Abstract
THA revisions using standard cups are at risk of dislocation (5.1% to 14.4% incidence), especially in patients over 70 years of age. Constrained tripolar cups have reduced this risk (6% incidence) but are associated with substantial loosening rates (9%). The nonconstrained dual mobility cup was designed to improve prosthetic stability (polyethylene head ≥ 40 mm diameter) without increasing loosening rates by reducing wear and limiting impingement (rotation range of 108°). We implanted 88 cemented dual mobility cups for THA revisions in 82 patients at high risk of dislocation. Average patient age was 72 years (range, 65–86 years). Eighty-five of the 88 hips were reviewed at 2 to 5 years followup. One patient (1.1%) had a traumatic dislocation at 2 years postoperatively. Two patients (2.3%) had asymptomatic early loosening and three patients (3.5%) had localized radiographic lucencies. These results confirm those with press-fit dual mobility cups suggesting a low dislocation rate at 5 years and a cup survival of 94.6%. At middle term followup, cemented dual mobility cup achieved better results than constrained cups in cases at risk of dislocation and recurrent loosening.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Dislocation is the second most common cause for surgical THA revision [7]. After primary THA, the incidence of dislocation is approximately 2%, ranging from 1.7% in community hospitals [19] to 3.2% in specialist centers [35], which deal with more difficult cases, and up to 3.9% in Medicare centers [31], which treat older patients with poor medical status. However, the incidence of dislocation after revision surgery is two to three times higher than that following primary surgery (5.1% [19], 7.4% [2], 14.4% [31]). Furthermore, for patients older than 70 years of age, the risk of dislocation increases twofold [7] and an American Association of Anesthesiologists (ASA) grade of 3 or more can increase the risk by 10 times [17].
Revision surgery in elderly patients should aim to reduce the risk of dislocation. In these cases, a dislocation rate of about 7% has been achieved with standard prostheses and optimized approach [2]. Specifically designed constrained prostheses better prevent dislocations, but the increased strains on the prostheses have been responsible for a high rate of loosening. Tripolar cups reduced the dislocation rate to 6% but with a loosening rate of 9% at 10 years [6]. Farizon et al. [13] developed the press-fit dual mobility cup (DMC) in 1976 to reduce the risk of dislocation by increasing head functional diameter without increasing loosening by reducing impingement, distraction strains, and wear. It was initially used in primary and revision surgery and associated with only moderate osteolysis. However, in more complex revisions with substantial bone loss, we believe reconstruction using either impaction grafting or defect filling with bone cement should be used. From this perspective, a cemented DMC was conceived.
We therefore asked whether this device was associated with a low dislocation rate. We next ascertained the loosening rate in these patients at high risk for dislocation. We finally compared our results with those published concerning standard cups and constrained cups (retentive, tripolar) in such revision surgery conditions.
Material and Methods
We retrospectively reviewed 82 patients with 88 bipolar (acetabular and femoral) THA revisions with substantial acetabular bone loss performed between January 2002 and December 2004. The patients had an average age of 72 years (range, 65–86 years). We used the cemented DMC when we judged osteolysis was too severe to achieve stable fixation with a press-fit cup without screws. We excluded patients with only cavitary bone in which press-fit cups could achieve sufficient primary stability. Seventy of the 88 revisions (82%) were repeat revisions. Five patients had recurrent dislocations and seven had trochanteric nonunion. Thirty-five of the 82 patients were ASA Grade 3 or greater [17]. The bone loss, according to American Association of Orthopedic Surgeons grading [11], was segmental in 21 hips, combined (segmental and cavitary) in 50 hips, complex (several segmental defects) in 14 hips, and with pelvic discontinuity in three hips. Three patients died of causes unrelated to the surgery before the end of the mean 3-year survey, without any orthopaedic complication. None of the remaining 79 patients (85 hips) was lost to followup. The minimum followup was 2 years (mean, 3 years; range, 2–5 years).
The Medial Cup® DMC (Aston, St Etienne, France) consists of an unconstrained cemented metallic cup, articulating with a dual mobility polyethylene (PE) insert, which is clipped on the 22-mm head of a Charnley-type stem (Aston, St Etienne, France) (Fig. 1). This principal creates two articulations: the small inner one between the femoral head and the insert and the large outer one between the insert and the metallic cup. In flexion/extension, most of the motion occurs in the inner articulation in which the friction torque is lower than that of the larger articulation. For abduction/adduction, or external and internal rotations, movement initiates in the smaller articulation up to 25°. Then, the femoral neck impinges on the rim of the PE insert and drives it. Impingement of the femoral neck on the metal cup occurs only at 54° from cup axis (total range of motion = 108°). Therefore, most movement occurs in the inner articulation, explaining why the wear of the bearing surfaces of this prosthesis is similar to that of Charnley prosthesis [1].
Fig. 1.

The cemented Medial Cup® DMC consists of a steel inox polished sphericocylindrical cemented metal cup and a PE cup insert. Flexion occurs mainly in the small inner articulation. Abduction/adduction and rotation start in the small articulation (up to 25°) and continue up to 54° in the large peripheral articulation between the PE cup and the metallic socket. The PE cup (≥ 8-mm thickness) is not constrained in the metallic socket (no retentive rim), but the PE inner articulation is retentive. The femoral head, usually metallic (22 mm), is clipped inside the PE cup intraoperatively.
Dislocation is prevented by the femoral head being clipped inside the PE insert; hence, the femoral component acts as a large unconstrained head inside a large cup. The distance necessary for dislocation is 23 to 33 mm (with a cup diameter of 40 to 60 mm plus the cylindrical segment of the cup of 3 mm). Moreover, there are very few risks of dislocation by fulcrum of the neck on the metallic cup in extreme rotation, as impingement occurs only after 54° (which is beyond the physiologic range of abduction or rotation). This design also limits strains (and therefore risk of loosening) on the cup fixation because there are no distraction forces on the metal cup as the outer articulation is not retentive. There are few shear strains due to impingement on cup fixation. Importantly, the metal cup is thickest at the equator (4 mm) and thinnest at the pole (1.5 mm). The center of rotation of the PE insert is therefore medialized by about 5 mm in comparison with the center of the acetabular cavity. Mobile cups with concentric centers tend to tilt in a varus position. However, when the center of the PE insert is medialized, this creates a valgus force on the insert counterbalancing the tendency to tilt into varus, maintaining a satisfactory alignment between the femoral neck and the PE cup.
Surgery was performed on each case by one of the five authors. The surgical approach depended mainly on the difficulty of the femoral revisions. Forty cases having a simple femoral implant exchange were treated by a posterior approach. In more difficult femoral revisions (32 cases), we used a trochanteric slide. A femoral flap [22] (extended trochanteric slide osteotomy, 16 cases) was used in cases with severe bone loss (Paprosky Grade 3) and in cases where stem or cement removal was difficult. We used a Charnley-Kerboull cemented femoral stem (Aston, St Etienne, France) [23] in less severe cases (n = 62) and a hydroxyapatite-coated interlocked stem (Aston, St Etienne, France) in cases with major osteolysis (n = 26).
Bone loss was reconstructed by impaction grafting using morsellized allografts (cryopreserved or freeze-dried) (Fig. 2) in 77 cases (91%). In 11 hips, bone loss was palliated only with bone cement (for patients older than 75 years of age or in poor general health). A Kerboull cross-plate (Aston, St Etienne, France) [18, 26] was used in 81 hips (Fig. 3); its main roles were to facilitate positioning of the cup (as its hook catches the upper rim of the obturator foramen) and to increase the cup’s primary stability in impaction grafting.
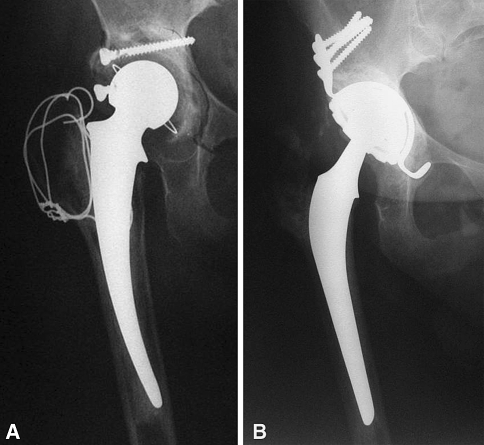
Fig. 2A–B.
Revision of a loose THA with a cemented DMC is shown. (A) This 77-year-old patient had bilateral combined segmental and cavitary acetabular osteolysis with severe instability. (B) The patient underwent bipolar revision with a femoral Charnley prosthesis. Impaction grafting plus structural allografts, reinforced by a Kerboull cross-plate, were used. Three years postoperatively, a good clinical result (Merle d’Aubigné-Postel functional score, 5–6–4) was achieved with no loosening or osteolysis.
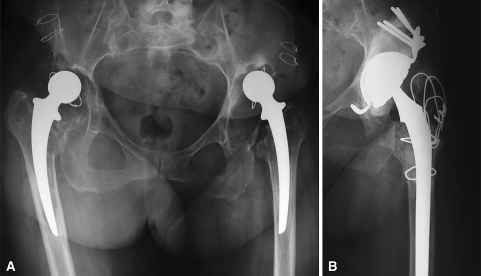
Fig. 3A–B.
Revision of a loose THA with a cemented DMC is shown. (A) This 65-year-old patient had combined segmental and cavitary acetabular osteolysis after first revision. (B) Bipolar revision was performed using a femoral Charnley prosthesis. Impaction grafting and a Kerboull cross-plate reinforcement device were used. Two years postoperatively, the patient’s Merle d’Aubigné-Postel functional score was 6–6–5, and the minute demarcation between allografts and host bone was similar to the immediate postoperative aspect.
Four authors (FLL, MR, FG, TM) undertook the postoperative evaluation of the patients at a followup visit for this study. We used the Merle d’Aubigné-Postel clinical rating [27] in which a maximum score of 6 points each is given for least pain, best range of motion, and best stability and a total score of 15 points (of a maximum 18 points) is considered a good result. Other complications (infection, fracture under the stem) were also noted at postoperative evaluation.
We (FLL, MR, FG, TM) radiographically assessed the cup fixation according to Harris et al. [16] (for femoral lucencies) and DeLee and Charnley [12] (for acetabular zones). KKaplan-Meyer survival analysis was used to assess probability of survival for the cup fixation.
Results
The average clinical Merle d’Aubigné-Postel score was 5.5/6 for pain, 5.4/6 for motion, and 5.2/6 for stability, with 91% having a total score over 15/18. The average range of motion was flexion 120°, abduction 30°, adduction 25°, external rotation 25°, and internal rotation 20°. Regarding dislocations, only one hip dislocation occurred 2 years after revision for loosening and recurrent dislocations in a 75-year-old patient following trauma. After review of the initial revision surgery, the metallic cup was judged too vertical (55°). The dislocation occurred between the femoral head and the PE insert. No wear of the PE insert rim was identified at revision. This was the only case of dislocation in the series of 88 DMCs, which included treatment of five recurrent dislocations.
We had a 5-year cup survival of 94.6%. We observed no radiographic demarcation in 64 hips (75.4%) (Fig. 2) either between allograft and acetabulum or between cement and bone. No osteolysis or lucency changes were detected on comparison of postoperative and followup radiographs in 72 of 86 cases (84.8%). Two hips (2.3%) had asymptomatic radiographic signs of loosening, one with partial lysis of the allograft in Charnley-DeLee Zone I and one with a cement-bone lucency over 2 mm in Zones I, II, and III. In three hips (3.5%), a lucency less than 2 mm in Zone I or III was observed at 3 months but had not progressed at followup. In eight hips (9.4%), a minute bone-cement demarcation less than 1 mm was visible on postoperative radiographs and nonprogressive. In eight hips (9.4%), a reactive dense line was observed at 2 years and did not appear progressive (Fig. 3). Radiographic abnormalities were observed in five cases (5.8% osteolysis or lucencies). We observed minor dense lines in eight cases (9.4%).
Six (8%) of the 85 hips suffered major complications requiring repeat surgery: two for infection and three for traumatic fracture below the stem tip.
Discussion
The risk of dislocation reported in THA revisions with standard cups ranges from 5.1% to 14.4% in patients of an average age of 63 years [2, 19, 31]. This risk increases twofold after the age of 70 years [7] and even more for patients with poor medical status [17]. Constrained cups better prevent dislocation than standard cups but with an increased loosening rate. To judge the effectiveness of the DMC in preventing dislocation and associated morbidity (especially loosening), this cup should be compared with other types of antidislocation cups. The aim of this study was to assess functional outcome and efficiency of this cemented DMC in terms of preventing dislocation in high-risk cases (revisions in elderly patients).The second aim was to estimate loosening rates in those revision surgery cases.
Our study is limited by the relatively short followup (mean, 3 years; range, 2–5 years) and therefore dislocation and loosening rates will likely increase over time. However, data on other devices indicate dislocations and lucencies usually occur in the first 3 postoperative years [13]. A longer followup is necessary to assess the longevity of the cement fixation of the medial cup. The shock-absorbing effect achieved by a PE insert 8-mm-thick or more and the motion between the metal cup and the PE insert (absorbing shear stresses) may reduce strains on the cement fixation and favor longevity.
Previous reports have dealt only with treatment of recurrent dislocations [15, 33], while other authors [13] associate curative treatments for recurrent dislocations and prevention of dislocations in at-risk cases (especially when intraoperative instability was observed during revision). We believe the results of constrained cups such as the S-ROM® (DePuy Orthopaedics, Inc, Warsaw, IN) (Table 1) are disappointing, given 37% required repeat surgery at 10 years (14% for dislocation, 10% loosening, 13% for other causes) [4]. These results may have been the consequence of limited range of motion in rotation (41°) and an insufficient thickness of PE (3–5 mm). Tripolar cups had a slightly improved range of motion (45°) and better dislocation rates (4.5%–6%) [3, 8, 9, 34], but loosening rates remained excessive (requiring 8.6% to 9% of revisions, plus 9% substantial radiolucencies) as these devices were still constrained. Novel constrained cups [5] with a larger range of movement (55°) had less than 1 year of followup. In preventing dislocation, the major difference between the currently available cups (S-ROM®, Tripolar® [Osteonics Corp, Allendale, NJ], Freedom® [Biomet Inc, Warsaw, IN]) and the DMC is that the DMC is an unconstrained device; the metal cup is not retentive, and the prevention of dislocation is only achieved by the increased jumping distance (23–33 mm) and the large range of motion (54°), reducing the likelihood of impingement. Furthermore, the PE is thicker in the DMC (≥ 8 mm), reducing flow and wear. We had 88 revisions with severe bone loss (67 combined segmental and cavitary, several with complex segments or discontinuity), requiring 77 allografts in older patients (average age, 72 years), with 35 having an ASA grade greater than 3. Five patients had recurrent dislocations before surgery. Hence, our results in terms of dislocation (one case) at 3 years are favorable.
Table 1.
Constrained cups (S-Rom®, Tripolar®) versus unconstrained dual mobility cups (Medial Cup®)
| Study | Cup type* | Number of cases | Average follow up (years) | Dislocations (%) | Revisions/loosening (%) | Lucencies (%) |
|---|---|---|---|---|---|---|
| Berend et al. [4] | S-ROM® | Total 755 | > 10 | 17.5 | 10 | |
| At risk 574 | 14.2 | |||||
| Recurrences 188 | 28.5 | |||||
| Bremner et al. [6] | Tripolar® | Total 101 | 10 | 6 | 9 | |
| At risk 50 | ||||||
| Recurrences 51 | ||||||
| Callaghan et al. [8] | Tripolar® | Total 132 | 4–10 | 4.5 | 8.7 | 9 |
| At risk 60 | 7 | |||||
| Recurrences 72 | ||||||
| Cooke et al. [9] | Tripolar® | Total 58 | About 3 | 5 | 8.6 | |
| Shrader et al. [34] | Tripolar® | Total 110 | < 3 | 0 | 9 | 14 |
| At risk 31 | ||||||
| Recurrences 79 | ||||||
| Our series | Medial Cup® | Total 88 | 3 | 1.1 | 2.2 | 3.3 |
| At risk 83 | ||||||
| Recurrences 5 |
* S-ROM® was manufactured by DePuy Orthopaedics, Inc, Warsaw, IN; Tripolar® by Osteonics Corp, Allendale, NJ; and Medial Cup® by Aston, St Etienne, France.
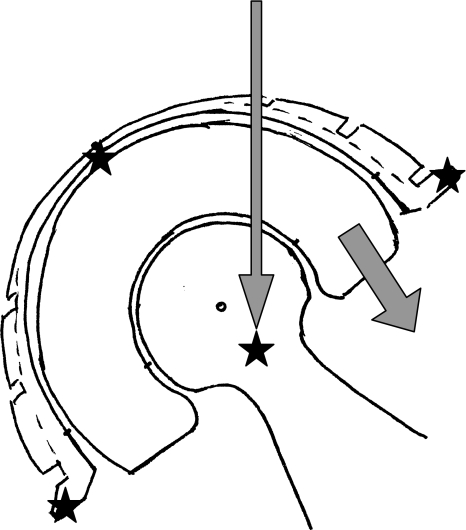
The current cemented DMC is an evolution of the original Bousquet DMC [13, 24]; its primary fixation was achieved by press-fit, enhanced by one supraacetabular screw and two studs (pubic and ischial). The DMC described by Farizon et al. [13] 30 years ago has been widely used in its original press-fit design. It generally prevented dislocation, but the original design led to wear of the PE rim and varus tilting of the PE cup [25]. A series of original Bousquet cups for primary surgery [30] (106 cases at 10 years’ followup) had a 2% incidence of dislocation and a 94.6% survival (for cup revision for loosening). Another series of 40 retrieved Bousquet cups at an average of 8 years identified minute wear on the inner and outer PE bearing surfaces [1]. However, wear of the rim of the PE was of primary importance and occurred by two mechanisms. The first was the wear of the entire PE rim due to the impingement of a poorly matched femoral neck (thick and rough), leading to osteolysis by PE wear debris. The second was the consequence of tilting of the PE cup in a varus position; the head abraded the superior part of the tilted insert resulting in a dislocation of the head inside the metal cup (intraarticular dislocation) [28]. The original cup was modified for the cemented DMC, in particular by medialization of the center of rotation of the PE cup (Fig. 4) in the acetabular cavity (avoiding varus tilting), by reducing the consequences of the impingement of the neck on the PE rim, and by improvement of the neck design (conformity of neck and of rim; low head:neck ratio of 22 mm:10 mm) and of the neck surface finish (polished steel).
Fig. 4.
The DMC design was improved by medialization of the PE cup. The center of rotation of the surface bearing of the metal socket is more medial than the center of the socket periphery. Therefore, resultant forces acting on the PE cup avert its tilting in varus (and thus the risk of upper PE cup rim wear, leading to subluxation). To reduce the wear of the entire rim, the head:neck ratio is 22 mm:10 mm, which allows more than 25° of abduction or rotation in the inner articulation. The femoral neck is polished, and its good congruity with the PE rim limits chipping during impingement.
The original Bousquet cup proved unsatisfactory in 120 revisions [13] for severe cup loosening, as press-fit was not always sufficient to achieve primary stability (18% recurrent loosening at 5 years). A cemented version of the Bousquet cup was therefore designed for revisions with cement. In our experience, this new device, the Medial Cup® cemented DMC, was initially used for cases of primary THA at risk of dislocation. We used these DMCs in three circumstances: patients at risk of dislocation for neurologic (hemiplegia), muscular (poliomyelitis), or oncologic (metastasis) reasons; infection revisions with a large excision of periprosthetic soft tissues and use of antibiotic bone cement [21]; or primary THA in octogenarians.
A concern for long-term fixation of cemented DMCs is the poor results observed with cemented metal cups and with cemented metal-backed PE cups. In one study, cemented metal-backed PE cups [32] were associated with early loosening. A series of 124 cases at 2 years identified 23.5% progressive lucencies and a 2.5% incidence of revisions for loosening [29]. Some cemented metal-on-metal cups (McKee-Farrar) have survived over 20 years, but most were revised within 10 years for cup loosening [36]. In contrast, at the same 2 to 5 years followup with the cemented DMC, we did not observe radiographic signs of impending loosening. Fully cemented retentive cups have been abandoned for poor longevity [20]. Large heads [10] can now be used with highly crosslinked PE [28]. Early results [14] (48 hips; average followup, 3.3 years) are encouraging (2% dislocation) with minute head penetration. However, clinical tolerance of highly crosslinked PE at 10 years has not yet been extensively assessed. For similar passive stability, this device requires acetabular overreaming in comparison to the DMC to obtain the same PE thickness. Metal-on-metal bearings could be of major interest due to their minute wear on large heads. However, there may be a risk of fatigue fracture in the cement, as these devices do not include any PE shock absorber.
Our midterm data (average followup, 3 years) of 88 cemented DMCs for revisions in older patients (average age, 72 years) are encouraging as they had a low incidence of dislocation (1.1%) and asymptomatic incipient loosening (2.2%), with 94.6% having stable fixation. These results compare favorably with the 7.4% incidence of dislocation using standard cups for revision (5.1%–14.4%) [31] and with the 6% incidence of dislocation at 10 years achieved by tripolar cups (13% revisions for aseptic loosening) [3]. At present, this technique appears favorable in comparison to tripolar cups and has been assessed with longer followup than large metal heads with highly crosslinked PE as well as metal-on-metal designs. Currently, we recommend its use in THA revisions in patients over 65 years of age, especially for loosening with severe bone loss and in cases requiring exchange due to infection.
Acknowledgments
We thank Rajiv Kaila for his help in translating this manuscript.
Footnotes
†Prof Langlais died on June 16, 2007.
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Adam P, Farizon F, Fessy MH. Dual articulation retentive acetabular liners and wear: surface analysis of 40 retrieved polyethylene implants [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:627–636. [DOI] [PubMed]
- 2.Alberton GM, High WA, Morrey BF. Dislocation after revision total hip arthroplasty: an analysis of risk factors and treatment options. J Bone Joint Surg Am. 2002;84:1788–1792. [PubMed]
- 3.Beaulé PE, Roussignol X, Schmalzried TP, Udomkiat P, Amstutz HC, Dujardin FH. Tripolar arthroplasty for recurrent total hip prosthesis dislocation [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2003;89:242–249. [PubMed]
- 4.Berend KR, Lombardi AV Jr, Mallory TH, Adams JB, Russell JH, Groseth KL. Long-term outcome of 755 consecutive constrained acetabular components in total hip arthroplasty. J Arthroplasty. 2005;20(7 Suppl 3):93–102. [DOI] [PubMed]
- 5.Berend KR, Lombardi AV Jr, Welch M, Adams JB. A constrained device with increased range of motion prevents early dislocation. Clin Orthop Relat Res. 2006;447:70–75. [DOI] [PubMed]
- 6.Bremner RB, Goetz DO, Callaghan JJ, Capello WN, Johnston RC. Use of constrained acetabular components for hip instability: an average 10-year follow-up study. J Arthroplasty. 2003;18(7 Suppl 1):131–137. [DOI] [PubMed]
- 7.Brytröm S, Espehaug B, Furnes O, Havelin LI. Femoral size is a risk factor for total hip dislocation (a study of 42987 primary THR from the Norwegian Arthroplasty Register). Acta Orthop Scand. 2003;74:514–524. [DOI] [PubMed]
- 8.Callaghan JJ, O’Rourke MR, Goetz DD, Lewallen DG, Johnston RC, Capello WN. Use of a constrained tripolar acetabular liner to treat intraoperative instability and postoperative dislocation after total hip arthroplasty. Clin Orthop Relat Res. 2004;429:117–123. [DOI] [PubMed]
- 9.Cooke CC, Hozack W, Lavernia C, Sharkey P, Shastri S, Rothman RH. Early failure mechanisms of constrained tripolar acetabular sockets used in revision total hip arthroplasty. J Arthroplasty. 2003;18:827–833. [DOI] [PubMed]
- 10.Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don’t do. Clin Orthop Relat Res. 2004;429:102–107. [DOI] [PubMed]
- 11.D’Antonio JA, Capello WN, Borden LS, Bargar WL, Bierbaum BF, Boettcher WG, Steinberg ME, Stulberg SD, Wedge JH. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989;243:126–137. [PubMed]
- 12.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 13.Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup wear mobility. Int Orthop. 1998;22:219–224. [DOI] [PMC free article] [PubMed]
- 14.Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop Relat Res. 2006;447:53–59. [DOI] [PubMed]
- 15.Goetz DD, Bremner BR, Callaghan JJ, Capello WN, Johnston RC. Salvage of a recurrently dislocating total hip prosthesis with use of a constrained acetabular component. J Bone Joint Surg Am. 2004;86:2419–2423. [DOI] [PubMed]
- 16.Harris WH, McCarthy JC Jr, O’Neill DA. Femoral component loosening using contemporary techniques of femoral cement fixation. J Bone Joint Surg Am. 1982;64:1063–1067. [PubMed]
- 17.Jolles BM, Zangger P, Leyvraz PF. Factors predisposing to dislocation after primary total hip arthroplasty: a multivariate analysis. J Arthroplasty. 2002;17:282–288. [DOI] [PubMed]
- 18.Kerboull M, Hamadouche M, Kerboull L. The Kerboull acetabular reinforcement device in major acetabular reconstructions. Clin Orthop Relat Res. 2000;378:155–168. [DOI] [PubMed]
- 19.Khatod M, Barber T, Paxton E, Namba R, Fithian D. An analysis of the risk of hip dislocation with a contemporary total joint registry. Clin Orthop Relat Res. 2006;447:19–23. [DOI] [PubMed]
- 20.Lagrange J, Letournel E, Pupin P. Review of patients with total hip replacement after a period of more than ten years (author’s transl) [in French]. Chirurgie. 1979;105:733–737. [PubMed]
- 21.Langlais F, Belot N, Ropars M, Thomazeau H, Lambotte JC, Cathelineau G. Antibiotic cements in articular prostheses: current orthopaedic concepts. Int J Antimicrob Agents. 2006;28:84–89. [DOI] [PubMed]
- 22.Langlais F, Kerboull M, Sedel L, Ling RS. The “French paradox.”. J Bone Joint Surg Br. 2003;85:17–20. [DOI] [PubMed]
- 23.Langlais F, Lambotte JC, Collin PH, Langlois F, Fontaine JW, Thomazeau H. Trochanteric slide osteotomy in revision total hip arthroplasty for loosenings. J Bone Joint Surg Br. 2003,85:510–516. [DOI] [PubMed]
- 24.Leclercq S, el Blidi S, Aubriot JH. Bousquet’s device in the treatment of recurrent dislocation of a total hip prosthesis: apropos of 13 cases [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1995;81:389–394. [PubMed]
- 25.Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90:249–255. [DOI] [PubMed]
- 26.Lunn JV, Kearns SS, Quinlan W, Murray P, Byrne JO. Impaction allografting and the Kerboull acetabular reinforcement device: 35 hips followed for 3–7 years. Acta Orthop. 2005;76:296–302. [PubMed]
- 27.Merle d’Aubigné R, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed]
- 28.Muratoglu OK, Bragdon CR, O’Connor D, Perinchief RS, Estok DM 2nd, Jasty M, Harris WH. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 Suppl 1):24–30. [DOI] [PubMed]
- 29.Peraldi P, Vandenbussche E, Augereau B. Bad clinical results of cemented caps with metal-backed acetabular components: 124 cases with 21 months follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83:561–568. [PubMed]
- 30.Philippot R, Adam P, Farizon F, Fessy MH, Bousquet G. Survival of cementless dual mobility sockets: ten-year follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:326–331. [DOI] [PubMed]
- 31.Phillips CB, Barrett JA, Losina E, Mahomed NN, Lingard EA, Guadagnoli E, Baron JA, Harris WH, Poss R, Katz JN. Incidence rates of dislocation, pulmonary embolism, and deep infection during the fist six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85:20–26. [DOI] [PubMed]
- 32.Ritter MA. The cemented acetabular component of a total hip replacement: all polyethylene versus metal backing. Clin Orthop Relat Res. 1995;311:69–75. [PubMed]
- 33.Shapiro GS, Weiland DE, Markel DC, Padgett DE, Sculco TP, Pellicci PM. The use of constrained acetabular component for recurrent dislocation. J Arthroplasty. 2003;18:250–258. [DOI] [PubMed]
- 34.Shrader MW, Parvizi J, Lewallen DG. The use of a constrained acetabular component to treat instability after THR. J Bone Joint Surg Am. 2003;85:2179–2183. [DOI] [PubMed]
- 35.Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64:1295–1306. [PubMed]
- 36.Zahiri CA, Schmalzried TP, Ebramzadeh E, Szuszczewicz ES, Salib D, Kim C, Amstutz HC. Lessons learned from loosening of the McKee-Farrar metal-on-metal total hip replacement. J Arthroplasty. 1999;14:326–332. [DOI] [PubMed]