Abstract
Respiratory distress syndrome (RDS) remains a major cause of perinatal morbidity and mortality in premature infants. To avoid iatrogenic RDS, The American College of Obstetricians and Gynecologists (ACOG) recommends that obstetricians confirm fetal pulmonary maturity prior to elective delivery less than 39 weeks’ gestation. However, ACOG based such recommendations primarily on studies carried out prior to the routine use of antenatal corticosteroids to accelerate lung maturity and the liberal use of ultrasound to confirm gestational age. Recent studies suggest that testing for fetal lung maturity at more advanced gestational ages is neither reliable nor cost effective. This article reviews the current literature on the use of amniocentesis to confirm fetal lung maturity prior to elective delivery at 36 to 39 weeks’ gestation.
Key words: Fetal lung maturity, Respiratory distress syndrome, Prematurity, Amniocentesis
Neonatal respiratory distress syndrome (RDS) refers to respiratory compromise presenting at or shortly after birth due specifically to a deficiency of pulmonary surfactant, a naturally occurring phospholipid detergent required to decrease surface tension within the alveoli thereby preventing alveolar collapse. Originally described by Avery and Mead in 1959,1 RDS remains a major cause of neonatal morbidity and mortality. A recent epidemiological study in the United States estimated that there are 80,000 cases of neonatal RDS each year, resulting in 8500 deaths and hospital costs in excess of $4.4 billion.2 Neonatal RDS affects approximately 1% of all live births2–6; however, not all infants are at equal risk. Infants with RDS are nearly always premature, and it remains a major cause of perinatal morbidity and mortality in extremely premature infants. Although preterm delivery may be indicated in many situations, elective delivery of fetuses with immature pulmonary systems should be avoided whenever possible.
Incidence
As mentioned above, neonatal RDS affects approximately 1% of all live births, but affects 10% to 15% of all infants with a birth weight less than 2500 g.4,5,7–9 In the days before antenatal ultrasound to confirm gestational age, iatrogenic prematurity was responsible for approximately 10% to 20% of all cases of RDS; however, recent data demonstrate that this is now an uncommon cause of RDS. Indeed, the increased use of obstetric ultrasound to confirm gestational age and the routine use of antenatal corticosteroids to accelerate fetal lung maturity in women threatening to deliver before 34 weeks’ gestation has resulted in a dramatic decrease in the incidence of RDS over the past 30 years (Figure 1).3–5,10,11
Figure 1.
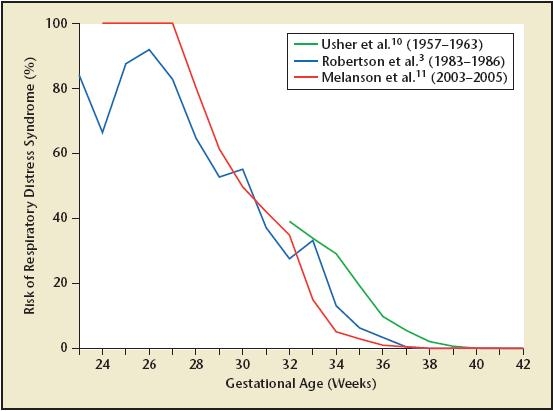
Incidence of RDS by gestational age. These data offer an estimate of the gestational age-specific incidence of RDS in the postcorticosteroid era. The most likely explanation for the observed decrease in neonatal RDS over the past 30 years is the increased utilization of antenatal corticosteroids in women at risk for preterm birth prior to 34 weeks’ gestation, from 0% (data collected 1957–196310) to 20% (data collected 1983–19863) and finally to 84% (data collected 2003–200511).
Despite these improvements, infants who are born in the late preterm period (35–37 weeks’ gestation) are at increased risk of respiratory morbidity compared with infants born at term. Similarly, infants born at 37 weeks are at increased risk of respiratory morbidity compared with infants born at 39 weeks. For example, in 1 recent prospective observational study of 34,458 low-risk births in Denmark, the relative risk of composite neonatal respiratory morbidity (which included transitory tachypnea of the newborn, RDS, and persistent pulmonary hypertension of the newborn) for cesarean delivery prior to the onset of labor during the week 37-0/7 to 37-6/7 compared with the week 38-0/7 to 38-6/7 was 1.74 (95% confidence interval [95% CI], 1.1–2.8; P < .02) and during the week 38-0/7 to 38-6/7 compared with the week 39-0/7 to 39-6/7 was 2.4 (95% CI, 1.2–4.8; P < .02).9
Risk Factors
The pulmonary system is among the last of the fetal organ systems to become functionally mature. As such, RDS is primarily—although not exclusively—a disease of premature infants with an incidence and severity that is highly dependent on gestational age. Other factors that are independently associated with an increased risk of developing RDS include low birth weight, advanced maternal age, cesarean delivery prior to the onset of labor, male sex, maternal diabetes and hypertension, and ethnicity (Table 1). In contrast, antenatal steroid administration, pregnancies complicated by preeclampsia and intrauterine growth restriction, maternal smoking, and documented fetal lung maturity are associated with a decreased risk of developing RDS.
Table 1.
Factors Associated With Neonatal Respiratory Distress Syndrome
| Factors Associated With an Increased |
| Risk of Respiratory Distress Syndrome |
|
| Factors Associated With a Decreased |
| Risk of Respiratory Distress Syndrome |
|
| Factors Associated With No Change |
| in the Risk of Respiratory Distress |
| Syndrome |
|
Current Recommendations for the Prevention of Neonatal RDS
Surfactant refers to the endogenous detergentlike compounds that reduce surface tension at the air-water interface within the alveolar spaces. The pressure needed to distend the lungs and prevent alveolar collapse is thereby diminished, allowing for continuous and maximally effective gas exchange. Surfactant is composed primarily of phosphatidylcholine (lecithin), but also other phospholipids (phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol), cholesterol, and a variety of proteins. To avoid iatrogenic RDS, The American College of Obstetricians and Gynecologists (ACOG) defined a series of clinical and/or sonographic criteria that allow an obstetric care provider to infer fetal pulmonary maturity based on a gestational age greater than or equal to 39 weeks prior to elective delivery (Table 2).4,5 If such criteria are not met and elective delivery is being considered prior to 39 weeks’ gestation, ACOG recommends that fetal pulmonary maturity be confirmed prior to delivery.4,5
Table 2.
Acceptable Criteria for Confirmation of Gestational Age
| Clinical Criteria |
|
| Laboratory Criteria |
|
Data from The American College of Obstetricians and Gynecologists.4
Fetal pulmonary maturity can be assessed by the direct or indirect measurement of surfactant phospholipids secreted by the fetal lungs into amniotic fluid. Several such tests have been developed in an attempt to prevent iatrogenic neonatal RDS. All require sampling of amniotic fluid, usually by amniocentesis. These tests can be categorized broadly into 2 groups: (1) biochemical tests, including lecithin/sphingomyelin ratio (L/S ratio), phosphatidylglycerol (PG), and surfactant-to-albumin ratio (TDx FLM II [Abbott Laboratories, Abbott Park, IL]); and (2) biophysical tests, including the foam stability index (FSI) and lamellar body count, which tests the surface-active effects of these bioactive phospholipids. These tests have equivalent accuracy and are better at predicting the likely absence rather than the presence of neonatal RDS. No test is infallible and all perform less well at earlier gestational ages, which should be taken into account when interpreting results. These tests are summarized in Table 3.
Table 3.
Tests Available for Confirmation of Fetal Lung Maturity
| Predictive | Predictive | ||||
|---|---|---|---|---|---|
| Positive | Value for | Value for | |||
| Discriminating | Pulmonary | Pulmonary | Relative | ||
| Test | Value | Maturity (%)* | Immaturity (%)* | Cost | Comments |
| L/S ratio | > 2.0 | 95–100 | 33–50 | High | Time-consuming study (4 h) |
| Large interlaboratory variation | |||||
| Affected by blood or meconium | |||||
| Can use a pooled vaginal sample | |||||
| PG | Present | 95–100 | 23–53 | High | Not affected by blood or meconium |
| Can use a pooled vaginal sample | |||||
| TDx FLM II | ≥ 55 mg surfactant/ | 96–100 | 47–61 | Moderate | Simple, rapid test (10 min) |
| assay (Abbott | 1 g albumin | Minimal inter- and intra-assay variability | |||
| Laboratories, | |||||
| Abbott Park, IL) | |||||
| Foam stability | > 47 | 95 | 51 | Low | Affected by blood or meconium |
| index | Simple, rapid test | ||||
| Use of silicon tubes can give a | |||||
| false-positive result | |||||
| Lamellar | > 50,000 lamellar | 97–98 | 29–35 | Low | Investigational |
| body count | bodies/µL |
Data are reported independent of gestational age.
L/S, lethicin/sphingomyelin ratio; PG, phosphatidylglycerol.
Lecithin/Sphingomyelin Ratio
The L/S ratio is one of the oldest, best validated, and most widely used of these tests. It is based on the original observations by Gluck and colleagues12,13 that lecithin (but not sphingomyelin) in the fetal pulmonary secretions increases after 32 to 33 weeks’ gestation, and that neonatal RDS was uncommon after the L/S ratio reached 2.0, which occurs around 35 weeks in uncomplicated pregnancies. This cutoff has remained an accepted threshold for the determination of fetal pulmonary maturity in nondiabetic pregnancies regardless of gestational age. That said, however, a threshold value for prediction of lung maturity should be established independently at each tertiary care center by correlating the L/S ratio with clinical outcome because the variation within and between laboratories can be considerable. This should be done separately for diabetic and nondiabetic pregnancies.
The L/S ratio is a thin layer chromatography assay, which is difficult to perform and interpret, and requires approximately 4 hours of effort from a dedicated laboratory technician. Care at each step of sample handling is critical for consistent results. For example, if transport to a remote laboratory is required, the amniotic fluid sample should be maintained on ice or refrigerated to avoid activation of enzymes that, at room temperature, would adversely affect the L/S ratio. Moreover, the presence of blood or meconium in amniotic fluid can interfere with test interpretation.
Phosphatidylglycerol
This test measures the presence or absence of PG in the amniotic fluid. PG is a minor constituent of surfactant that begins to increase appreciably in amniotic fluid several weeks after the rise in lecithin. Because PG enhances the dispersion of phospholipids throughout the alveoli, its presence indicates an advanced state of fetal lung development and function. Although not widely used, 1 advantage of this test is that PG determination is not generally affected by blood, meconium, or other contaminants.
Surfactant-to-Albumin Ratio
The TDx FLM II assay uses polarized light to quantify the competitive binding of a probe to both albumin and surfactant in amniotic fluid using an automatic analyzer; thus, it is a direct measurement of surfactant concentration. Results are expressed as milligrams of surfactant per gram of albumin. An elevated ratio has been correlated with the presence of fetal lung maturity, although the presence of blood and/or meconium in amniotic fluid can interfere with the interpretation of the test. According to the recommendations of the manufacturer, the threshold for lung maturity is 55 mg/g or higher; a measurement of less than 40 mg/g should be regarded as immature and levels of 40 to 54 mg/g should be regarded as borderline and requiring a secondary test to decide whether or not to proceed with delivery (often the L/S ratio).
Foam Stability Index
This test predicts fetal lung maturity based upon the ability of surfactant to generate stable foam in the presence of ethanol. To perform this test, 0.5 mL of amniotic fluid is added to each well in the test kit containing serial dilutions of ethanol. The amniotic fluid-ethanol mixture is then shaken. If surfactant is present, a stable ring of foam will be evident in the presence of 100% ethanol. The FSI is then read as the highest value well in which a ring of stable foam persists. The discriminating value indicative of lung maturity is usually set at 47 or higher. Because this is a functional test, a positive result will virtually exclude the risk of subsequent neonatal RDS; however, a negative test can often be seen even in the presence of mature lungs. The presence of blood or meconium will interfere with the results of the FSI test, and the test should not be relied upon in such situations.
Lamellar Body Count
This test is a direct measurement of surfactant production by type II pneumocyte cells within the fetal lungs. Because the size of lamellar bodies is similar to that of platelets, a standard Coulter counter can be used to identify and quantify their presence in amniotic fluid. Lamellar body counts of less than 15,000 per microliter suggest pulmonary immaturity, whereas counts of 50,000 per microliter or greater suggest pulmonary maturity. An intermediate value requires the use of a secondary test to decide whether to proceed with delivery (often the L/S ratio).
Revisiting Recommendations for the Prevention of Neonatal RDS at 36–39 Weeks of Gestation
Tests on amniotic fluid are designed to predict pulmonary maturity (viz, the absence of subsequent neonatal RDS) with a greater certainty than pulmonary immaturity (viz, the presence of subsequent neonatal RDS).2 Indeed, the reported positive predictive values of an immature test result range from less than 20% to over 60%.5,7,14–16 This wide variation is not unexpected, because the predictive value of any test is dependent on the prevalence of the disease in the population, and the prevalence of RDS is dependent in large part on gestational age.
In the absence of clinical conditions that may delay fetal pulmonary maturation thereby increasing the risk of neonatal RDS (such as maternal diabetes, nonhypertensive renal disease, fetal isoimmunization [Table 1]), parturients delivering after 36 weeks represent a population with a very low risk of delivering a fetus with RDS.3,4,10 Even in the unlikely event that an assay for the assessment of fetal lung maturity was developed that had a 99% sensitivity and 99% specificity, the predictive value for such a test near term would be less than 50%.17,18 This mathematical model is borne out by reports showing that around 50% of all fetal lung maturity test results indicating immaturity are inaccurate.4,14 A falsely immature test after 34 weeks’ gestation may delay an otherwise appropriate early delivery.
ACOG states that, irrespective of the test used, the predictive value of a mature test result is over 95%.4 Stated differently, if the result of any test indicates pulmonary maturity, the likelihood that that infant will develop RDS is less than 5%. However, this statement fails to account for gestational age. Despite extensive data demonstrating that gestational age is a major and independent risk factor for RDS, individual fetal lung maturity tests all have a single recommended cutoff value regardless of gestational age. The limitations of such an approach have been elegantly demonstrated in several recent studies.18,19 For example, McElrath and colleagues19 studied 415 mother-neonate pairs who delivered at the Brigham and Women’s Hospital (Boston, MA) between 1998 and 2000 and who had had fetal lung maturity assessment within 72 hours of delivery. Fetal lung maturity testing was by TDx FLM II assay. The overall incidence of RDS in this cohort was 6.7% (28/415). Using these data, logistic regression model was generated to predict the probability of RDS for any combination of gestational age and TDx FLM II data pairs. The theoretic TDx FLM II cutoff for a defined probability for RDS at any given gestational age was calculated. For example, if we assume that the desired clinical cutoff of predicted probability for RDS is 15%, this would correspond to a TDx FLM II result of 60 mg/g or higher at 28 weeks, 50 mg/g or higher at 30 weeks, 40 mg/g or higher at 33 weeks, 30 mg/g or higher at 35 weeks, 25 mg/g or higher at 36 weeks, and 20 mg/g or higher at 37 weeks (Table 4).19 These data support the conclusion that a single cutoff value for the TDx FLM II assay irrespective of gestational age is not appropriate. The same is likely true of the other fetal lung maturity tests. Indeed, a meta-analysis published in 1984 reported that an L/S ratio of less than 2.0 after 34 weeks’ gestation “is only slightly better than accurate assessment of gestational age” at predicting RDS.17 In this meta-analysis, the positive predictive value of an immature L/S test at 34 to 37 weeks was 20% (10/50) with an overall incidence of RDS of 12%; at 37 or more weeks, the positive predictive value was 9% (3/35) with an overall incidence of RDS of 2%.17
Table 4.
Predicted Risk of RDS by Gestational Age and TDx FLM II Surfactant-to-Albumin Ratio II Value
| Gestational | TDx FLM II Surfactant-to-Albumin Ratio Cutoff Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | (mg Surfactant/g Albumin) | |||||||||
| (Weeks) | 0 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| 28 | 88% | 67% | 53% | 38% | 25% | 15% | 9% | 5% | 3% | 2% |
| 29 | 84% | 61% | 46% | 32% | 20% | 12% | 7% | 4% | 2% | 1% |
| 30 | 81% | 55% | 40% | 26% | 16% | 10% | 5% | 3% | 2% | 1% |
| 31 | 76% | 48% | 34% | 22% | 13% | 7% | 4% | 2% | 1% | 1% |
| 32 | 71% | 42% | 28% | 18% | 10% | 6% | 3% | 2% | 1% | 1% |
| 33 | 66% | 36% | 23% | 14% | 8% | 5% | 3% | 1% | 1% | <1% |
| 34 | 59% | 30% | 19% | 11% | 6% | 4% | 2% | 1% | 1% | <1% |
| 35 | 53% | 25% | 15% | 9% | 5% | 3% | 2% | 1% | <1% | <1% |
| 36 | 46% | 20% | 12% | 7% | 4% | 2% | 1% | 1% | <1% | <1% |
| 37 | 40% | 16% | 10% | 5% | 3% | 2% | 1% | <1% | <1% | <1% |
| 38 | 34% | 13% | 8% | 4% | 2% | 1% | 1% | <1% | <1% | <1% |
| 39 | 28% | 10% | 6% | 3% | 2% | 1% | 1% | <1% | <1% | <1% |
| 40 | 23% | 8% | 5% | 3% | 1% | 1% | <1% | <1% | <1% | <1% |
A single TDx FLM II value cutoff is currently recommended to predict risk for neonatal RDS for all pregnancies regardless of gestational age.
This is clearly inappropriate. The TDx FLM II cutoff value to predict a risk of neonatal RDS of approximately 15% at each gestational age is highlighted.
RDS, respiratory distress syndrome.
Reprinted with permission from McElrath TF et al.19
Although testing for fetal lung maturity may be useful in many clinical situations, it is not clear from the data presented above that there is benefit after 36 weeks’ gestation. Moreover, although the risks of amniocentesis at this late gestational age (including preterm premature rupture of the membranes, placental abruption, isoimmunization, infection, direct trauma to the fetus, and/or fetal death) are likely small and, as such, difficult to quantify, this procedure is not without risk.
Reexamining the Severity of Neonatal RDS
Neonatal RDS has typically been regarded as a binary clinical event (viz, present or absent). As such, little has been published on the gestational age-specific severity of neonatal RDS. Existing data suggest that there is an almost linear decline in mechanical ventilatory support and oxygen therapy 24 and 28 weeks’ gestation, which decreases even further between 29 and 35 weeks, and thereafter approaches 0.20 These data demonstrate a dramatic fall in the severity of RDS between 24 and 28 weeks of gestation. After 28 weeks, the severity of RDS appears to be mild and changes little with increasing gestational age. These observations suggest that a physiologic developmental milestone may be reached within the pulmonary system at around 27 to 28 weeks. This represents the early alveolar (terminal sac) period of fetal pulmonary development, which follows the canalicular period (16–24 weeks) and is characterized by bronchiolar division leading to the development of thin spherical saccules (alveoli) that are lined by type II pneumocytes, the cells responsible for surfactant production.
Cost-Effectiveness of Amniocentesis at 36 to 39 Weeks of Gestation to Prevent Iatrogenic Neonatal RDS
In addition to performance, other test characteristics—such as cost, ease of test performance, availability, and reproducibility—are important factors in considering whether or not to proceed with amniocentesis and fetal lung maturity testing (Table 3).4,15 Unfortunately, the data on cost-effectiveness of fetal lung maturity testing are limited. Using a Markov decision analytic model, Myers and colleagues14 investigated the cost-effectiveness of 3 separate strategies for the prevention of RDS resulting from preterm labor: (a) routine tocolysis and corticosteroid therapy (TREATALL); (b) amniocentesis and testing for fetal lung maturity using the TDx FLM II assay with treatment based on test results (TESTALL); and (c) no treatment. The probabilities of delivery and subsequent RDS over the 7-day time frame of the analysis demonstrated that the most cost-effective strategy varied with the probability of RDS and, as such, with gestational age. TREATALL was the most cost-effective strategy when the probability of developing RDS was greater than 17% (before 34 weeks), whereas TESTALL was most cost effective when the probability was 2% to 17% (34–36 weeks). Both TREATALL and TESTALL were cost saving as compared with no treatment when the probability was higher than 2% (before 36 weeks). However, even in this model, where the gestational age-specific risks of RDS were likely over-estimated due to the use of older literature to obtain the probability variables, it was most cost effective to use no fetal lung maturity testing and no treatment when the probability of developing RDS was 2% or less (after 36 weeks).14 A similar cost-effectiveness analysis published in 1984 showed no benefit to treatment after 34 weeks’ gestation, although this report did not use formal decision analysis techniques.21
Two alternative approaches should be considered: first, the use of a probabilistic model or formula that incorporates both gestational age and the results of the fetal lung maturity test to more accurately predict the risk of neonatal RDS. Such a model has been developed,19 but has not yet been tested prospectively. Second, existing data suggest that it would be reasonable to proceed with delivery at 36 to 39 weeks’ gestation after a detailed discussion with the patient and her family regarding the risks and benefits of amniocentesis for documentation of fetal lung maturity. This discussion should include such issues as the risks of amniocentesis (rupture of membranes, bleeding, injury to the fetus, isoimmunization) as well as the absolute risk of developing RDS at this gestational age (< 5%), and the severity of RDS at this gestational age should it develop (on average, 1 day of respiratory support with no long-term sequelae). The discussion and informed consent should be documented in the patient’s medical record.
Conclusions
Optimal timing of delivery is a critical determinant of perinatal outcome. When faced with a pregnancy complication and possibility of a premature birth, the obstetric care provider’s knowledge of the lung maturity status of the fetus may assist in deciding about timing of delivery. Amniocentesis and use of one or more biochemical tests to measure the surface-active properties of surfactant phospholipids secreted by the fetal lungs into the amniotic fluid remains the only reliable technique for predicting risk for neonatal RDS. If elective delivery is being considered prior to 39 weeks’ gestation, ACOG currently recommends that fetal pulmonary maturity be confirmed to minimize the incidence of iatrogenic RDS.4,5 However, such recommendations are based primarily on studies carried out prior to the routine use of antenatal corticosteroids to accelerate lung maturity and the liberal use of ultrasound to confirm gestational age. Moreover, despite technological advances in the field, recent studies suggest that testing for fetal lung maturity at a more advanced gestational age (> 36 weeks) is neither reliable17,18 nor cost effective.14 Taken together, these data mandate reconsideration of our current recommendation of amniocentesis to confirm fetal lung maturity prior to elective delivery at 36 to 39 weeks’ gestation in well-dated pregnancies.
Main Points.
Neonatal respiratory distress syndrome (RDS) is a major cause of neonatal morbidity and mortality.
Infants who are born in the late preterm period (35–37 weeks’ gestation) are at increased risk of respiratory morbidity compared with infants born at term.
RDS is primarily a disease of premature infants with an incidence and severity that is highly dependent on gestational age. Other factors that are independently associated with an increased risk of developing RDS include low birth weight, advanced maternal age, cesarean delivery prior to the onset of labor, male sex, maternal diabetes and hypertension, and ethnicity.
Several tests have been developed in an attempt to prevent iatrogenic neonatal RDS. All require sampling of amniotic fluid, usually by amniocentesis. These tests can be categorized into 2 groups: (1) biochemical tests, including lecithin/sphingomyelin ratio, phosphatidylglycerol, and surfactant-to-albumin ratio; and (2) biophysical tests, including the foam stability index and lamellar body count.
Despite extensive data demonstrating that gestational age is a major and independent risk factor for RDS, individual fetal lung maturity tests all have a single recommended cutoff value regardless of gestational age.
Despite technological advances in the field, recent studies suggest that testing for fetal lung maturity at a more advanced gestational age (> 36 weeks) is neither reliable nor cost effective. Data mandate reconsideration of our current recommendation of amniocentesis to confirm fetal lung maturity prior to elective delivery at 36 to 39 weeks’ gestation in well-dated pregnancies.
References
- 1.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Clermont G, et al. Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Resp Crit Care Med. 2001;164:1154–1160. doi: 10.1164/ajrccm.164.7.2012126. [DOI] [PubMed] [Google Scholar]
- 3.Robertson PA, Sniderman SH, Laros RK, Jr, et al. Neonatal morbidity according to gestational age and birth weight from five tertiary care centers in the United States, 1983 through 1986. Am J Obstet Gynecol. 1992;166:1629–1641. doi: 10.1016/0002-9378(92)91551-k. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists, authors. Fetal Maturity Assessment Prior to Elective Repeat Cesarean Delivery. Washington, DC: American College of Obstetricians and Gynecologists; 1991. Committee Opinion No. 98. [Google Scholar]
- 5.American College of Obstetricians and Gynecologists, authors. Assessment of fetal lung maturity. (Educational Bulletin No. 230).Int J Gynaecol Obstet. 1997;56:191–198. [PubMed] [Google Scholar]
- 6.Fantz CR, Powell C, Karon B, et al. Assessment of the diagnostic accuracy of the TDx FLM II to predict fetal lung maturity. Clin Chem. 2002;48:761–765. [PubMed] [Google Scholar]
- 7.Lewis DF, Futayyeh S, Towers CV, et al. Preterm delivery from 34 to 37 weeks of gestation: is respiratory distress syndrome a problem? Am J Obstet Gynecol. 1996;174:525–528. doi: 10.1016/s0002-9378(96)70421-0. [DOI] [PubMed] [Google Scholar]
- 8.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstet Gynecol Scand. 2007;86:389–394. doi: 10.1080/00016340601159256. [DOI] [PubMed] [Google Scholar]
- 9.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. Br Med J. 2008;336:85–87. doi: 10.1136/bmj.39405.539282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usher RH, Allen AC, McLean FH. Risk of respiratory distress syndrome related to gestational age, route of delivery, and maternal diabetes. Am J Obstet Gynecol. 1971;111:826–832. doi: 10.1016/0002-9378(71)90495-9. [DOI] [PubMed] [Google Scholar]
- 11.Melanson SE, Berg A, Jarolim P, et al. Validation of a formula that calculates the estimated risk of respiratory distress syndrome. Obstet Gynecol. 2006;108:1471–1476. doi: 10.1097/01.AOG.0000245785.70216.8a. [DOI] [PubMed] [Google Scholar]
- 12.Gluck L, Kulovich MV, Borer RC, Jr, et al. Diagnosis of the respiratory distress syndrome by amniocentesis. Am J Obstet Gynecol. 1971;109:440–445. doi: 10.1016/0002-9378(71)90342-5. [DOI] [PubMed] [Google Scholar]
- 13.Gluck L, Kulovich MV, Borer RC, Jr, Keidel WN. The interpretation and significance of the lecithin-sphingomyelin ratio in amniotic fluid. Am J Obstet Gynecol. 1974;120:142–155. doi: 10.1016/0002-9378(74)90194-x. [DOI] [PubMed] [Google Scholar]
- 14.Myers ER, Alvarez JG, Richardson DK, Ludmir J. Cost-effectiveness of fetal lung maturity testing in preterm labor. Obstet Gynecol. 1997;90:824–829. doi: 10.1016/S0029-7844(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 15.Herbert WNP, Chapman JF. Clinical and economic considerations associated with testing for fetal lung maturity. Am J Obstet Gynecol. 1986;155:820–823. doi: 10.1016/s0002-9378(86)80029-1. [DOI] [PubMed] [Google Scholar]
- 16.Brown LM, Duck-Chong CG. Methods of evaluating fetal lung maturity. Crit Rev Clin Lab Sci. 1982;16:85. doi: 10.3109/10408368209107026. [DOI] [PubMed] [Google Scholar]
- 17.Creasy GW, Simon NV. Sensitivity and specificity of the L/S ratio in relation to gestational age. Am J Perinatol. 1984;1:302–305. doi: 10.1055/s-2007-1000026. [DOI] [PubMed] [Google Scholar]
- 18.Tanasijevic MJ, Wybenga DR, Richardson D, et al. A predictive model for fetal lung maturity employing gestational age and test results. Am J Clin Pathol. 1994;102:788–793. doi: 10.1093/ajcp/102.6.788. [DOI] [PubMed] [Google Scholar]
- 19.McElrath TF, Colon I, Hecht J, et al. Neonatal respiratory distress syndrome as a function of gestational age and an assay for surface-to-albumin ratio. Obstet Gynecol. 2004;103:463–468. doi: 10.1097/01.AOG.0000113622.82144.73. [DOI] [PubMed] [Google Scholar]
- 20.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101:178–193. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 21.Korenbrot CC, Aalto LH, Laros RK. The cost effectiveness of stopping preterm labor with beta-adrenergic treatment. N Engl J Med. 1984;310:691–696. doi: 10.1056/NEJM198403153101105. [DOI] [PubMed] [Google Scholar]


