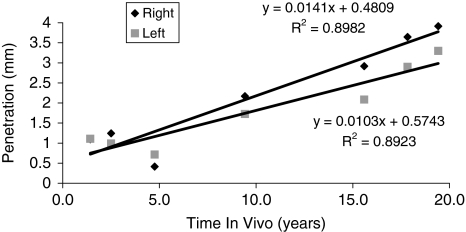
For each patient, penetration against time in vivo as measured from each radiograph was plotted in a scatter chart and a line of best fit plotted (for example, Fig. 2). Such graphs were plotted for all hip arthroplasties analyzed, and the slope of line of best fit and intercept were calculated. The slope provides information about the penetration rate (ie, higher slope is a higher penetration rate) and the intercept point on the y-axis provides an indication of the degree of creep (higher intercept point, more creep of the polyethylene). The relationship between penetration (slope) and creep (intercept) was further analyzed.
Fig. 2.
Penetration against time in vivo as measured from each radiograph (data shown for Patient 1) is plotted in a scatter chart with a line of best fit.
Penetration (P) was used to calculate the volumetric wear (V) using the tunneling equation described by Hall et al. [9, 10] in which R1 is the head diameter and ΔR is the clearance between the head and cup:
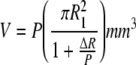 |
Equation 1 |
Radiographic penetration measurements were carried out at the different time intervals using in-house software previously validated by Kennard et al. [13] (Tables 2, 3). Penetration against time plots for each patient was analyzed. The slope (mean ± 95% confidence limits) provided the penetration rate and the intercept the amount of creep. This was completed for the whole series.
All limits, both textural and graphic, are 95% confidence limits unless otherwise stated.
Results were analyzed for statistically significant differences using the Student’s t test (p < 0.05).
Results
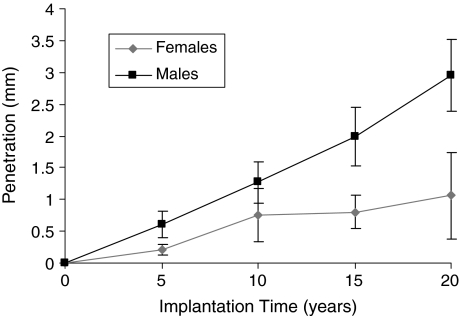
Degradation of the polyethylene and hence acceleration of wear did not occur over time. The overall penetration on the final radiograph was 2.28 ± 0.51 mm (range, 0.13–4.83 mm). The overall mean penetration rate for the same radiographs was 0.11 ± 0.03 mm/year (range, 0.01–0.23 mm/year). Using Equation 1, the overall change in mean volume was 796 ± 132 mm3 and the overall mean volumetric change rate was 38 ± 3.4 mm3/year. The slope (mean ± 95% confidence limits) was 0.076 ± 0.033 mm/year, and the intercept (mean ± 95% confidence limits), which provides information about the level of creep for the whole series, was 0.24 ± 0.09 mm. We observed no correlation between the slope and intercept (R2 = 0.02). The mean overall penetration increased with time. The mean penetration rate remained at (approximately) 0.1 mm/year at each interval (Figs. 3, 4).
Fig. 3.
A graph plots penetration against time for male and female patients at different time intervals (Table 2). Error bars = 95% confidence limits.
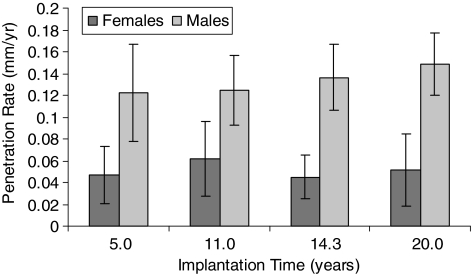
Fig. 4.
A graph plots penetration rate against time for male and female patients at different time intervals (Table 3). Error bars = 95% confidence limits.
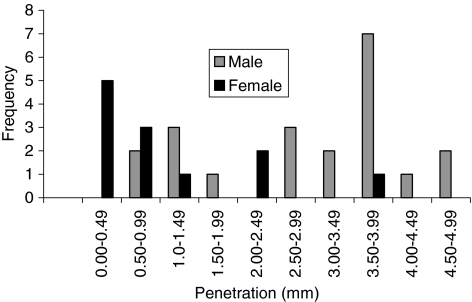
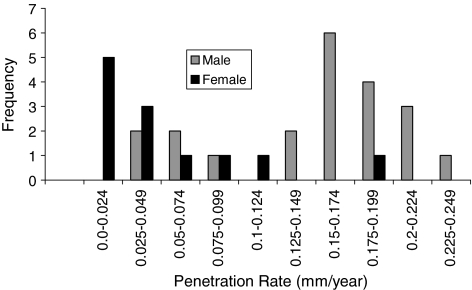
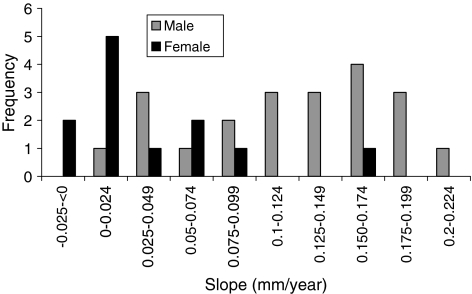
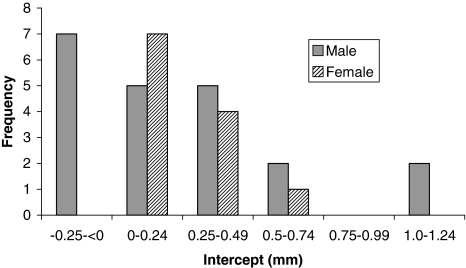
Penetration was influenced by gender; however, creep was not. The overall penetration and penetration rate had a bimodal distribution (Figs. 5, 6). The mean penetrations were 1.06 ± 0.61 mm for female patients and 2.98 ± 0.54 mm for male patients. The mean penetration rate for women was 0.05 ± 0.03 mm/year, which was less (p < 0.05) than that for men (0.15 ± 0.03 mm/year). The slope value was plotted against frequency for men and women (Fig. 7). The, the, mean slope for female patients was 0.04 ± 0.03 mm/year, which was greater (p < 0.05) than that for male patients (0.12 ± 0.03 mm/year). The mean intercepts for women (0.27 ± 0.11 mm) and men (0.27 ± 0.15 mm) did not differ (p = 0.9) (Fig. 8). The penetration slopes from the right and left hips of patients with bilateral arthroplasties were similar.
Fig. 5.
Penetration was influenced by gender. Overall penetration (from longest radiograph) is plotted against frequency for male and female patients.
Fig. 6.
Penetration rate was influenced by gender. Overall penetration rate (from longest radiograph) is plotted against frequency for male and female patients.
Fig. 7.
Penetration rate was influenced by gender. Slope value (penetration rate) is plotted against frequency for male and female patients.
Fig. 8.
Intercept value was not influenced by gender. Intercept value (polyethylene creep) is plotted against frequency for male and female patients.
Discussion
Previous in vitro studies [2] suggest increased wear of polyethylene acetabular cups resulting from oxidative degradation of polyethylene during on-the-shelf storage before implantation. This has raised concerns about the potential of polyethylene cups to undergo oxidative degradation in vivo over prolonged periods. The aim of this study was to assess the in vivo long-term performance of Charnley Ogee® polyethylene cups implanted for more than 20 years by evaluating the penetration of polyethylene cups over time and the time dependency of any variation in penetration rates. Specifically, we asked whether polyethylene degradation continues in vivo over time, thereby accelerating wear. The effect of patient demographics on polyethylene penetration and creep was also assessed.
We measured penetration using two-dimensional anteroposterior radiographs. Our study should be treated with a degree of caution, because we could not detect wear out of the plane of the radiograph, which can be a considerable proportion of the overall wear in a small percentage of cups [8]. Hence, radiographic methods tend to underestimate the true level of wear. Nevertheless, comparison with other radiographic studies is still a valid and useful comparison. Additionally, this study has some bias in that the patients included were randomly selected from a subset of patients who were well enough to travel for radiographic examination. However, all patients in the original series were clinically assessed with good results.
We found a difference in wear rates between male and female patients, which has been observed previously, most recently by Faris et al. [6], who cited gender as the factor explaining the greatest percentage of variance in volumetric. However, other studies have considered overall penetration rate (Table 4). In contrast, we reported the slope and intercept as measurements of wear and creep, respectively. We found the wear for men and women were considerably different, whereas the values for creep were not. The reasons for this are unclear. The overall penetration rate measured (0.11 mm/year) in our study is comparable to those in other studies (Table 4). However, our penetration rate is approximately half of the rate reported by Isaac et al. [12] (0.20 mm/year) in a group of patients in which a Charnley prosthesis had been implanted. We postulate this difference is the result of the increased influence of the initial creep in the shorter study (mean followup, 8.8 years compared with 20 years in our study) and the fact that the patients in that study had all been revised for aseptic loosening. An earlier study [4] reported a penetration rate of 0.15 mm/year in a group of 72 patients with early Charnley implants; it is highly unlikely these cups were gamma-irradiated. It is reassuring our wear rates are not dramatically different from this. Interestingly, a similar study by Griffith et al. [7] reporting on a well-functioning group of patients with gamma-irradiated air cups, calculated a penetration rate of 0.07 mm/year after 8.3 years in vivo.
Table 4.
Comparison of similar studies measuring long-term polyethylene wear from radiographs
We determined penetration rates for each patient using all of the data points to calculate a slope and an intercept (which equate to wear rate and initial creep, respectively). Using this measure, there appears to be a relationship between penetration rate and time. The initial penetration or creep for this group was 0.24 mm and the slope or wear rate was 0.076 mm/year. The wear rate is less than the 0.181 mm/year slope previously reported by Isaac et al. [12]. The same study reported the intercept (creep) to be 0.16 mm. However, that study consisted of 87 patients with a single data point per patient and a weighted regression analysis was used because of the preponderance of points with a short followup. A similar analysis was carried out by Sychterz et al. [17] with a group of 1300 radiographs from 315 hips. They reported on four different designs of cementless acetabular cup all with 32-mm-diameter cobalt-chrome heads and followup of 3.0 to 10.5 years. Three designs were gamma-irradiated in air and the slopes ranged from 0.08 to 0.12 mm/year and the intercepts from 0.09 to 0.33 mm. The ethylene oxide-sterilized group had a slope of 0.13 mm and an intercept of 0.26 mm. These results are similar to ours despite the differences in followup time, head material, head diameter, and fixation method.
Mean volumetric wear was 796 mm3 for 20 years, equating to a mean rate of approximately 40 mm3/year. Hip simulator studies [1] have reported wear rates of approximately 40 mm3/million cycles for 28-mm bearings gamma irradiation sterilized in air, which is comparable to this study if one million cycles/year is assumed and the increased wear associated with increased head size is considered [12].
In our series, the mean penetration rate for male patients was substantially higher than that for female patients. Additionally, the weight, height, and body mass index of the male patients were higher than those of the female patients. Increased load would lead to increased polyethylene penetration. However, when the plotted slopes for wear with time for each patient were analyzed (Fig. 6), there was a difference in the slope values for men and women (Fig. 7) but not in the intercept values (Fig. 8). This indicates there is a difference in the wear penetration between the genders, but not the creep penetration. Intrinsically, load would also be expected to influence creep; however, this was not demonstrated in this study.
Gamma irradiation has been used for a long time (since approximately 1969) and most of the clinical data so far compiled have been with material gamma-irradiated in air. These data indicate, although this sterilization method may be detrimental to the overall wear performance [2, 5], this problem has already manifested itself and is not catastrophic. Furthermore, if the degradation of UHMWPE is continuing in vivo, this has had no measurable effect on the penetration rate over the 20-year implantation period.
Acknowledgments
We thank Laura McCann (iMBE, University of Leeds) and David Sharples (Leeds Teaching Hospitals) for assistance in digitizing the radiographs and Drs Richard Hall and Ruth Wilcox in using the measurement method described.
Footnotes
Four authors (SW, JF, NP, JO) certify they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. One author (GI) is an employee of DePuy International.
Each author certifies that his or her institution did not require approval for this investigation and that all investigations were conducted in conformity with the ethical principles of research.
References
- 1.Barbour PS, Stone MH, Fisher J. A hip joint simulator study using simplified loading and motion cycles generating physiological wear paths and rates. Proc Inst Mech Eng H. 1999;213:455–467. [DOI] [PubMed]
- 2.Besong AA, Hailey JL, Ingham E, Stone M, Wroblewski BM, Fisher J. A study of the combined effects of shelf-ageing following irradiation in air and counterface roughness on the wear of UHMWPE. Biomed Mater Eng. 1997;7:59–65. [PubMed]
- 3.Charnley J. Low Friction Arthroplasty of the Hip. Berlin, Germany: Springer-Verlag; 1979.
- 4.Charnley J, Halley DK. Rate of wear in total replacement. Clin Orthop Relat Res. 1975;112:170–179. [DOI] [PubMed]
- 5.Collier JP, Sutula LC, Currier BH, Currier JH, Wooding RE, Williams IR, Farber KB, Mayor MB. Overview of polyethylene as a bearing material: comparison of sterilization methods. Clin Orthop Relat Res. 1996;333:76–86. [DOI] [PubMed]
- 6.Faris PM, Ritter MA, Pierce AL, Davis KE, Faris GW. Polyethylene sterilization and production affects wear in total hip arthroplasties. Clin Orthop Relat Res. 2006;453:305–308. [DOI] [PubMed]
- 7.Griffith MJ, Seidenstein MK, Williams D, Charnley J. Socket wear in Charnley low friction arthroplasty of the hip. Clin Orthop Relat Res. 1978;137:37–47. [PubMed]
- 8.Hall RM, Siney P, Craig PS, Unsworth A, Wroblewski BM. Discrepancy between penetration depths derived from radiographic and direct measurement of acetabular components. Proc Inst Mech Eng H. 1998;212:57–64. [DOI] [PubMed]
- 9.Hall RM, Siney P, Unsworth A, Wroblewski BM. The association of wear in retrieved acetabular components and the radius of the femoral head. Proc Inst Mech Eng H. 1998;212:321–326. [DOI] [PubMed]
- 10.Hall RM, Unsworth A, Craig PS, Hardaker C, Siney P, Wroblewski BM. Measurement of wear in retrieved acetabular sockets. Proc Inst Mech Eng H. 1995;209:233–242. [DOI] [PubMed]
- 11.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. [DOI] [PubMed]
- 12.Isaac GH, Dowson D, Wroblewski BM. An investigation into the origins of time-dependent variation in penetration rates with Charnley acetabular cups—wear, creep or degradation? Proc Inst Mech Eng H. 1996;210:209–216. [DOI] [PubMed]
- 13.Kennard E, Wilcox RK, Hall RM. Validation of a digital image processing software package for in vivo measurement of wear in cemented Charnley total hip arthroplasties. Med Eng Phys. 2006;28:356–362. [DOI] [PubMed]
- 14.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene: a hip-simulator study. J Bone Joint Surg Am. 2000;82:1708–1725. [DOI] [PubMed]
- 15.Ries MD, Weaver K, Rose RM, Gunther J, Sauer W, Beals N. Fatigue strength of polyethylene after sterilization by gamma irradiation or ethylene oxide. Clin Orthop Relat Res. 1996;333:87–95. [DOI] [PubMed]
- 16.Shaver SM, Brown TD, Hillis SL, Callaghan JJ. Digital edge-detection measurement of polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am. 1997;79:690–700. [DOI] [PubMed]
- 17.Sychterz CJ, Engh CA Jr, Yang A, Engh CA. Analysis of temporal wear patterns of porous-coated acetabular components: distinguishing between true wear and so-called bedding-in. J Bone Joint Surg Am. 1999;81:821–830. [DOI] [PubMed]