Abstract
Different bearing surfaces, including alumina-on-alumina, have been used to avoid osteolysis.
We prospectively followed 288 patients (319 hips) in which an alumina-on-alumina cup was used with a hydroxyapatite stem. The patients’ mean age was 52.7 (range, 14–70 years), and the minimum followup was 3 years (mean, 4.7 years; range, 3–8 years). At final followup, five cups (including one with an alumina liner fracture) and two stems underwent revision. The cumulative probability of not having a revision of one or both components for any cause was 97% (95% confidence interval, 94.7%–99.1%). No patient spontaneously reported any noises from the hip and none reported noises when specifically questioned. All patients who had not undergone revision had good clinical results, but five of these patients had radiographic cup loosening at last followup. These data suggest alumina-on-alumina prostheses had reasonable outcomes after 5 years. One acetabular component fractured from trauma. We observed no linear femoral head penetration. Continued followup will be required to determine if reduction in wear between the alumina-on-alumina bearings results in less osteolysis and loosening.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Polyethylene wear produces osteolysis and loosening in cemented as well as cementless prostheses, especially in young, active patients [13]. Different bearing surfaces, including alumina-on-alumina [1–3, 6, 7, 14, 15, 19, 20, 31–33], have been used to avoid these complications. Alumina-on-alumina coupling was introduced by Boutin [5] in France in 1970, and different authors [3, 19, 20, 32, 34] have reported good results after 30 years. At the end of the 1970s, cementless prostheses with a threaded ceramic cup were widely used. Published series [14, 21, 30, 33] report no ceramic wear but report poor results because of acetabular fixation failure and ceramic fractures. Current ceramics are very different from old ceramics in regard to porosity, purity, and grain size and the quality has improved considerably, reducing the risk of fracture [32]. Consequently, fixation of the socket has become the weak link of an alumina-on alumina arthroplasty [2]. However, in addition, cup loosening, noises [36], and alumina fractures are still major concerns, and, although long-term results are needed to obtain definite data, short-term results are relevant because nearly all such fractures occur in the first 18 months [1, 15, 20].
We hypothesized loosening and fracture are related to acetabular component size and cup orientation.
Materials and Methods
We prospectively followed 288 patients, active and younger than 70 years old, with 319 primary cementless Cerafit-Multicone prostheses (hydroxyapatite; Ceraver, Roissy, France) implanted between January 1999 and December 2003 at four institutions by four surgeons (EGC, AMM, ABP, EM). Of the 319 hips, we excluded five: one hip with a deep infection (0.3%); one hip with a cup revision after a posttraumatic acetabular fracture; one hip with a femoral stem revision after a periprosthetic fracture; and two patients (two hips) died of nonimplant-related causes 2 and 3 years after surgery. The other 314 hips formed the basis of the study. The minimum clinical and radiographic followup at the last evaluation was 3 years (mean, 4.7 years; range, 3–8 years). Patients were relatively young and active with good bone quality at the time of surgery (Table 1). The activity level was classified on the Devane et al. [9] scale and femoral bone quality was classified according to Dorr et al. [10]. Oral and written informed consent was obtained in all patients, and they were also informed preoperatively that they might receive a new biomaterial without well-known results (alumina-on-alumina).
Table 1.
Patient data
| Variable | Number |
|---|---|
| Gender (men/women) | 192/127 |
| Mean age (years)* | 52.7 ± 13.6 |
| Mean weight (kg)* | 74 ± 12.5 |
| Femoral shape [13] | |
| Type A (champagne cup) | 192 |
| Type B (intermediate) | 95 |
| Type C (cylindrical) | 32 |
| Activity [12] | Hips |
| Level 1-2-3 | 59 |
| Level 4 | 161 |
| Level 5 | 99 |
| Diagnosis | Hips (%) |
| Osteoarthrosis | 135 (42.3%) |
| Developmental arthritis | 18 (5.6%) |
| Posttraumatic arthritis | 25 (7.8.1%) |
| Dysplasia Crowe Grades I-II | 24 (7.5%) |
| Dysplasia Crowe Grades III-IV | 11 (3.4%) |
| Inflammatory arthritis (rheumatoid arthritis) | 41 (12.8%) |
| Avascular necrosis | 63 (19.7%) |
| Other | 2 (0.6%) |
*Mean ± standard deviation.
The cementless Multicone stem used in this series (Ceraver Osteal, Paris, France) has a tapered straight stem, rectangular geometry, is made of TiAl6V4, and is fully coated with hydroxyapatite (HAP). The stem was paired with a Cerafit-Triradius press-fit, fully HAP-coated TiAl6V4 rough shell with optional screws fitted with an Al2O3 liner (Ceraver). The liner is inserted using a Morse taper shape with an angle of 5°. This permits excellent fixation with no risk of liner dislocation. Characteristics of the alumina are 99.8% purity, 3.98 density, 2-μm grain size, and hipped in all cases. The HAP coating was applied by plasma spray and, according to the manufacturer’s specifications, the coating had a relative crystallinity of 37%, thickness of 80 ± 20 μm, bond shear strength of 15/25 MPa, and a mean roughness of 3 μm. Regarding stem and shell surface finish, the arithmetic rugosity Ra beneath HAP was Ra 3 to 4 μm.
We performed all operations with the same technique using a posterior approach. A total of 282 (88.4%) 32-mm Al2O3 femoral heads (Ceraver) and 37 (11.6%) 28-mm femoral heads (patients with congenital dysplasia of the hip or with rheumatoid arthritis) were implanted with three neck lengths. With a cup diameter size of less than 50 mm, the femoral head diameter must be 28 mm. The minimum ceramic liner thicknesses are 4.2 mm for 46-mm (28-mm femoral head) and 50-mm (32-mm femoral head) shells and 6.2 mm for 48-mm (28-mm femoral head) and 52-mm (32-mm femoral head) shells. The median diameter of the cups and the size of the stems were 52 mm and 10 mm, respectively. Small cups (28-mm femoral head) were more frequently used in women than in men and in patients with congenital dysplasia. Short femoral neck lengths were more commonly used in association with a small cup and medium necks in association with the larger cups (32-mm femoral head) (Table 2). Two screws for cup fixation were used in 107 hips (33.5%). The surgeons decided on the use of the screws or femoral head allografts depending on the presence of acetabular protrusion or partial uncovering of the cup, which was more frequent in women than in men; in small cups (28-mm femoral head), screw fixation was also more frequent in patients with acetabular protrusion and congenital dysplasia (Table 3). Postoperatively, all patients received an antibiotic and low-molecular-weight heparin subcutaneously to prevent thromboembolic incidents. Patients walked with toe-touch and partial weightbearing with crutches for 3 weeks postoperatively, after which they were allowed to walk using two crutches for the next 6 weeks.
Table 2.
Number of hips in which a small cup size (28-mm femoral head) and larger cup size (32-mm femoral head) were used
| Variable | Size of femoral head | Total | p | |
|---|---|---|---|---|
| 28 mm | 32 mm | |||
| Total number | 37 | 282 | 319 | 0.001 |
| Male gender, number (%) | 12 (32.4%) | 179 (63.5%) | 191 | |
| Female gender, number (%) | 25 (67.6%) | 103 (36.5%) | 128 | |
| Age in years, mean (range) | 47.7 (14–70) | 53.3 (15–70) | 52.7 (14–70) | 0.062 |
| Diagnoses, number (%) | <0.001 | |||
| Osteoarthrosis | 8 (21.6%) | 127 (45.0%) | 135 | |
| Developmental arthritis | 4 (10.8%) | 14 (5.0%) | 18 | |
| Posttraumatic arthritis | 1 (2.7%) | 24 (8.5%) | 25 | |
| Acetabular protrusion | 2 (5.4%) | 12 (4.2%) | 14 | |
| CDH (Crowe Grades 1–2) | 6 (16.2%) | 18 (6.4%) | 24 | |
| CDH (Crowe Grades 3–4) | 7 (21.9%) | 4 (1.4%) | 11 | |
| Rheumatoid arthritis | 4 (12.5%) | 23 (8.1%) | 27 | |
| Avascular necrosis | 5 (15.6%) | 58 (20.6%) | 63 | |
| Others | 0 (0%) | 2 (0.7%) | 2 | |
| Femoral neck length, number (%) | 0.093 | |||
| Short neck | 14 (37.8%) | 70 (24.8%) | 84 | |
| Medium neck | 12 (32.4%) | 144 (51.1%) | 156 | |
| Long neck | 11 (29.7%) | 68 (24.1%) | 79 | |
| Cup fixation, number (%) | 0.004 | |||
| No screws | 18 (48.6%) | 206 (73.0%) | 224 | |
| Screws | 19 (51.3%) | 76 (27.0%) | 95 | |
CDH = congenital dislocation of the hip.
Table 3.
Number of hips in which screws were used for cup fixation
| Variable | Cup fixation | Total | p | |
|---|---|---|---|---|
| No screws | Screws | |||
| Total number | 224 | 95 | 319 | 0.026 |
| Male gender, number | 143 (63.8%) | 49 (51.6%) | 192 | |
| Female gender, number | 81 (36.2%) | 46 (48.4%) | 127 | |
| Age in years, mean (range) | 52.4 (18–70) | 53.3 (14–74) | 52.7 (14–70) | 0.622 |
| Diagnoses, number (%) | 0.026 | |||
| Osteoarthrosis | 96 (42.9%) | 39 (41%) | 135 | |
| Developmental arthritis | 11 (4.9%) | 7 (7.2%) | 18 | |
| Posttraumatic arthritis | 18 (8%) | 7 (7.2%) | 25 | |
| Acetabular protrusion | 4 (1.8%) | 10 (10.5%) | 14 | |
| CDH (Crowe Grades 1–2) | 19 (8.5%) | 5 (5.3%) | 24 | |
| CDH (Crowe Grades 3–4) | 6 (2.7%) | 5 (5.3%) | 11 | |
| Rheumatoid arthritis | 19 (8.5%) | 8 (8.4%) | 27 | |
| Avascular necrosis | 50 (22.3%) | 13 (13.7%) | 63 | |
| Other | 1 (0.4%) | 1 (1%) | 2 | |
CDH = congenital dislocation of the hip.
We (EGR, AMM, ABP, EM) clinically evaluated patients for pain, function, and range of motion using the six-level scale described by Merle D’Aubigné and Postel [26]. Patients were also asked about the location of pain. Standard anteroposterior radiographs of the pelvis and lateral radiographs of the hip were made immediately after the operation, at 6 weeks, at 3, 6, and 12 months, and then annually thereafter following the same protocol. The patient was positioned supine with his or her feet together. The xray tube was positioned over the symphysis pubis 1 m from and perpendicular to the table. To reduce interobserver error, measurements were made by a single author (EGR) who had not been involved in the surgery.
We (EGR) assessed the cup position according to the acetabular abduction angle, the height of the center of the hip (as measured from the center of the femoral head to the interdrop line), and the horizontal distance of the cup (measured from the center of the femoral head to the Köhler line) [22]. The distribution of any radiolucent gaps on the initial postoperative radiograph and of radiolucent lines or osteolysis at the acetabular bone-prosthesis interface on the subsequent radiographs was recorded in the three zones described by DeLee and Charnley [8]. Because of the difficulty in detecting radiolucent lines around uncemented cups, the current method of determining radiographic bone ingrowth into an acetabular component is by indirect inference based on the absence of the two classic signs of loosening, radiolucent lines, and cup migration [27]. Linear wear was estimated according to the method of Kim et al. [24], which was used because of its simplicity. Penetration of the prosthetic head into the liner was measured by a computer-assisted edge-detection system and specially designed software (AutoCAD 2000; AutoDesk Inc, Sausalito, CA). Radiographs were digitized using a scanner (Epson Expression 1680; Seiko Epson Corp, Hirooka, Japan).
The position of the stem was defined as neutral, valgus (0.3° of lateral deviation), or varus (0.3° of medial deviation). Femoral canal filling, measured as the ratio of the width of the stem to the width of the medullary canal, was determined at three levels: Level A (at the metaphyseal part of the stem), Level B (at the middle of the stem), and Level C (1 cm proximal to the tip). The distribution of any radiolucent lines or osteolysis as seen on the anteroposterior radiographs was recorded in the zones described by Gruen et al. [18]. Femoral osteolysis was classified according to the criteria described by Goetz et al. [17]. Femoral osteopenia resulting from stress shielding was graded according to the system described by Engh et al. [11]. Femoral component fixation was graded according to the criteria for porous prostheses described by Engh et al. [12].
Kaplan-Meier survivorship analysis [23], with 95% confidence intervals, was used to estimate the cumulative probability of not having a revision of one or both prosthetic components in the whole series and to estimate the cumulative probability of not having cup loosening. The analysis is the worst case scenario for revision surgery because all operated hips are included and there were no patients lost to followup. Pearson’s chi square test was used to compare cup sizes (28-mm and 32-mm femoral head) and the use of screws for cup fixation. Fisher’s exact test was used to compare shell diameters and in relation to cup revision and gender in relation to cup loosening. Mann-Whitney test was used to compare acetabular abduction angle in relation to cup loosening.
Results
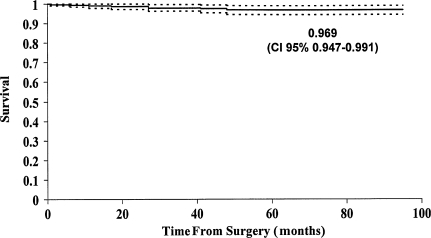
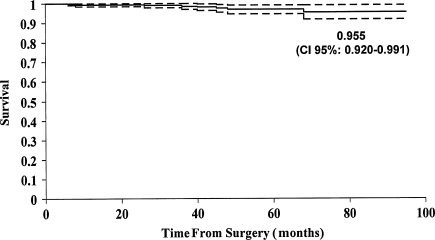
The probability of not having a revision of one or both components for any reason (eight hips) was 97% (95% confidence interval, 94.7%–99.1%) (Fig. 1). The survival with radiographic cup loosening as an endpoint was 95.5% (95% confidence interval, 92.0%–99.1%) (Fig. 2). We revised five cups: three for loosening; one for an acetabular periprosthetic fracture after a high-energy trauma; and one for an alumina liner fracture occurring from a fall 40 months after the operation. The cup with the liner fracture has a 50-mm outer diameter cup (4.2-mm alumina liner) and a 35° acetabular abduction angle. Of the three cups revised for loosening, two loosened as a result of poor press-fit and the third 3.7 years after surgery. All revised cups had an outer diameter of 50 mm or less. We revised two stems for aseptic loosening: one in a patient with acromegaly after implanting a very thin stem and subsequent early subsidence; and one owing to a periprosthetic fracture from a fall 2 months after surgery (Table 4).
Fig. 1.
Cumulative probability of not having a revision of one or both prosthetic components for any cause in all patients is shown. The upper and lower curves represent the 95% confidence limits.
Fig. 2.
Cumulative probability of not having cup loosening is shown. The upper and lower curves represent the 95% confidence limits.
Table 4.
Data on revised hips
| Case number | Revision | Moment (months) | Cause of failure | Age (years) | Gender | Diagnosis | Cup size | Head diameter | Screws | Abduction angle | Stem size |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cup | 27 | Acetabular fracture | 49 | Male | Avascular necrosis | 50 | 32 | — | 45° | 9 |
| 2 | Stem | 2 | Periprosthetic fracture | 53 | Male | Posttraumatic arthritis | 58 | 32 | Yes | 45° | 12 |
| 3 | Cup | 37 | Cup loosening | 64 | Female | Hip dysplasia | 46 | 28 | — | 45° | 9 |
| 4 | Cup | 41 | Alumina fracture | 65 | Female | Posttraumatic arthritis | 50 | 32 | — | 39° | 9 |
| 5 | Cup | 6 | Cup loosening | 52 | Male | Developmental arthritis | 48 | 28 | — | 55° | 10 |
| 6 | Cup | 16 | Cup loosening | 45 | Female | Rheumatoid arthritis | 50 | 32 | Yes | 75° | 8 |
| 7 | Stem | 0.75 | Stem subsidence | 57 | Male | Acromegaly | 56 | 32 | — | 45° | 7 |
| 8 | Stem | 27 | Infected stem | 73 | Female | Osteoarthrosis | 56 | 32 | Yes | 47° | 11 |
The mean diameter of the revised cups was less (p = 0.002) than the mean diameter of cups not undergoing revision. We considered eight cups as radiographically loose: three were revised and five cups had radiolucency in two or three zones. The mean diameter of the loosened cups was similar (p = 0.263) to that of the nonloosened cups (51.2 ± 3.4 vs 52.8 ± 3.4, respectively). Radiographically loosened cups were more frequent (p = 0.047) in females (six hips) than in males (two hips). With the number of available hips, no other risk factors were associated with loosening.
The mean acetabular abduction angle was higher (p = 0.018) in loosened cups than in nonloosened cups (56.4° ± 17.8° vs 45.9° ± 4.5°, respectively). The mean acetabular abduction angle was 46.3° ± 5.4°, the mean height of the center of the hip was 21.9 ± 6.9 mm, and the mean horizontal distance of the cup was 33.5 ± 6.7 mm. There were 278 cups in neutral position (acetabular abduction angle between 40° and 50°), 32 in vertical position (greater than 50°), and nine in horizontal position (less than 40°). Forty-six hips presented cup lucency on the initial postoperative radiograph. At the end of followup (3–8 years), nine of these hips had lucency in one zone and four in two zones, and only one hip had circumferential lucency 4 years postoperatively. The lucencies had disappeared in the other hips (Fig. 3). There was no osteolysis around any component. There were 268 stems (84%) in neutral position, 47 (14.7%) in varus position, and four (1.3%) in valgus position. The average femoral canal filling was 83% ± 7.1% at the proximal level of the stem (Level A), 90.8% ± 8.8% at the middle third of the stem (Level B), and 86.8% ± 8.7% at the distal level of the stem (Level C). There were no instances of Grade 2 or higher proximal femoral osteopenia or cortical widening in any case. Radiographic penetration of the femoral head into the alumina liner was measured in 261 of the 314 hips (83.1%) in which the femoral head could be differentiated from the cup on radiographs. The available technology could not detect wear after the 6-week postoperative reference radiograph for postoperative femoral head penetration.
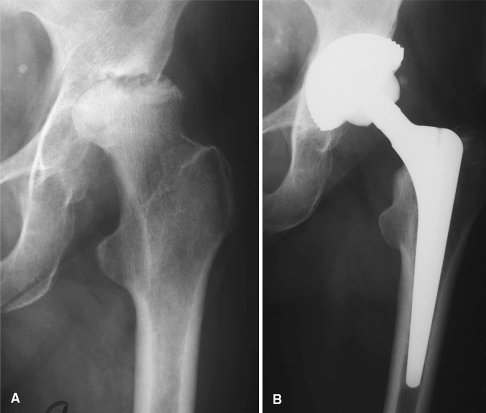
Fig. 3A–B.
(A) Preoperative radiograph of a 69-year-old-woman shows a dysplastic hip. (B) Five-year followup radiograph shows Cerafit-Multicone cementless THA.
One patient had intermittent pain in the thigh during the first year after surgery. At the last followup evaluation, there was one hip with Level 4 pain, 27 hips with Level 5 pain, and 285 hips with Level 6 pain. The mean preoperative pain rating was 2.6 and it had improved to 5.9 at the last followup evaluation. The walking function rating increased from 3.0 to 5.8 and range of motion improved from 3.0 to 5.4. Complications included one intraoperative femoral fracture in a patient with juvenile rheumatoid arthritis who was treated with cerclage, one pelvic perforation, and one fracture of the greater trochanter. All these hips had good clinical results and were included in the followup study. There were also two dislocations that resolved with closed treatment and were included in the followup study. One stem had a deep infection and was revised and therefore excluded from the study. There were no eccentric liner seatings or ceramic liner chip fractures at the time of insertion requiring intraoperative replacement.
Discussion
The theoretical advantages of alumina-on-alumina bearings are related to tribologic properties such as the scratch resistance and wettability of the material. In addition to superior wear properties, ceramics are biologically inert. These favorable qualities are particularly desirable for implants in a young and active population [31, 33]. Modern third-generation alumina is produced with a finer grain size with fewer impurities, thereby improving the material and increasing durability [1, 2]. These new generations of alumina-on-alumina couplings have been associated with press-fit metal-backed shells and tapered cementless stems coated with HAP [20].
The main limitation to our study was the short-term followup. However, although long-term results are needed to obtain definitive data, short-term data results are also important because alumina-specific complications such as fractures [1, 2, 7, 15, 20] and noises occur in the first 2 years [36]. Another limitation of the current study includes the length of the enrollment period needed to obtain a sufficient number of patients. The short followup and the number of patients limited the ability to make definitive conclusions, especially in relation to different diagnoses and cup loosening. The nonvalidated method of wear measurement, especially when considering such small figures in the order of microns, is also a main limitation to this study.
We observed one alumina fracture in a 60-year-old woman in whom a thin alumina liner (4.2 mm) was inserted in an acetabular cup (a 50-mm outer diameter matched with a 32-mm femoral head) that had been implanted in a horizontal position (acetabular abduction angle, 35°); the fracture occurred 40 months postoperatively after a trauma (fall). Alumina has excellent tribologic properties but poor bending strength [35]. Alumina ceramics are brittle and have no way to deform without breakage. It is difficult to know the exact number of alumina fractures because they are not well-recognized and could be underestimated. Fracture risk is now approximately one in 2000 for a 10-year period [35]. Liner chipping/fracture could be related to different causes such as cup malposition, neck-cup impingement, and dislocation [7]. Alumina fractures can be produced by the propagation of subcritical crack growth when subjected to unexpected high-load pressure. Park et al. [28] suggest repetitive impingement can occur in some hips, especially those in which the components are suboptimally positioned and in those with a high range of motion. Poggie et al. [29] believe the failures of the ceramic liner in the so-called sandwich-design ceramic-on-ceramic cups were caused by high torque transmitted from the femoral head to the ceramic liner causing dislodgment of the ceramic liner from the polyethylene socket. Striped wear damage of the head occurs as a result of edge loading and rim wear as a result of subluxation and microseparation. The high frictional torque transmitted across the articulation interface probably occurs during deep flexion or other high-load range-of-motion activities [29]. Subsequent to dislodging, the ceramic liner is completely displaced and will eventually fracture in most cases [29, 35]. A potential solution to ceramic liner displacement is the addition of a geometric irregularity such as a central peg [29]. The alumina liner used in this study has this central peg to increase the torque required to displace the liner and reduce this mode of failure. Hannouche et al. [20] reported three liner fractures in a series using Cerafit and Multicone implants. All these fractures occurred very early (8–16 months postoperatively). These authors advise of the necessity for adequate component positioning and avoidance of small implants (50 mm/32 mm). In the current series, the fractured liner was 4.2 mm wide (32-mm femoral head) and was associated with a small shell (50-mm diameter). The new titanium alloy shells are 3 mm instead of 5 mm thick so as to allow placement of a 2-mm thick alumina liner and consequently improve mechanical resistance. Therefore, the new minimum ceramic liner thickness is 6.2 mm for small acetabular cups (46/28 mm and 52/32 mm) and 8.2 mm for other cups (48/28 mm and 52/32 mm). Revision surgery for a fracture of an alumina implant is controversial. Failure is infrequent and is easy to solve with a limited revision procedure if performed promptly because osteolysis rarely is encountered. However, early diagnosis of liner fracture is rare, except when ceramic fragments are visible on radiographs. The breakage of a ceramic ball may alter the surface of the Morse taper, and this would lead to fracture of the newly implanted head [20]. Toni et al. [35] reported that a noisy ceramic-bearing THA can be an early clinical sign of liner chipping, fracture, or head stripe wear. They proposed computed tomography to identify cup malposition, ceramic fragments, and low range of mobility and impingement. They also proposed needle aspiration in an attempt to find ceramic fragments. Because the risk of ceramic component fracture still exists, it is mandatory to use a precise surgical technique. Garino [15] suggested placing the cup at 45° or less, increasing anteverted cup placement (greater than 20°), and removal of osteophytes and/or part of the anterior wall of the acetabulum to avoid impingement. Patients should also be informed about the potential for this complication before receiving an alumina-on-alumina coupling.
There was no noise during movement in the current series, although noise generation by an alumina-on-alumina bearing THA is a major concern with this material. Walter et al. [36] report a 0.66% incidence of squeaking in a series of 2397 ceramic-on-ceramic arthroplasties. Patients with squeaking hips were younger, heavier, and taller when compared with patients without squeaking hips [36]. Toni et al. [35] reported a clinically audible hip noise may be correlated with ceramic fracture. Hip squeaking is a peculiar phenomenon unique to hard-on-hard total hips. The causes and implications of squeaking are yet to be determined.
Because of the difficulty in detecting radiolucent lines around uncemented cups, the current method of determining radiographic bone ingrowth into an acetabular component is by indirect inference based on the absence of the two classic signs of loosening, radiolucent lines and cup migration [27]. Although a press-fit metal-backed shell has been used in the current series, the failure of acetabular fixation was the most common reason for revision. Of the five cups that have been revised in this study, three were the result of cup loosening: two loosened as a result of poor press-fit and one loosened 3.7 years postsurgery. There were also five hips with good clinical results at this time but with signs (radiolucent lines around the cup, one with circumferential lucency and four with radiolucent lines in two zones) that may indicate possible loosening. Although the midterm results of HAP-coated femoral stems are promising [6, 7, 16], the HAP-coated smooth hemispheric cup, even with screw fixation, has a high failure rate. HAP coating cannot replace stable mechanical fixation. Soballe [34] reported an HAP coating on a smooth surface had a higher risk of delamination than when applied to an implant with a porous surface. Micromovements between the implant and surrounding tissues would accelerate HAP coating resorption. Capello et al. [6] believed HAP-coated threaded cups performed better than HAP-coated press-fit cups, suggesting implant design is more important than the HAP coating. Lai et al. [25] reported technical errors resulting in a poor press-fit of the cup and/or eccentric placement of the screws produce stresses that allow cup micromovement increasing the risk of cup loosening. The relatively high rate of acetabular lucencies encountered in our series may be problematic, although so far most have been partial, nonprogressive, and not associated with socket migration. We conclude partial socket lucencies possibly indicate limited bone ingrowth through the HAP-coated cup but the areas are sufficient for durable fixation [2]. This has been seen in another animal series using press-fit metal-backed cups with polyethylene liners [4]. Bizot et al. [2] encountered no problems related to modularity of the socket. The locking mechanism of the alumina insert with conical sleeving appeared safe and reliable provided a careful technique was used. For the femoral component, minor technical errors that leave gaps between the prosthesis and the host bone are more easily overcome because the funnel-shaped geometry of the proximal femur is designed to allow osseointegration. In the acetabulum, the geometry is hemispheric with little resistance to rotational stress, and screw fixation alone is not optimal for an HAP-coated hemispheric cup. Press-fit metal-backed sockets provide a major improvement in fixation of the acetabular component in an alumina-on-alumina hip arthroplasty if the surgical technique is adequate and primary stability of the shell is achieved. Vertical placement of the cup was more frequent in loosened cups in our series. The manufacturer has recently improved socket fixation by adjusting surface and alumina liner width. The new titanium alloy shells (implanted since 2004) are 3 mm thick instead of 5 mm, making it possible to increase alumina thickness by 2 mm and consequently improve mechanical resistance. Moreover, the outer macrostructure of the new shells provides a larger contact area for osteointegration, further enhancing secondary stability.
Although the followup of this series is too short to allow definite conclusions, our data suggest these Cerafit alumina-on-alumina prostheses had reasonable outcomes after 5 years. We observed one component fracture associated with trauma and no linear femoral head penetration. Continued followup is required to determine if reduction in wear between the alumina-on alumina bearings results in less osteolysis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Bal BS, Aleto TJ, Garino JP, Toni A, Hendricks KJ. Ceramic-on ceramic versus ceramic-on-polyethylene bearings in total hip arthroplasty: results of a multicenter prospective randomized study and update of modern ceramic total hip trials in the United States. Hip International. 2005;15:129–135. [DOI] [PubMed]
- 2.Bizot P, Hannouche D, Nizard R, Witvoet J, Sedel L. Hybrid alumina total hip arthroplasty using press-fit metal-backed socket in patients younger than 55 years. A six- to 11-year evaluation. J Bone Joint Surg Br. 2004;86:190–194. [DOI] [PubMed]
- 3.Bizot P, Nizard R, Hamadouche M, Hannouche D, Sedel L. Prevention of wear and osteolysis. Alumina-on-alumina bearing. Clin Orthop Relat Res. 2001;393:85–93. [DOI] [PubMed]
- 4.Bobyn JD, Toh KK, Hacking SA, Tanzer M, Krygier JJ. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty. 1999;14:347–354. [DOI] [PubMed]
- 5.Boutin P. Total hip arthroplasty using a ceramic prosthesis. The Classic. Clin Orthop Relat Res. 2000;379:3–11. [DOI] [PubMed]
- 6.Capello WN, D’Antonio JA, Manley MT, Feinberg JR. Hydroxyapatite in total hip arthroplasty. Clinical results and critical issues. Clin Orthop Relat Res. 1998;355:200–211. [DOI] [PubMed]
- 7.D’Antonio J, Capello W, Manley M, Bierbaum B. New experience with alumina-on-alumina ceramic bearings for total hip arthroplasty. J Arthroplasty. 2002;17:390–397. [DOI] [PubMed]
- 8.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 9.Devane PA, Horne JG, Martin K, Coldham G, Krause B. Three-dimensional polyethylene wear of a press-fit titanium prosthesis. Factors influencing generation of polyethylene debris. J Arthroplasty. 1997;12:256–266. [DOI] [PubMed]
- 10.Dorr LD, Faugere M-C, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231–242. [DOI] [PubMed]
- 11.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding and clinical results. J Bone Joint Surg Br. 1987;69:45–55. [DOI] [PubMed]
- 12.Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants. The femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed]
- 13.Garcia-Cimbrelo E, Cruz-Pardos A, Cordero J, Sánchez-Sotelo J. Low-friction arthroplasty in patients younger than 40 years. 20- to 25-year results. J Arthroplasty. 2000;15:825–832. [DOI] [PubMed]
- 14.García-Cimbrelo E, Martínez-Sayanes JM, Minuesa A, Munuera L. Mittelmeier ceramic-ceramic prosthesis after ten years. J Arthroplasty. 1996;11:773–781. [DOI] [PubMed]
- 15.Garino J. Modern ceramic-on-ceramic total hip systems in the United States. Clin Orthop Relat Res. 2000;379:41–47. [DOI] [PubMed]
- 16.Geesink RG, Hoefnagels NHM. Six-year results of hydroxyapatite-coated total hip replacement. J Bone Joint Surg Br. 1995;77:534–547. [PubMed]
- 17.Goetz DD, Smith JJ, Harris WH. The prevalence of femoral osteolysis associated with components inserted with or without cement in total hip replacements. J Bone Joint Surg Am. 1994;76:1121–1128. [DOI] [PubMed]
- 18.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed]
- 19.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty. A minimum 18.5-year follow-up study. J Bone Joint Surg Am. 2002;84:69–77. [PubMed]
- 20.Hannouche D, Nich C, Bizot P, Meunier A, Nizard R, Sedel L. Fractures of ceramic bearings. History and present status. Clin Orthop Relat Res. 2003;417:19–26. [DOI] [PubMed]
- 21.Huo MH, Martin RP, Zatorsky LE, Keggi KJ. Cementless total hip arthroplasties using ceramic-on-ceramic articulation in young patients: a minimum 5-year follow-up study. J Arthroplasty. 1996;11:673–678. [DOI] [PubMed]
- 22.Johnston RC, Fitzgerald RH, Harris WH, Poss R, Müller ME, Sledge CB. Clinical and radiographic evaluation of total hip arthroplasty. A standard system of terminology for reporting results. J Bone Joint Surg Am. 1990;72:161–168. [PubMed]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [DOI]
- 24.Kim Y-H, Kim J-S, Cho S-H. A comparison of polyethylene wear in hips with cobalt-chrome or zirconia heads. A prospective, randomised study. J Bone Joint Surg Br. 2001;83:742–750. [DOI] [PubMed]
- 25.Lai K-A, Shen W-J, Chen C-H, Yang C-Y, Hu W-P, Chang G-L. Failure of hydroxyapatite-coated acetabular cups. Ten-year follow-up of a 85 Landos Atoll arthroplasties. J Bone Joint Surg Br. 2002;84:641–646. [DOI] [PubMed]
- 26.Merle D’Aubigné R, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed]
- 27.Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;444:176–183. [DOI] [PubMed]
- 28.Park Y-S, Hwang S-K, Choy W-S, Kim Y-S, Moon Y-W, Lim S-J. Ceramic failure after total hip arthroplasty with an alumina-on-alumina bearing. J Bone Joint Surg Am. 2006;88:780–787. [DOI] [PubMed]
- 29.Poggie RA, Turgeon TR, Coutts RD. Failure analysis of a ceramic bearing acetabular component. J Bone Joint Surg Am. 2007;89:367–380. [DOI] [PubMed]
- 30.Riska EB. Ceramic endoprosthesis in total hip arthroplasty. Clin Orthop Relat Res. 1993;297:87–94. [PubMed]
- 31.Sedel L. Evolution of alumina-on-alumina implants. A review. Clin Orthop Relat Res. 2000;379:48–54. [DOI] [PubMed]
- 32.Sedel L, Kerboull L, Christel P. Alumina on alumina hip replacement results and survivorship in young patients. J Bone Joint Surg Br. 1990;72:658–663. [DOI] [PubMed]
- 33.Sedel L, Nizard RS, Kerboull L, Witwoet J. Alumina-alumina hip replacement in patients younger than 50 years old. Clin Orthop Relat Res. 1994;298:175–183. [PubMed]
- 34.Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Acta Orthop Scand. 1993;255(Suppl):1–58. [DOI] [PubMed]
- 35.Toni A, Traina F, Stea S, Sudanese A, Visentin M, Bordini B, Squarzoni S. Early diagnosis of ceramic liner fracture. Guidelines based on a twelve-year clinical experience. J Bone Joint Surg Am. 2006;88(Suppl 4):55–63. [DOI] [PubMed]
- 36.Walter WL, O’Toole GC, Walter WK, Ellis A, Zicat BA. Squeaking in ceramic-on-ceramic hips. The importance of acetabular component orientation. J Arthroplasty. 2007;22:496–503. [DOI] [PubMed]