Abstract
Over the past decade, significant advances have occurred in the diagnosis and treatment of reproductive disorders. In this review, we discuss the routine testing performed to diagnose unexplained infertility. We also discuss additional testing, such as assessment of ovarian reserve, and the potential role of laparoscopy in the complete workup of unexplained infertility. Finally, we outline the available therapeutic options and discuss the efficacy and the cost-effectiveness of the existing treatment modalities. The optimal treatment strategy needs to be based on individual patient characteristics such as age, treatment efficacy, side-effect profile, and cost considerations.
Key words: Infertility, unexplained; Semen analysis; Ovulation assessment; Ovarian reserve; Intrauterine insemination; Controlled ovarian hyperstimulation; In vitro fertilization
Significant advances have occurred in the diagnosis and, more importantly, in the treatment of reproductive disorders over the past decade. The overall incidence of infertility has remained stable1; however, the success rates have markedly improved with the widespread use of assisted reproductive technologies. Treatment options and success vary with the cause of infertility. Approximately 15% to 30% of couples will be diagnosed with unexplained infertility after their diagnostic workup.2 In this review, we specifically discuss the routine testing performed to diagnose unexplained infertility. We also discuss additional testing, such as assessment of ovarian reserve, and the potential role of laparoscopy in the complete workup of unexplained infertility. Finally, we outline the available therapeutic options and discuss the efficacy and the cost-effectiveness of the existing treatment modalities.
Diagnosis of Unexplained Infertility: The Basic Infertility Evaluation
Infertility is customarily defined as the inability to conceive after 1 year of regular unprotected intercourse. The infertility evaluation is typically initiated after 1 year of trying to conceive, but in couples with advanced female age (> 35 years), most practitioners initiate diagnostic evaluation after an inability to conceive for 6 months. The Practice Committee of the American Society for Reproductive Medicine (ASRM) has published guidelines for a standard infertility evaluation.3 It includes a semen analysis, assessment of ovulation, a hysterosalpingogram, and, if indicated, tests for ovarian reserve and laparoscopy. When the results of a standard infertility evaluation are normal, practitioners assign a diagnosis of unexplained infertility. Although estimates vary, the likelihood that all such test results for an infertile couple are normal (ie, that the couple has unexplained infertility) is approximately 15% to 30%.2
Assessment of Male Infertility
Male factor infertility is the only cause of infertility in approximately 30% of couples and a contributing factor in another 20% to 30%.4 Assessment of the infertile couple includes evaluation of the male partner by history, examination, and semen analysis. Important elements of the history include prior paternity, a history of cryptorchidism, medical and surgical history, sexual dysfunction, and any use of medications, tobacco, alcohol, or illicit drugs. On the physical examination, testicular abnormalities such as a varicocele or absence of the vas deferens can be detected.
If the semen analysis is abnormal, it should be repeated after at least 1 month by a laboratory that adheres to World Health Organization (WHO) guidelines, with a quality control program ensuring accurate testing. Guidelines from WHO regarding the reference ranges for the number, morphology, and motility in a semen sample have been published5 and are shown in Table 1. A number of commercial laboratories use a variety of ranges for the different components of the semen analysis. Careful attention should be paid to these ranges and the semen values should be interpreted in the right context. Although semen analysis is routinely used to evaluate the male partner in infertile couples, the discriminatory ranges are not clearly defined. A study by Guzick and colleagues concluded “threshold values for sperm concentration, motility, and morphology can be used to classify men as subfertile, of indeterminate fertility, or fertile. None of the measures, however, are diagnostic of infertility.”6 Table 2 shows the reference values for fertile, indeterminate, and subfertile ranges for semen analysis parameters as identified by regression analysis. Despite its limitations, semen analysis remains the most important tool in the investigation of male factor infertility. If any abnormalities are repeatedly detected on a semen analysis, referral to a urologist may be warranted. The treatment of severe male factor infertility including azoospermia has been revolutionized with the combination of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).
Table 1.
World Health Organization Criteria for a Normal Semen Analysis
| Criteria | Parameters |
|---|---|
| Volume | 2.0–5.0 mL |
| pH | 7.2–7.8 |
| Sperm concentration | ≥ 20 × 106/mL |
| Total sperm count | ≥ 40 × 106 spermatozoa |
| Motility | ≥ 50% with forward progression or ≥ 25% with rapid |
| linear progression within 60 min after collection | |
| Morphology | ≥ 50% with normal morphology |
| Viability | ≥ 75% live (ie, excluding dye) |
| White blood cells | ≤ 1 × 106/mL |
| Fructose (total) | ≥ 13 mol/ejaculate |
Adapted from Ann Ist Super Sanita. 2001;37:I–XII, 1–123.5
Table 2.
Fertile, Indeterminate, and Subfertile Ranges for Semen Analysis Parameters as Identified by Regression Analysis
| Semen Measurement |
|||
|---|---|---|---|
| Concentration | Motility | Morphology | |
| Variable | (× 106/mL) | (%) | (% Normal) |
| Fertile range | > 48.0 | > 63 | > 12 |
| Indeterminate range | 13.5–48.0 | 32–63 | 9–12 |
| Univariate odds ratio | 1.5 (1.2–1.8) | 1.7 (1.5–2.2) | 1.8 (1.4–2.4) |
| for infertility (95% CI) | |||
| Subfertile range | < 13.5 | < 32 | < 9 |
| Univariate odds ratio | 5.3 (3.3–8.3) | 5.6 (3.5–8.3) | 3.8 (3.0–5.0) |
| for infertility (95% CI) | |||
95% CI, 95% confidence interval.
Reprinted with permission from Guzick DS et al. N Engl J Med. 2001;345:1388–1393. Copyright © 2001 Massachusetts Medical Society. All rights reserved.
Previously, the postcoital test (PCT) assessing the sperm motility in a sample of postcoital cervical mucus was considered an integral part of the basic infertility evaluation. However, past investigations revealed a poor correlation between postcoital sperm motility and pregnancy outcome.7 In addition, a 1995 blinded, prospective study demonstrated poor reproducibility of the test among trained observers, further questioning the validity of the PCT as a diagnostic tool.8 Today, the PCT has been largely abandoned and we do not recommend it as a component of the standard infertility investigation.3
Assessment of Ovulation
Ovulatory defects are present in 40% of infertile women and in approximately 15% of couples with infertility.3 Often a defect in ovulatory function manifests itself in menstrual disturbances and can be identified by history in the majority of women. A patient with menstrual abnormalities should be investigated for underlying causes such as polycystic ovarian syndrome, thyroid disease, hyperprolactinemia, and hypothalamic causes secondary to weight changes. Eumenorrhea—normal menstrual cycles by history—is a highly accurate marker of ovulation and anovulatory levels of serum progesterone (< 3 ng/mL) are found in only a very small minority of eumenorrheic patients.9
In addition to a thorough menstrual history, other methods used to evaluate ovulation include basal body temperature (BBT) recordings, urinary luteinizing hormone (LH) ovulation predictor kits, mid luteal serum progesterone testing, and endometrial biopsy to assess for secretory endometrial development. Although BBT recordings are the least costly tool in a reliable patient (Figure 1), they are difficult to interpret and often frustrating for the patient. Ovulation predictor kits are useful for women who do not have very long menstrual cycles and can be used by couples to appropriately time intercourse. Mid luteal progesterone levels are measured around day 21 in women with regular (∼ 28 day) cycles. However, they are often poorly timed if they are drawn on cycle day 21 in women with irregular menses. In such women it is better to use an ovulation kit and measure the progesterone levels 7 to 8 days after the LH surge is detected. Serum progesterone levels higher than 3 ng/mL suggest that ovulation has occurred and levels higher than 10 ng/mL are optimum. Although endometrial biopsy results were previously used to diagnose luteal phase defect, they do not correlate with fertility status and hence are no longer recommended.10
Figure 1.

An example of a basal body temperature recording chart. Reprinted with permission from Hyde and DeLamater, Understanding Human Sexuality, 6th ed. Copyright ©1997 The McGraw-Hill Companies, Inc. All rights reserved.
Assessment of Ovarian Reserve
Another test added to the workup of couples with infertility includes assessment of ovarian reserve. Women with advanced age or history of prior ovarian surgery are at risk for diminished ovarian function or reserve. Given the relatively noninvasive nature of the testing, several practitioners are including the evaluation of ovarian reserve as first-line workup for infertility. The testing includes a cycle day 3 serum follicle-stimulating hormone (FSH) and estradiol level, clomiphene citrate challenge test, and/or an ultrasonographic ovarian antral follicle count.3 The results of these tests are not absolute indicators of infertility but abnormal levels correlate with decreased response to ovulation induction medications and lowered live birth rates after IVF.
Assessment of Uterus and Fallopian Tubes
Assessment of the uterine contour and the tubal patency is an integral part of the basic infertility evaluation.3 This may be achieved by hysterosalpingography (HSG) (Figure 2). An HSG consists of radiographic evaluation of the uterine cavity and fallopian tubes after injection of a radio-opaque medium through the cervical canal. Along with laparoscopic dye pertubation, it can best assess tubal patency: the concordance of HSG with laparoscopic dye pertubation is estimated as close to 90%.11 However, patent fallopian tubes on HSG do not confirm that ovum pickup will occur. For example, women with severe endometriosis may have adherent ovaries in the cul de sac with normal fallopian tubes.
Figure 2.
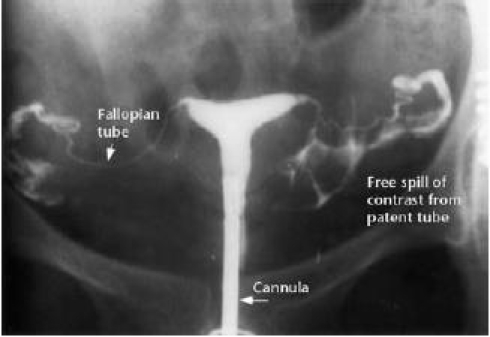
Normal hysterosalpingogram. Reprinted from Fertility and Sterility, Volume 83, Baramki TA, Hysterosalpingography, pages 1595–1606, Copyright 2005, with permission from Elsevier.
Ultrasound evaluation in the follicular phase is used to identify uterine fibroids, polyps, and congenital cavitary anomalies such as a septate uterus. At the same time, information on ovarian volume and antral follicle counts can be obtained, making pelvic ultrasound part of the initial workup for infertility. A complete cavitary assessment, however, necessitates either sonohysterography for conditions such as uterine polyps, submucus leiomyomas, or Asherman’s syndrome (uterine synechiae).12 Sonohysterography, an office procedure, involves assessing the uterine cavity with ultrasound with concurrent instillation of sterile water. Some practitioners prefer diagnostic office hysteroscopy as it allows direct visualization of the uterine cavity.
The Role of Laparoscopy in the Infertility Evaluation
The role of laparoscopy in the investigation of infertility has changed over the past decade. Whereas laparoscopy used to be part of the basic infertility workup, it is now reserved for selected cases. Given that it allows direct visual examination of the pelvic reproductive anatomy, it is the test of choice to identify otherwise unrecognized peritoneal factors that influence fertility, specifically endometriosis and pelvic adhesions. According to the guidelines of the ASRM, laparoscopy should be performed in women with unexplained infertility or signs and symptoms of endometriosis or in whom reversible adhesive tubal disease is suspected.3
Recommendations for the Basic Infertility Evaluation
We recommend that couples have a semen analysis, testing for detection of ovulation (mid luteal progesterone, LH kit), assessment of ovarian reserve, transvaginal ultrasound, and HSG. With this expanded testing, fewer than 15% to 30% of couples will have unexplained infertility.
Treatment of Unexplained Infertility
A diagnosis of unexplained infertility is made after the above-recommended testing fails to reveal any abnormality. The treatment for unexplained infertility is therefore, by definition, empiric because it does not address a specific defect or functional impairment.2 The principal treatments for unexplained infertility include expectant observation with timed intercourse and lifestyle changes, clomiphene citrate and intrauterine insemination (IUI), controlled ovarian hyperstimulation (COH) with IUI, and IVF. We present the different treatment options and discuss the advantages and disadvantages of various treatment strategies.
Expectant Management and Lifestyle Changes
Epidemiological studies indicate cigarette smoking, abnormal body mass index (BMI), and excessive caffeine and alcohol consumption reduce fertility in the female partner. The female partner should be counseled to achieve a normal BMI, reduce caffeine intake to no more than 250 mg daily (2 cups of coffee), and reduce alcohol intake to no more than 4 standardized drinks per week.13
The likelihood of pregnancy without treatment among couples with unexplained infertility is less than that of fertile couples but greater than zero. It is possible that unexplained infertility represents the lower extreme of the normal distribution of fertility with no defect present. However, it could also be imagined that the routine infertility evaluation misses subtle defects because of imperfect or incomplete testing methods. Studies of couples with unexplained infertility who are followed without any treatment report a broad variation in cumulative pregnancy rates. A retrospective review of 45 studies by Guzick and colleagues found an average cycle fecundity of 1.3% to 4.1% in the untreated groups, which was lower than most treatment interventions.14 In a recent study, couples with unexplained infertility on a waiting list for IVF in the Netherlands had a 10% to 15% cumulative chance of pregnancy over a 12-month period. As expected, the age of the female partner influenced the pregnancy rate associated with expectant management.15 The currently available evidence suggests that methods prospectively identifying the window of fertility are likely to be more effective for optimally timing intercourse than calendar calculations or BBT. Although expectant management is associated with the lowest cost, it results in the lowest cycle fecundity rates, and is therefore inferior to the commonly available reproductive techniques outlined below. It may provide an option for a couple with unexplained infertility in whom the female partner is young and the problem of oocyte depletion is not an immediate concern.
Laparoscopy as a Treatment
Whether operative laparoscopy improves pregnancy outcomes in a subject with unexplained or minimal/mild endometriosis is of debate. In 1997, Marcoux and colleagues reported the results of a randomized, controlled trial (RCT) in a population of 341 infertile women 20 to 39 years of age with minimal or mild endometriosis.16 During diagnostic laparoscopy the women were randomly assigned to undergo resection or ablation of visible endometriosis or diagnostic laparoscopy only. They were followed for 36 weeks after the laparoscopy or, for those who became pregnant during that interval, for up to 20 weeks of pregnancy. In the intervention group, 50 of the 170 women became pregnant in the follow-up period, compared with only 29 of 169 in the diagnostic laparoscopy group. The corresponding rates of fecundity were 4.7 and 2.4 per 100 person-months (rate ratio, 1.9; 95% confidence interval, 1.2–3.1). The authors concluded “laparoscopic resection or ablation of minimal and mild endometriosis enhances fecundity in infertile women.”16 Interestingly, a smaller RCT from Italy with a similar study design could not confirm these results. In the study reported by Parazzini,17 the 1-year birth rate in the resection/ablation group was 10 out of 51 women (19.6%), compared with 10 out of 45 women (22.2%) in the no-treatment group. A Cochrane review on the topic published in 2002 concluded that laparoscopic surgery in the treatment of minimal and mild endometriosis may improve pregnancy success rates, but that the “relevant trials have some methodological problems and further research in this area is needed.”18 If laparoscopy is performed in a patient with unexplained infertility and minimal/mild endometriosis is identified, we recommend ablation of endometriosis. However, the current literature does not support performing a diagnostic laparoscopy in all patients with unexplained infertility.
IUI
Intrauterine insemination involves the placement of washed sperm into the uterine cavity around the time of ovulation. It can be performed in conjunction with natural ovulation timed with LH kit, ovulation induction using clomiphene citrate, or injectable gonadotropins. Few data exist on the use of IUI without ovarian hyperstimulation (OH). In 1991, Kirby and colleagues reported a RCT of 73 couples with unexplained infertility who were either randomized to IUI or timed intercourse.19 Conceptions occurred in 6 of 145 (4.1%) of the IUI cycles and 3 of 123 (2.4%) of the timed intercourse cycles. A large RCT showed that intracervical insemination alone had lower pregnancy rates per couple compared with IUI alone.20 It has been estimated that 37 cycles of IUI without additional ovarian stimulation would be needed to obtain an additional pregnancy compared with control cycles.2 A recent Cochrane review on this topic confirmed that IUI with ovulation induction increased the live birth rate compared with IUI alone.21 Therefore, IUI without additional treatment with clomiphene citrate or gonadotropins is not routinely performed in couples with unexplained infertility.
COH and IUI
Over the past decades, there has been a marked increase in the use of COH, with or without IUI, in the treatment of unexplained infertility. Both clomiphene citrate and gonadotropins have been used for COH, in combination with IUI or alone. The theoretical rationale for COH in women with a normal ovulatory assessment is that subtle ovulatory defects missed by standard testing may be overcome, and that an increased number of eggs available for fertilization may increase the likelihood of pregnancy. In a similar fashion, introducing washed sperm into the uterine cavity using IUI may increase the density of motile sperm available to ovulated oocytes, which should maximize the chance of fertilization.
Use of clomiphene citrate with timed intercourse in patients with unexplained infertility has been shown to have a small effect on pregnancy rates: combined analysis of the available evidence revealed that 40 cycles with empiric clomiphene citrate therapy were necessary to achieve 1 additional pregnancy.2 Gonadotropin therapy is superior to clomiphene citrate therapy, and both are most effective when combined with IUI. A meta-analysis of 27 studies involving 2939 cycles revealed that the pregnancy rate per cycle was 8% with gonadotropin treatment alone and 18% with gonadotropin treatment combined with IUI.14 The cumulative pregnancy rate rises with the number of attempted COH/IUI cycles; however, there is some evidence suggesting that the number of COH/IUI cycles prior to treatment with IVF should be limited to 3.22 Aboulghar and colleagues performed an observational prospective study on 594 couples with unexplained infertility to determine the optimum number of COH/IUI cycles. They found that 1 to 3 cycles of COH/IUI resulted in 182 pregnancies, with a cycle fecundity of 16.4% and a cumulative pregnancy rate (PR) of 39.2% (total of 1112 cycles with a mean of 1.9 cycles/patient).22 Up to 3 further trials of COH/IUI in 91 of these women resulted in only 9 more pregnancies, with a cycle fecundity of 5.6%, significantly lower than that in the first 3 attempts (additional 161 cycles with a mean of 1.8 cycles/patient). The cumulative PR rose to 48.5% by cycle 6, a further increase of only 9.3%. A historical comparison group with 131 patients with 3 failed cycles of COH/IUI who underwent 1 cycle of IVF at the same center resulted in 48 pregnancies, with a cycle fecundity of 36.6% per cycle, suggesting that patients should be offered IVF if they fail to conceive after 3 trials of COH and IUI.
There are several studies addressing the effect of IUI on 2 consecutive days over single IUI.23–25 Available trials on this issue are difficult to interpret because they are not restricted to patients with unexplained infertility, but also included subjects with other types of infertility, such as male factor and cervical factor. Although some studies suggested marginal benefits of double IUI over single, the most recent randomized trial concluded that among patients undergoing COH/IUI, results of single and double IUI do not statistically differ.23 Therefore, double IUI is not routinely offered.
IVF/ICSI
The most expensive, but also most successful treatment of unexplained infertility consists of the spectrum of assisted reproductive technology including IVF, with or without ICSI. IVF is the treatment of choice for unexplained infertility when the less costly, but also less successful treatment modalities outlined above have failed. In the 2006 SART data for unexplained infertility, there were 126,726 completed cycles with 40.4% live birth rate for women younger than 35 years of age and 38.9% for women 35 to 37 years of age. In addition to offering the highest success rate, IVF also explains infertility in some of these couples. In some IVF programs ICSI is performed in all couples with unexplained infertility (for an undetected fertilization problem), whereas other programs may perform ICSI in 50% of the retrieved oocytes.
Comparison of Different Treatments for Unexplained Infertility
A randomized trial comparing each of these treatment alternatives against one another and a nontreated control group has not been performed. The European Society for Human Reproduction and Embryology (ESHRE) Multicentre Trial reported in 1991 that pregnancy rates per cycle were 15.2% in gonadotropin-only cycles, 27.4% in gonadotropin and IUI cycles, and 25.7% in IVF cycles.26 The pregnancy rate for IVF cycles has since increased with availability of improved and sequential media, embryo micromanipulation, and extended embryo culture. The ASRM Practice Committee published an analysis on the appropriate roles and the cost-effectiveness of the various procedures in the management of unexplained infertility by analysis of previously published data.2,14 There is limited information on the cost-effectiveness of various treatments; however, it appears that there is a correlation between the cost of a treatment modality and its pregnancy rates. Therefore, depending on the individual couple and their particular clinical situation, COH with IUI may be attempted first, with transition to IVF/ICSI if pregnancy is not achieved in a timely manner.
A RCT of clomiphene citrate/IUI versus IVF (Fast Track and Standard Treatment [FASTT] Trial) has recently been completed. In this trial, Reindollar and colleagues27 studied 503 couples assigned to conventional infertility treatment or an accelerated track to IVF. All couples had unexplained infertility and underwent infertility treatment for the first time. Patients were randomized to receive either a conventional treatment regimen of 3 cycles of clomiphene/IUI, 3 cycles of FSH/IUI, and up to 6 cycles of IVF or to receive an accelerated treatment course of 3 cycles of clomiphene citrate/IUI and then up to 6 cycles of IVF. In the conventional arm, 247 couples underwent 646 clomiphene citrate/IUI, 439 FSH/IUI, and 261 IVF treatment cycles; in the accelerated arm, 256 couples received 642 clomiphene citrate and 357 IVF cycles. As of April 2007, 43/232 (18.5%) of the women in the conventional arm became clinically pregnant after clomiphene citrate/IUI, 43/170 (25.3%) after FSH/IUI cycle, and 71/111 (64%) after IVF. In the accelerated arm, 50/242 (20.7%) became pregnant after clomiphene citrate/IUI and 117/171 (68.4%) in an IVF cycle. The median time to pregnancy in the accelerated arm was shorter than the conventional arm. The complete study has not yet been published.
Summary and Conclusions
A thorough but time-efficient investigation of the infertile couple is required prior to a diagnosis of unexplained infertility. Couples should undergo a semen analysis, ovulation testing, assessment of ovarian reserve, and imaging to assess for tubal and uterine factors before a diagnosis of unexplained infertility is made. This workup can be completed within 1 menstrual cycle. In the couples with unexplained infertility, various treatment modalities are available, including expectant management with lifestyle changes, operative laparoscopy, COH (clomiphene citrate or gonadotropins) with IUI, and IVF (with or without ICSI). The optimal treatment strategy needs to be based on individual patient characteristics such as age, treatment efficacy, side-effect profile such as multiple pregnancy, and cost considerations.
Main Points.
Couples should undergo a semen analysis, ovulation testing, assessment of ovarian reserve, and imaging to assess for tubal and uterine factors before a diagnosis of unexplained infertility is made.
The principal treatments for unexplained infertility include expectant observation with timed intercourse and lifestyle changes, clomiphene citrate and intrauterine insemination (IUI), controlled ovarian hyperstimulation with IUI, and in vitro fertilization (IVF).
Although expectant management is associated with the lowest cost, it results in the lowest cycle fecundity rates. It may provide an option for a couple with unexplained infertility in whom the female partner is young and the problem of oocyte depletion is not an immediate concern.
The most expensive, but also most successful treatment of unexplained infertility consists of the spectrum of assisted reproductive technology including IVF, with or without intracytoplasmic sperm injection. IVF is the treatment of choice for unexplained infertility when the less costly, but also less successful treatment modalities have failed.
The optimal treatment strategy needs to be based on individual patient characteristics such as age, treatment efficacy, side-effect profile such as multiple pregnancy, and cost considerations.
References
- 1.Stephen EH, Chandra A. Updated projections of infertility in the United States: 1995–2025. Fertil Steril. 1998;70:30–34. doi: 10.1016/s0015-0282(98)00103-4. [DOI] [PubMed] [Google Scholar]
- 2.The Practice Committee of the American Society for Reproductive Medicine, authors. Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86(5 suppl):S111–S114. doi: 10.1016/j.fertnstert.2006.07.1475. [DOI] [PubMed] [Google Scholar]
- 3.The Practice Committee of the American Society for Reproductive Medicine, authors. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(5 suppl):S264–S267. doi: 10.1016/j.fertnstert.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Kolettis PN. Evaluation of the subfertile man. Am Fam Physician. 2003;67:2165–2172. [PubMed] [Google Scholar]
- 5.[Laboratory manual of the WHO for the examination of human semen and sperm-cervical mucus interaction] Ann Ist Super Sanita. 2001:1–123. I-XII. [PubMed] [Google Scholar]
- 6.Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 7.Collins JA, So Y, Wilson EH, et al. The postcoital test as a predictor of pregnancy among 355 infertile couples. Fertil Steril. 1984;41:703–708. [PubMed] [Google Scholar]
- 8.Glatstein IZ, Best CL, Palumbo A, et al. The reproducibility of the postcoital test: a prospective study. Obstet Gynecol. 1995;85:396–400. doi: 10.1016/0029-7844(94)00390-Y. [DOI] [PubMed] [Google Scholar]
- 9.Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol. 2003;102:317–318. doi: 10.1016/s0029-7844(03)00527-1. [DOI] [PubMed] [Google Scholar]
- 10.Coutifaris C, Myers ER, Guzick DS, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–1272. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 11.Exacoustos C, Zupi E, Carusotti C, et al. Hysterosalpingo-contrast sonography compared with hysterosalpingography and laparoscopic dye pertubation to evaluate tubal patency. J Am Assoc Gynecol Laparosc. 2003;10:367–372. doi: 10.1016/s1074-3804(05)60264-2. [DOI] [PubMed] [Google Scholar]
- 12.Roma Dalfó A, Ubeda B, Ubeda A, et al. Diagnostic value of hysterosalpingography in the detection of intrauterine abnormalities: a comparison with hysteroscopy. AJR Am J Roentgenol. 2004;183:1405–1409. doi: 10.2214/ajr.183.5.1831405. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri RL. The initial fertility consultation: recommendations concerning cigarette smoking, body mass index, and alcohol and caffeine consumption. Am J Obstet Gynecol. 2001;185:1168–1173. doi: 10.1067/mob.2001.117667. [DOI] [PubMed] [Google Scholar]
- 14.Guzick DS, Sullivan MW, Adamson GD, et al. Efficacy of treatment for unexplained infertility. Fertil Steril. 1998;70:207–213. doi: 10.1016/s0015-0282(98)00177-0. [DOI] [PubMed] [Google Scholar]
- 15.Eijkemans MJ, Lintsen AM, Hunault CC, et al. Pregnancy chances on an IVF/ICSI waiting list: a national prospective cohort study. Hum Reprod. 2008;23:1627–1632. doi: 10.1093/humrep/den132. [DOI] [PubMed] [Google Scholar]
- 16.Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337:217–222. doi: 10.1056/NEJM199707243370401. [DOI] [PubMed] [Google Scholar]
- 17.Parazzini F Gruppo Italiano per lo Studio dell’Endometriosi, authors. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod. 1999;14:1332–1334. doi: 10.1093/humrep/14.5.1332. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson TZ, Barlow DH, Koninckx PR, et al. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD001398. CD001398. [DOI] [PubMed] [Google Scholar]
- 19.Kirby CA, Flaherty SP, Godfrey BM, et al. A prospective trial of intrauterine insemination of motile spermatozoa versus timed intercourse. Fertil Steril. 1991;56:102–107. [PubMed] [Google Scholar]
- 20.Guzick DS, Carson SA, Coutifaris C, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. National Cooperative Reproductive Medicine Network. N Engl J Med. 1999;340:177–183. doi: 10.1056/NEJM199901213400302. [DOI] [PubMed] [Google Scholar]
- 21.Verhulst SM, Cohlen BJ, Hughes E, et al. Intrauterine insemination for unexplained subfertility. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD001838.pub3. CD001838. [DOI] [PubMed] [Google Scholar]
- 22.Aboulghar M, Mansour R, Serour G, et al. Controlled ovarian hyperstimulation and intrauterine insemination for treatment of unexplained infertility should be limited to a maximum of three trials. Fertil Steril. 2001;75:88–91. doi: 10.1016/s0015-0282(00)01641-1. [DOI] [PubMed] [Google Scholar]
- 23.Alborzi S, Motazedian S, Parsanezhad ME, Jannati S. Comparison of the effectiveness of single intrauterine insemination (IUI) versus double IUI per cycle in infertile patients. Fertil Steril. 2003;80:595–599. doi: 10.1016/s0015-0282(03)00980-4. [DOI] [PubMed] [Google Scholar]
- 24.Ransom MX, Blotner MB, Bohrer M, et al. Does increasing frequency of intrauterine insemination improve pregnancy rates significantly during superovulation cycles? Fertil Steril. 1994;61:303–307. doi: 10.1016/s0015-0282(16)56522-4. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg KM, Johnson JV, Olive DL, et al. A prospective, randomized trial comparing two different intrauterine insemination regimens in controlled ovarian hyperstimulation cycles. Fertil Steril. 1992;57:357–361. doi: 10.1016/s0015-0282(16)54845-6. [DOI] [PubMed] [Google Scholar]
- 26.Crosignani PG, Walters DE, Soliani A. The ESHRE multicentre trial on the treatment of unexplained infertility: a preliminary report. European Society of Human Reproduction and Embryology. Hum Reprod. 1991;6:953–958. doi: 10.1093/oxfordjournals.humrep.a137468. [DOI] [PubMed] [Google Scholar]
- 27.Reindollar RH, Regan MM, Neumann PJ, et al. A randomized controlled trial of 503 couples assigned to conventional infertility treatment or an accelerated track to IVF: preliminary results of the Fast Track and Standard Treatment (FASTT) Trial. Fertil Steril. 2007;88(suppl 1):S41. [Google Scholar]


