Abstract
Femoral stems with reduced stiffness have the potential of decreasing stress shielding and could be an alternative in revision surgery when restoration of bone stock is required. We retrospectively reviewed 38 patients (40 stems) with a central core of cobalt-chromium surrounded by a polymer and an outer titanium mesh layer containing a proximal coating of hydroxyapatite/tricalcium phosphate; 30 of the 38 patients (32 hips) had a minimum 2-year followup. We impacted morselized allograft around the stem in 28 of 32 revisions. Repeated radiostereometric examinations showed medial, distal, and posterior migration (median, 0.21 mm, 0.17 mm, and 0.96 mm, respectively) of the femoral head center for up to 6 months followed by stabilization. Measurements of bone mineral density in the seven Gruen zones at 6 months revealed either a decrease (down to a median of 3%), no change, or a slight increase (up to 5%) followed by a further increase up to 2 years in three of the regions (2, 3, and 5). Conventional radiography at 2 years demonstrated graft remodeling and incomplete radiolucent lines in 19 hips, mainly in Regions 1 and 7. Two hips were reoperated on as a result of dislocation, but none of the stems had been revised.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Revision of a failed femoral stem to eliminate pain and to restore function requires stable fixation of the new stem. Restoration of the bone surrounding the failed component is also important to minimize the risk of recurrent failures and to facilitate any future revision procedure. To maintain bone stock, the proximal part of the femur must carry the load transferred from the stem and proximal muscular attachments. However, in the revision situation, the proximal femur is more or less damaged and the remaining bone tissue compromised. To secure sufficient stem stability, the fixation area often requires enlargement. This may be accomplished with a long stem, which in a reproducible way can increase fixation in uncompromised bone distal to the failing implant. However, this decreases proximal load transfer and may increase the risk of stress shielding and more proximal bone loss. Most revisions of the femoral component will therefore become a compromise between two goals, fixation and proximal load transfer with fixation as the highest priority.
One possible solution to this problem is restoration of proximal bone stock using impaction grafting and a cemented stem. This technique has resulted in restoration of proximal bone mass and 0% to 6% loosening after 2 to 10 years mean followup, whereas the total reoperation rate was higher (up to 13%) mainly because of postoperative femoral fractures occurring distal to the stem [8, 10, 20, 22, 32, 35, 44]. Some studies have raised concern about early subsidence occurring in 10% to 44% of their cases, and intra- and postoperative fractures seem to remain a problem despite generous use of strut graft augmentation [8, 23, 26, 32]. These partly conflicting results probably mirror the complexity of these procedures. The new stem will be anchored partially into a region of the femur with more or less pronounced bone loss and osteopenia, which makes the surgery demanding and sensitive to technique. Impaction grafting has also been used with cementless fixation, but so far to a very limited extent [28, 36].
The tradeoff between distal load transfer and proximal stress shielding has been debated in the primary situation [15, 16, 24, 49]. Numerous composite stems made of polymer materials reinforced by a central metallic core, carbon fiber, or a flexible metallic construct were tried in a few studies [7, 12, 17, 21, 27, 29, 30, 38, 41, 43, 51, 52] and also in revisions [25, 31]. Even if some of these stems reduced postoperative loss of proximal femoral bone mineral density (BMD), most of them failed clinically as a result of poor fixation. In the early 1990s, a new variation of the low stiffness stem concept became available for clinical trials after preclinical testing [11, 50]. The most important change was probably the addition of porous surface mesh made of titanium attached to the polymer (polyaryletherketone). This type of stem achieved excellent fixation in primary THA based on radiostereometric measurements [18]. Compared with a solid stem, it was associated with less periprosthetic bone loss. The clinical results have been favorable, but the longest followup reported in the literature is only 6 years [1]. The documented ability of this stem to address proximal stress shielding in primary THA [18] makes it an attractive alternative in revision. There is, however, a need to document its use in revision. In these cases, there is more or less pronounced loss of bone resulting from distal load transfer induced by the previous stem and, in most instances, by a preceding loosening process or osteolysis. The bone turnover is increased and regardless of use of allograft bone, the metabolic response of the bone tissue to a revision stem can be expected to be different from the one observed after primary surgery.
In revision, the proximal part of the medullary canal is often more or less cavitary. This can be addressed by the use of bone cement, bone graft, or bone graft substitutes before a new stem is inserted. Another alternative is to make the canal bigger by reaming or broaching provided the femur is sufficiently wide and robust for further removal of bone. A third alternative when using a cementless stem is to compromise between the two techniques broaching until some contact is reached between the broach and the endocortex and then adding morselized allograft to improve contact and stability.
We therefore asked four questions: (1) Can this low stiffness stem with titanium mesh achieve stable fixation in revision when measured with radiostereometry? (2) If so, will it promote restoration of proximal bone density? (3) Will impacted allograft bone around this stem remodel on conventional radiographs as previously observed around cemented stems? (4) Could the amount of subsidence at 2 years to any extent be predicted by demographic factors, the magnitude of the bone defect, allograft volume used, stem size, and/or postoperative bone mineral density?
Materials and Methods
We prospectively followed 41 patients (43 hips) revised with the Epoch stem (Zimmer Inc, Warsaw, IN) by three surgeons (JK, JTH, HM) between January 1999 and October 2004. Because two of the surgeons only operated on two and one hip each, these cases were excluded from the evaluation, which left 38 patients (40 hips) operated on by one surgeon (JK) for our prospective review (Table 1). (The surgeon [JK] selected 41 hips for this procedure, but changed to a longer cemented stem during the operation in one of them as a result of a preoperative fracture, leaving 40 hips.) Before the minimum 2-year followup, five patients (five hips) died of causes unrelated to the index operation. Another three patients (three hips) did not want to report for followup at 2 years; two did not attend because of poor general health and one because of social problems. None of these stems had been exchanged. This left 30 patients (32 hips) for final radiographic review. One patient developed a trochanteric pseudarthrosis resulting in an insufficient number of stable tantalum markers in the distal femur. Another patient underwent surgery for reattachment of the trochanter and change of neck length that made further radiostereometric analysis studies uncertain, leaving 30 hips with complete radiostereometric followup at 2 years. One patient did not have a postoperative dual-energy xray absorptiometry examination and one patient’s 6-month dual-energy xray absorptiometry examination could not be evaluated, resulting in 28 patients (30 hips) with successful examinations postoperatively at 6 months and 2 years. This study was approved by the local ethical committee (S664-02).
Table 1.
Patient Data
| Variable | Numbers or Median (range) |
|---|---|
| Male/female | 25/15 |
| Age (years) | Mean, 70 (range, 36–87) |
| Body mass index (kg/m2) | Mean, 27 (range, 20–33) |
| Number of previous revisions (0/1/2/3) | 31/7/0/1 |
| Previously infected THA (no/yes) | 36/4 |
| Bone defect [14] (Grade I/II/III/IV) | |
| According to radiographs | 1/27/11/1 |
| According to surgeon after stem removal | 1/22/16/1 |
| Allograft volume* (mL) | 53 (range, 5–140) |
| Stem size (14/15/16/17/18) | 14/8/7/6/5 |
| Heterotopic bone formation (I/II/III/IV) [6] | 23/3/6/0 |
| Harris hip score | |
| Total scores | |
| Preoperatively | 57 (range, 17–100) |
| 2 years | 89 ( range, 47–100) |
| Difference 0–2 years | 30 (range, −5–73) |
| Pain | |
| Preoperatively | 20 (range, 0–44) |
| 2 years | 44 (range, 20–44) |
| Difference 0–2 years | 20 (range, −14–44) |
| Visual analog scale | |
| Pain at rest | |
| Preoperatively | 20 (range,0–80) |
| 2 years | 0 (range,0–70) |
| Difference 0–2 years | −20 (range,−80–40) |
| Pain during activity | |
| Preoperatively | 50 (range, 0–100) |
| 2 years | 3 (range, 0–80) |
| Difference 0–2 years | −50 (range, −90–60) |
*Measured intraoperatively.
The Epoch stem has a central metallic core made of cobalt-chromium alloy. The core is surrounded by a polymer (polyaeryletherketone). The polymer is covered by a porous layer made of titanium. In all of our cases, the proximal two-thirds of the mesh had a ceramic coating made of a mixture of hydroxyapatite and tricalcium phosphate [11].
The surgical indication to choose a low stiffness stem included amount of bone loss (mainly Grade II-III according to Gustilo and Pasternak [14]) and a femoral canal that, according to preoperative planning, could receive stem sizes 14 to 18 (the only sizes available). We also required a proximal femur with a contained defect or a defect that could be converted to a contained defect using mesh to surround the defect so a standard stem length could be used. The intention was to remove as little endosteal bone as possible. This implied the stem in almost all cases achieved some endosteal contact, usually distally. Bone graft was intended to fill out defects, which were most common in the proximal region where they jeopardized rotational stability.
An anterolateral or posterior approach was used with the patient on their side. One to three prophylactic cerclage wires were attached distal to the greater trochanter in all but two of the hips. In 36 hips, morselized allografts were impacted either before or after insertion of the stem. There were no impaction instruments available for the Epoch stem. If impaction was completed before stem insertion, a tibial Küntscher nail and polished phantoms intended for other types of stems were used. Otherwise, the graft was impacted around the stem after insertion using various types of tamps. This technique was used when only a small amount of graft was necessary and only in the proximal part of the femur. We used mesh to contain the defects in three hips. Intraoperatively, the surgeon recorded the amount of grafted bone in milliliters and reclassified the femoral defect [14] after the stem and any cement had been removed. These data were recorded in a standardized protocol immediately after the operation. There were six intraoperative complications: three trochanteric fractures (two greater trochanter, one lesser trochanter) and three calcar cracks detected after insertion of the stem. These complications were treated with more wires during the operation.
During the operation, six to nine spherical tantalum markers (ø = 0.8 mm or 1 mm) were inserted into the proximal femur. In this study, only a subset of stems was supplied with tantalum markers by the manufacturer. To standardize the radiostereometric measurements, we decided only to account for translation of the femoral head center (medial [+]/lateral [−], proximal [+]/distal [−], anterior [+]/posterior [−]). Stem motions in the postoperative period are usually a combination of translations and rotations. When several markers are inserted (eg, at the tip, shoulder, and collar) and the femoral head center is used as another marker, translations are usually reported as motions of a point situated at the gravitational center of these markers. This point of measurement is determined by the position of the markers in the stem and the head center. In practice, it is commonly located at a level one or a few centimeters below the lesser trochanter and close to the medial part of the femur. In this study, we measured translation at the head center.
Rotation could not be measured. However, anterior or posterior migration of the head center could be interpreted with high probability as an effect of anteversion or retroversion of the stem. Medial and lateral displacement of the head center could be an effect of varus or valgus tilt even if other motion (eg, rotation of the stem along its central core) could result in the same motions despite no varus or valgus tilt. Thus, the recorded medial/lateral and anteroposterior translations of the femoral head center reflect rotations, but the rotations should be interpreted cautiously when the translations are small.
Postoperatively, the patients were instructed to bear weight partially (up to 20 kg) using two crutches for 6 weeks. Thereafter, the patient was encouraged to bear as much weight as tolerated.
Radiostereometric examinations using a uniplanar technique were performed supine 3 to 6 days postoperatively, after 6 months, and after 1 and 2 years [19]. During the time period of this study, the software was updated to improve the accuracy of calculations [4]. However, the determination of the precision of the measurements was based on the older technique to reflect a worst case scenario. These calculations were based on double exposures [53] of the 31 different hips in this study. The 99% confidence intervals of the error (2.7 standard deviations) were 0.28 mm, 0.29 mm, and 1.16 mm (medial [+]/lateral [−], proximal [+]/distal [−], anterior [+]/posterior [−] head translation, respectively). The marker stability and scatter between the postoperative and the 2-year followup corresponded to a median value of the mean error of rigid body fitting of 0.19 mm (range, 0.06–0.32 mm) and 27 (range, 16–112) based on seven (range, 4–9) tantalum markers in the femoral segment [53].
Measurements of BMD were performed postoperatively and after 6 months, 1 year, and 2 years using a Lunar DPX IQ (GE Healthcare, Buckinghamshire, UK). The precision of these measurements (coefficient of variation %) has previously been calculated to vary up to 6.2% depending on the Gruen region analyzed [9]. Conventional radiographs (pelvic, anteroposterior, and lateral views) were taken postoperatively and after 2 years. The radiographic evaluation included heterotopic bone formation [6], presence of newly developed radiolucent lines, formation of rounded radiolucencies (osteolysis), and morphologic changes of the periprosthetic bone. Radiolucencies were graded within each Gruen region in four classes (no lucency, less than 50%, 50% to 99%, or 100% of the interface). Periprosthetic bone remodeling was also graded in four classes (no change, bone resorption, formation of new bone trabeculae or trabecular remodeling, and cortical repair or thickening). We accounted only for radiographic changes on the anteroposterior view because the variable quality of the lateral view, especially on the postoperative examination, made evaluation of this view uncertain. Harris hip score and two visual analog scales, one for pain at rest and one during weightbearing, were used. The patient was provided with a plastic stick in a transparent envelope supplied with a vertical red line. The patient placed the line anywhere along a black line with two vertical lines, one at each end representing no and maximum pain. On the back of the stick and not visible to the patient, there was a scale graded from 0 to 100. All examinations and instructions to the patient were performed by an orthopaedic surgeon.
Femoral head migration (translation from 3 to 4 days postoperative to final followup) and changes in BMD were evaluated using the Wilcoxon signed rank test. We used linear regression analysis to ascertain any associations among demographic factors, bone defect, allograft volume, stem size, postoperative BMD, and femoral head migration at 2 years.
Results
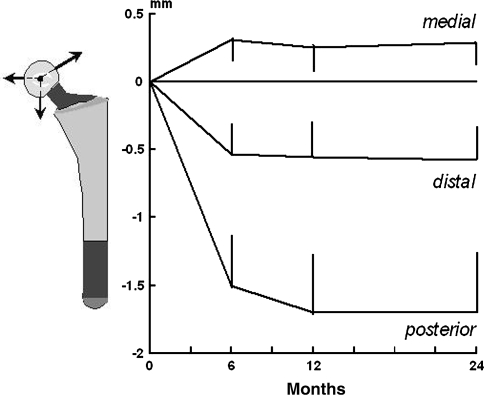
Overall, the stems migrated for up to 6 months after insertion and thereafter stabilized. Most of this migration occurred before 3 months. At this time, the femoral head center displaced a mean of 0.27 mm medially (range, −1.11–3.22 mm), −0.20 mm distally (range, −4.21–0.22 mm), and −0.73 mm posteriorly (range, −7.30–0.78 mm). Between 3 and 6 months postoperatively, there was a change (p = 0.003) in the femoral head position in the posterior direction (median, −0.86 mm; range, −8.75–0.81 mm), but no change in the medial/lateral or proximal/distal direction. After the 6-month followup, the stems stabilized (Fig. 1).
Fig. 1.
The graph shows the medial (+)/lateral (−), proximal (+)/distal (−), and anterior (+)/posterior (−) head translations up to 2 years (mean ± standard error).
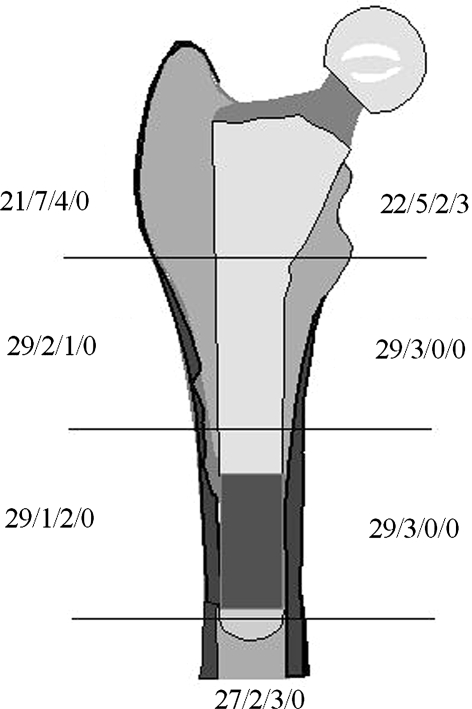
Radiographic evaluation confirmed stable fixation at 2 years. Radiolucent lines were limited and most commonly located in Regions 1 (11 cases) and 7 (10 cases) (Fig. 2). Complete lines were only found in Region 7 (three cases). In the other regions, incomplete lines were found with a lower frequency. Thirteen of the 32 hips available for examination at 2 years had no radiolucencies. Regional osteolysis or calcar granulomas were not observed.
Fig. 2.
The distribution of radiolucent lines in the seven Gruen regions for the 32 hips with radiographic followup is shown. The length of the radiolucent line is given in one of four grades: no radiolucency; 1% to 50%; 51% to 99%; or 100% of the interface.
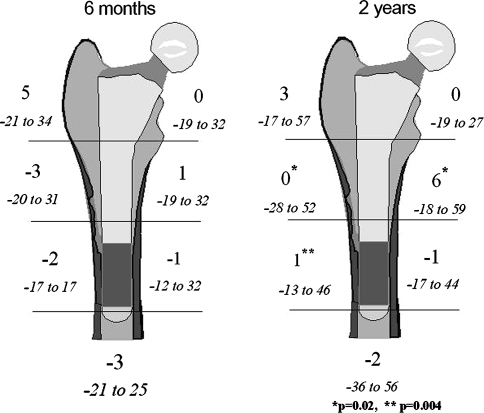
Six months postoperatively, the BMD showed a slight median decrease (1%–3%) in Regions 2 to 5, no change in Region 7, and a minimum increase of 1% and 5% in Regions 6 and 1, respectively (Fig. 3). Between the 6-month and 2-year followup, the BMD increased 3% in both Region 2 (p = 0.02) and Region 3 (p = 0.004) and 5% in Region 6 (p = 0.02).
Fig. 3.
Bone mineral density in the seven Gruen regions after 6 months and 2 years (right) for the 30 hips with full dual-energy xray absorptiometry data is shown. Median change and range related to the postoperative examination is shown in percent.
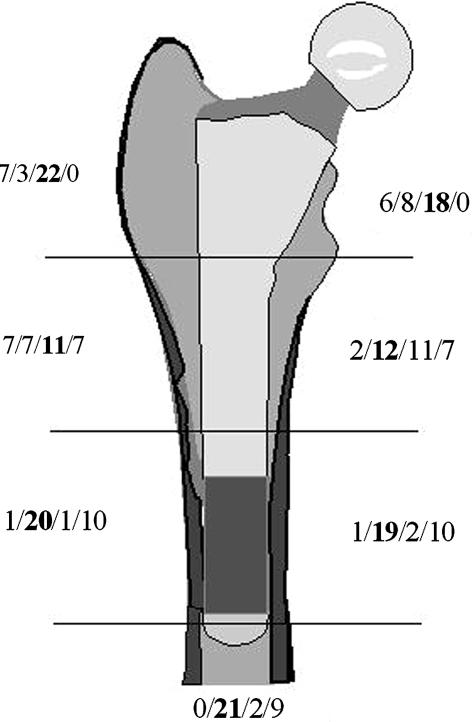
In the radiographic evaluation, we observed trabecular bone remodeling, particularly in Regions 1 and 7 (Fig. 4). It was also the most commonly observed change in Region 2. Cortical repair was mainly found in the distal regions (3, 4, and 5), but most frequently these regions did not change between the postoperative and 2-year examinations. When present, bone resorption was almost exclusively localized to the proximal regions (1, 2, and 7).
Fig. 4.
Distribution host bone and/or graft remodeling in the seven Gruen regions for the 32 hips with radiographic followup is shown. Bone remodeling was classified in one of four classes: resorption, no change, trabecular remodeling, or cortical restitution. We used the type of remodeling judged most predominant in each region.
The Harris hip score improved from a median of 57 points (range, 17–100 points) preoperatively to 89 points (range, 47–100 points) at 2 years (Table 1). There was also an overall improvement of the pain scores. Two patients reported their pain had increased.
We observed a weak association (r2 = 0.15; p = 0.04) between medial/lateral shift of the femoral head center at 2 years and the postoperative BMD in Region 7. The lower the density, the more the medial head had migrated at 2 years (r2 = 0.15; p = 0.04). None of the other factors included in the regression analysis had any influence on the proximal/distal or anteroposterior femoral head migration.
There were three fractures distal to the stem in three patients. All occurred 4 to 6 months postoperatively. Two of these patients died within 2 years of the index operation. Another patient had a supracondylar fracture 4 months before the 2-year followup but did not attend as a result of poor health. Three hips dislocated. Two of these underwent reoperation with a change to a bigger head; one of them underwent reattachment of the greater trochanter. This patient developed a deep infection after the second procedure that healed after open débridement and retention of the uncemented components. Six months after cessation of antibiotics, he had no symptoms, no signs of loosening, and a normal C-reactive protein level (< 5 mg/L).
Discussion
The rationale for our study was to evaluate if the Epoch stem could be used in the revision situation. To qualify, it should achieve sufficient stability and promote bone remodeling and restitution. Furthermore, we wanted to know if factors related to the surgical procedure or patient could be used to predict fixation and thereby facilitate patient selection.
The results of this study are encouraging but should be viewed against the background of our study design. Only one surgeon was involved, which is an advantage concerning influence of confounders such as patient selection and surgical technique. However, further studies are necessary to evaluate the reproducibility of the procedure with other surgeons and in other centers. Like in some previous series of impaction grafting with use of cement, the incidence of postoperative fractures distal to the tip of the stem was high [8, 34]. This might indicate there could be a place for a longer Epoch stem even if other measures such as improved patient selection, cortical struts, or other stem designs can address this problem. The followup is also comparatively short, and late-occurring problems such as regional osteolysis cannot be accounted for. We have not observed this complication in our primary Epoch stems with followup of 7 to 10 years. This observation is not quite transferable to revisions but suggests it will not become a major problem in the future. All patients had lateral radiographs, but their quality was not good enough for a consistent evaluation. We could not detect any certain ominous signs on any of the lateral radiographs, but their lack of quality leaves some uncertainty concerning the true outcome with regard to any problems that might occur with longer followup. The clinical followup could also be improved. Today, we prefer self-administered questionnaires to avoid any interference with the hospital personnel, who might have been present during the operation or at the repeated followup occasions. Finally, there are no dedicated impaction instruments to be used with the Epoch stem, which is a prerequisite for widespread use.
According to the radiostereometric measurements, the Epoch stem achieved stable fixation within 3 to 6 months. This observation was supported by the conventional radiographic followup. When present, the early loss of BMD was small. In three of the Gruen regions, the BMD increased between 6 months and 2 years. Use of impacted bone graft did not seem to jeopardize the stability and was associated with graft remodeling with limited or no development of radiolucent lines.
Uncemented stems rely on early stabilization to achieve ingrowth and fixation in the long term. In a clinical situation, it is unknown how long a stem can continue to subside and still achieve secondary stabilization and bony fixation. A substantial amount of migration was observed in many of our stems up to 3 months postoperatively followed by more limited posterior displacement of the femoral head (probably an effect of retroversion) up to 6 months postoperatively. This is an interesting observation that needs further studies to establish the upper time limit for migration as measured with radiostereometric analysis that is compatible with long-term stability of uncemented implants. Based on previous laboratory studies, use of hydroxyapatite seems advantageous because studies in the canine have shown such implants can stabilize despite an early period of micromotion [47].
The original Epoch stem is anteverted, which could be an advantage as long as most of the proximal anatomy of the femur is intact. If there is a Type 2 or 3 defect and loss of the entire neck region, it is more difficult to achieve rotational stability with and especially if the distal part is round like in the initial Epoch design. Recently, a straight stem design has been introduced. This stem could theoretically be advantageous but has at present only limited documentation. Another problem with the impaction grafting procedure we used was lack of dedicated instruments and the ability to test rotational stability with a torque wrench after impaction. To become a reasonable revision option, we believe it is desirable to make more sizes available and also to introduce and evaluate longer stems.
The stiffness of the proximal femur supplied with a femoral stem prosthesis increases with the stiffness of the cortical bone area, the size of the medullary canal and the stem, the rigidity and the shape of the implant used, and the quality of the interface [16, 45]. Use of solid metallic stems fixed without cement has consistently caused proximal bone loss [34, 39, 40, 46]. Increase of the stem diameter often necessitated by aging and a thin femoral cortex or reaming of the canal will further promote proximal stress shielding and distal load transfer [42, 45]. With smaller stem sizes, the relative stiffness of the stem will become less important, but some studies failed to find any correlation between BMD loss and stem diameter [2, 37] or that substantial loss of BMD also occurs with smaller stem sizes [46]. Thus, there might be indications for the use of flexible stems down to size 13, perhaps even smaller, if they can be manufactured without any increased risk of stem breakage.
We observed graft remodeling and some radiolucencies in the proximal zones between the remodeled graft and the stem after 2 years. It is possible the graft closest to the stem had not had yet been remodeled by 2 years. Previous studies by Sörensen et al. [48] suggest, however, this process is complete after 1 to 2 years. Eleven of the hips in our study have been followed for 5 years (Fig. 5). According to radiostereometric analysis, they all remain stable and the bone remodeling seems to continue without any progression of radiolucent lines. Thus, it seems our observations at 2 years are representative at least for the midterm outcome.
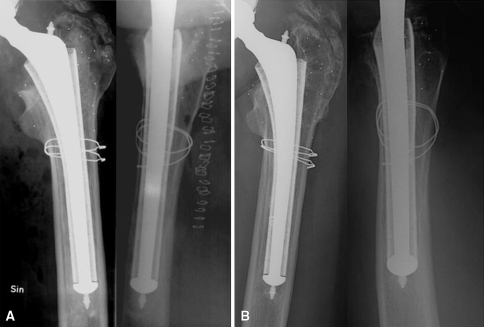
Fig. 5A–B.
A 39-year-old man received an uncemented Harris-Galante Type I stem, which was revised for loosening after 16 years. (A) Postoperative anteroposterior and lateral views are shown. At revision, morselized bone graft (85 mL) was impacted before and after insertion of the stem. The patient had renal transplantation 17 years earlier. At the 3-month followup, the femoral head center had migrated 0.8 mm medially, 0.5 mm distally, and 4.2 mm posteriorly. At subsequent examinations up to 5 years after the revision, there was no further migration. (B) Five years later, there were signs of graft remodeling (mainly visible on anteroposterior view) and some restitution of the anterior cortex especially (lateral view).
Previous studies of primary uncemented stems demonstrate proximal bone loss at 6 months [3, 5, 33], sometimes followed by further loss of BMD [13, 40] or a tendency for improvement in some regions [3, 40]. This tendency to early loss sometimes followed by various amount of increase might be one of several factors [40] that can explain the variable results previously reported [39]. Because the pattern of bone remodeling after insertion or change of a THA varies with the time to followup, comparison between different studies can only be performed if the same followup routines have been used. In two previous reports of primary Epoch stems with followup similar to our study [1, 18], proximal bone loss was observed after 2 years (Regions 1 and 7) in 6% and 27.5%, respectively. In our study, there was no change of the median value in Region 7 and a slight increase in Region 1 at both followups. Thus, the bone tissue seemed to react differently in the revision compared with the primary situation. If these data really represent a true difference, provided possible confounders are considered, they could indicate that the new revision stem restores load to the proximal femur. Together with findings of stem stability after 3 to 6 months and bone remodeling on conventional radiographs, they also suggest the graft tissue is partly or completely substituted with new bone.
The Epoch stem could become a reasonable option to treat small to moderate bone defects in revision surgery. In our series, this stem was associated with good fixation, favorable bone remodeling, and acceptable clinical results. It did not prevent some of the recognized complications to revision surgery such as intra- and postoperative fractures. At present, instruments and selection of stem sizes and lengths have limitations when used in the revision setting. Our findings suggest further clinical evaluation of this stem in revision surgery could be of interest, preferably in association with updated instruments for revision purposes and perhaps further development of the stem itself.
Acknowledgments
We thank Karin Danielsson for help with preparation of the manuscript.
Footnotes
One of more of the authors received funding from IngaBrit and the Arne Lundbergs Research Fund (JK), the Swedish Research Council (JK), and Zimmer, Inc (Warsaw, IN) (JK).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Akhavan S, Matthiesen MM, Schulte L, Penoyar T, Kraay MJ, Rimnac CM, Goldberg VM. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88:1308–1314. [DOI] [PubMed]
- 2.Aldinger PR, Sabo D, Pritsch M, Thomsen M, Mau H, Ewerbeck V, Breusch SJ. Pattern of periprosthetic bone remodeling around stable uncemented tapered hip stems: a prospective 84-month follow-up study and a median 156-month cross-sectional study with DXA. Calcif Tissue Int. 2003;73:115–121. [DOI] [PubMed]
- 3.Ang KC, Das De S, Goh JC, Low SL, Bose K. Periprosthetic bone remodeling after cementless total hip replacement. A prospective comparison of two different implant designs. J Bone Joint Surg Br. 1997;79:675–679. [DOI] [PubMed]
- 4.Börlin N, Röhrl SM, Bragdon CR. RSA wear measurements with or without markers in total hip arthroplasty. J Biomech. 2006;39:1641–1650. [DOI] [PubMed]
- 5.Brodner W, Bitzan P, Lomoschitz F, Krepler P, Jankovsky R, Lehr S, Kainberger F, Gottsauner-Wolf F. Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty. A five-year longitudinal study. J Bone Joint Surg Br. 2004;86:20–26. [PubMed]
- 6.Brooker AF, Bowerman JW, Robinson RA, Riler LH. Ectopic ossification following total hip replacement: incidence and method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed]
- 7.Bryan WJ, McCaskill BL, Tullos HS. Hip endoprosthesis stabilization with a porous low modulus stem coating: factors influencing stabilization. Clin Orthop Relat Res. 1981;157:125–132. [PubMed]
- 8.Cabanela ME, Trousdale RT, Berry DJ. Impacted cancellous graft plus cement in hip revision. Clin Orthop Relat Res. 2003;417:175–182. [DOI] [PubMed]
- 9.Digas G. New polymer materials in total hip arthroplasty. Evaluation with radiostereometry, bone densitometry, radiography and clinical parameters. Acta Orthop Suppl. 2005;76:3–82. [PubMed]
- 10.Gie GA, Linder L, Ling RS, Simon JP, Slooff TJ, Timperley AJ. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg Br. 1993;75:14–21. [DOI] [PubMed]
- 11.Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;393:128–136. [DOI] [PubMed]
- 12.Goosen JH, Castelein RM, Runne WC, Dartee DA, Verheyen CC. Long-term results of a soft interface- (Proplast-) coated femoral stem. Acta Orthop. 2006;77:585–590. [DOI] [PubMed]
- 13.Grant P, Aamodt A, Falch JA, Nordsletten L. Differences in stability and bone remodeling between a customized uncemented hydroxyapatite coated and a standard cemented femoral stem A randomized study with use of radiostereometry and bone densitometry. J Orthop Res. 2005;23:1280–1285. [DOI] [PubMed]
- 14.Gustilo RB, Pasternak HS. Revision total hip arthroplasty with titanium ingrowth prosthesis and bone grafting for failed cemented femoral component loosening. Clin Orthop Relat Res. 1988;235:111–119. [PubMed]
- 15.Hedia HS, Shabara MA, El-Midany TT, Fouda N. Improved design of cementless hip stems using two-dimensional functionally graded materials. J Biomed Mater Res B Appl Biomater. 2006;79:42–49. [DOI] [PubMed]
- 16.Huiskes R, Weinans H, Dalstra M. Adaptive bone remodeling and biomechanical design considerations for uncemented total hip arthroplasty. Orthopedics. 1989;12:1255–1267. [DOI] [PubMed]
- 17.Jacobsson SA, Djerf K, Gillquist J, Hammerby S, Ivarsson I. A prospective comparison of Butel and PCA hip arthroplasty. J Bone Joint Surg Br. 1993;75:624–629. [DOI] [PubMed]
- 18.Kärrholm J, Anderberg C, Snorrason F, Thanner J, Langeland N, Malchau H, Herberts P. Evaluation of a femoral stem with reduced stiffness. A randomized study with use of radiostereometry and bone densitometry. J Bone Joint Surg Am. 2002;84:1651–1658. [PubMed]
- 19.Kärrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses. Review of methodology and clinical results. Clin Orthop Relat Res. 1997;344:94–110. [PubMed]
- 20.Kärrholm J, Hultmark P, Carlsson L, Malchau H. Subsidence of a non-polished stem in revisions of the hip using impaction allograft. Evaluation with radiostereometry and dual-energy x-ray absorptiometry. J Bone Joint Surg Br. 1999;81:135–142. [DOI] [PubMed]
- 21.Keet GG, Runne WC. The Anaform endoprosthesis: a Proplast-coated femoral endoprosthesis. Orthopedics. 1989;12:1185–1190. [DOI] [PubMed]
- 22.Kim YH. Cemented revision hip arthroplasty using strut and impacted cancellous allografts. J Arthroplasty. 2004;19:726–732. [DOI] [PubMed]
- 23.Leopold SS, Berger RA, Rosenberg AG, Jacobs JJ, Quigley LR, Galante JO. Impaction allografting with cement for revision of the femoral component. A minimum four-year follow-up study with use of a precoated femoral stem. J Bone Joint Surg Am. 1999;81:1080–1092. [DOI] [PubMed]
- 24.Maistrelli GL, Fornasier V, Binnington A, McKenzie K, Sessa V, Harrington I. Effect of stem modulus in a total hip arthroplasty model. J Bone Joint Surg Br. 1991;73:43–46. [DOI] [PubMed]
- 25.Matricali GA, Thibaut H, Hendrickx M, Thibaut R. Revision of total hip arthroplasty using the RM isoelastic prosthesis. Acta Orthop Belg. 1993;59(Suppl 1):374–376. [PubMed]
- 26.Meding JB, Ritter MA, Keating EM, Faris PM. Impaction bone-grafting before insertion of a femoral stem with cement in revision total hip arthroplasty. A minimum two-year follow-up study. J Bone Joint Surg Am. 1997;79:1834–1841. [DOI] [PubMed]
- 27.Nagi ON, Kumar S, Aggarwal S. The uncemented isoelastic/isotitan total hip arthroplasty. A 10–15 year follow-up with bone mineral density evaluation. Acta Orthop Belg. 2006;72:55–64. [PubMed]
- 28.Nesse E, Nielsen EW, Bastian D. Cemented versus cementless revision femoral stems using morsellized allograft—a prospective randomized study with 5 years follow up. Z Orthop Ihre Grenzgeb. 2003;141:678–683. [DOI] [PubMed]
- 29.Niinimaki T, Jalovaara P. Bone loss from the proximal femur after arthroplasty with an isoelastic femoral stem. BMD measurements in 25 patients after 9 years. Acta Orthop Scand. 1995;66:347–351. [DOI] [PubMed]
- 30.Niinimaki T, Puranen J, Jalovaara P. Total hip arthroplasty using isoelastic femoral stems. A seven- to nine-year follow-up in 108 patients. J Bone Joint Surg Br. 1994;76:413–418. [PubMed]
- 31.Niinimaki TJ, Puranen JP, Jalovaara PK. Revision arthroplasty with an isoelastic uncemented femoral stem. Int Orthop. 1995;19:298–303. [DOI] [PubMed]
- 32.Oakes DA, Cabanela ME. Impaction bone grafting for revision hip arthroplasty: biology and clinical applications. J Am Acad Orthop Surg. 2006;14:620–628. [DOI] [PubMed]
- 33.Ohta H, Kobayashi S, Saito N, Nawata M, Horiuchi H, Takaoka K. Sequential changes in periprosthetic bone mineral density following total hip arthroplasty: a 3-year follow-up. J Bone Miner Metab. 2003;21:229–233. [DOI] [PubMed]
- 34.Ornstein E, Atroshi I, Franzen H, Johnsson R, Sandquist P, Sundberg M. Early complications after one hundred and forty-four consecutive hip revisions with impacted morsellized allograft bone and cement. J Bone Joint Surg Am. 2002;84:1323–1328. [DOI] [PubMed]
- 35.Ornstein E, Franzen H, Johnsson R, Karlsson MK, Linder L, Sundberg M. Hip revision using the Exeter stem, impacted morsellized allograft bone and cement: a consecutive 5-year radiostereometric and radiographic study in 15 hips. Acta Orthop Scand. 2004;75:533–543. [DOI] [PubMed]
- 36.Pekkarinen J, Alho A, Lepisto J, Ylikoski M, Ylinen P, Paavilainen T. Impaction bone grafting in revision hip surgery. A high incidence of complications. Bone Joint Surg Br. 2000;82:103–107. [DOI] [PubMed]
- 37.Petersen MB, Kolthoff N, Eiken P. Bone mineral density around femoral stems. DXA measurements in 22 porous-coated implants after 5 years. Acta Orthop Scand. 1995;66:432–434. [DOI] [PubMed]
- 38.Petrou G, Gavras M, Diamantopoulos A, Kapetsis T, Kremmydas N, Kouzoupis A. Uncemented total hip replacements and thigh pain. Arch Orthop Trauma Surg. 1994;113:322–326. [DOI] [PubMed]
- 39.Pitto RP, Schramm M, Hohmann D, Schmidt R. Clinical outcome and quantitative evaluation of periprosthetic bone-remodeling of an uncemented femoral component with taper design. A prospective study. Chir Organi Mov. 2001;86:87–97. [PubMed]
- 40.Rahmy AI, Gosens T, Blake GM, Tonino A, Fogelman I. Periprosthetic bone remodeling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporos Int. 2004;15:281–289. [DOI] [PubMed]
- 41.Ritter MA, Keating EM, Faris PM. A porous polyethylene-coated femoral component of a total hip arthroplasty. J Arthroplasty. 1990;5:83–88. [DOI] [PubMed]
- 42.Robinson D, Hendel D, Halperin N. Changes in femur dimensions in asymptomatic non-cemented hip arthroplasties. 20 cases followed for 5–8 years. Acta Orthop Scand. 1994;65:415–417. [DOI] [PubMed]
- 43.Runne WC, van Sambeek KJ, Stierum JL, van Tongerloo RB. Femoral endoprosthesis fixation with a soft, flexible low modulus stem coating. Four to six year clinical results. Orthopedics. 1989;12:529–535. [DOI] [PubMed]
- 44.Schreurs BW, Arts JJ, Verdonschot N, Buma P, Slooff TJ, Gardeniers JW. Femoral component revision with use of impaction bone-grafting and a cemented polished stem. J Bone Joint Surg Am. 2005;87:2499–2507. [DOI] [PubMed]
- 45.Skinner HB. Isoelasticity and total hip arthroplasty. Orthopedics. 1991;14:323–328. [PubMed]
- 46.Sköldenberg OG, Boden HS, Salemyr MO, Ahl TE, Adolphson PY. Periprosthetic proximal bone loss after uncemented hip arthroplasty is related to stem size: DXA measurements in 138 patients followed for 2–7 years. Acta Orthop. 2006;77:386–392. [DOI] [PubMed]
- 47.Søballe K, Mouzin OR, Kidder LA, Overgaard S, Bechtold JE. The effects of hydroxyapatite coating and bone allograft on fixation of loaded experimental primary and revision implants. Acta Orthop Scand. 2003;74:239–247. [DOI] [PMC free article] [PubMed]
- 48.Sörensen J, Ullmark G, Långström B, Nilsson O. Rapid bone and blood flow formation in impacted morsellized allografts: positron emission tomography (PET) studies on allografts in 5 femoral component revisions of total hip arthroplasty. Acta Orthop Scand. 2003;74:633–643. [DOI] [PubMed]
- 49.Sumner DR, Galante JO. Determinants of stress shielding: design versus materials versus interface. Clin Orthop Relat Res. 1992;274:202–212. [PubMed]
- 50.Sumner DR, Turner TM, Igloria R, Urban RM, Galante JO. Functional adaptation and ingrowth of bone vary as a function of hip implant stiffness. J Biomech. 1998;31:909–917. [DOI] [PubMed]
- 51.Trebse R, Milosev I, Kovac S, Mikek M, Pisot V. Poor results from the isoelastic total hip replacement: 14–17-year follow-up of 49 cementless prostheses. Acta Orthop. 2005;76:169–176. [DOI] [PubMed]
- 52.Tullos HS, McCaskill BL, Dickey R, Davidson J. Total hip arthroplasty with a low-modulus porous-coated femoral component. J Bone Joint Surg Am. 1984;66:888–898. [DOI] [PubMed]
- 53.Valstar ER, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop. 2005;76:563–572. [DOI] [PubMed]