Abstract
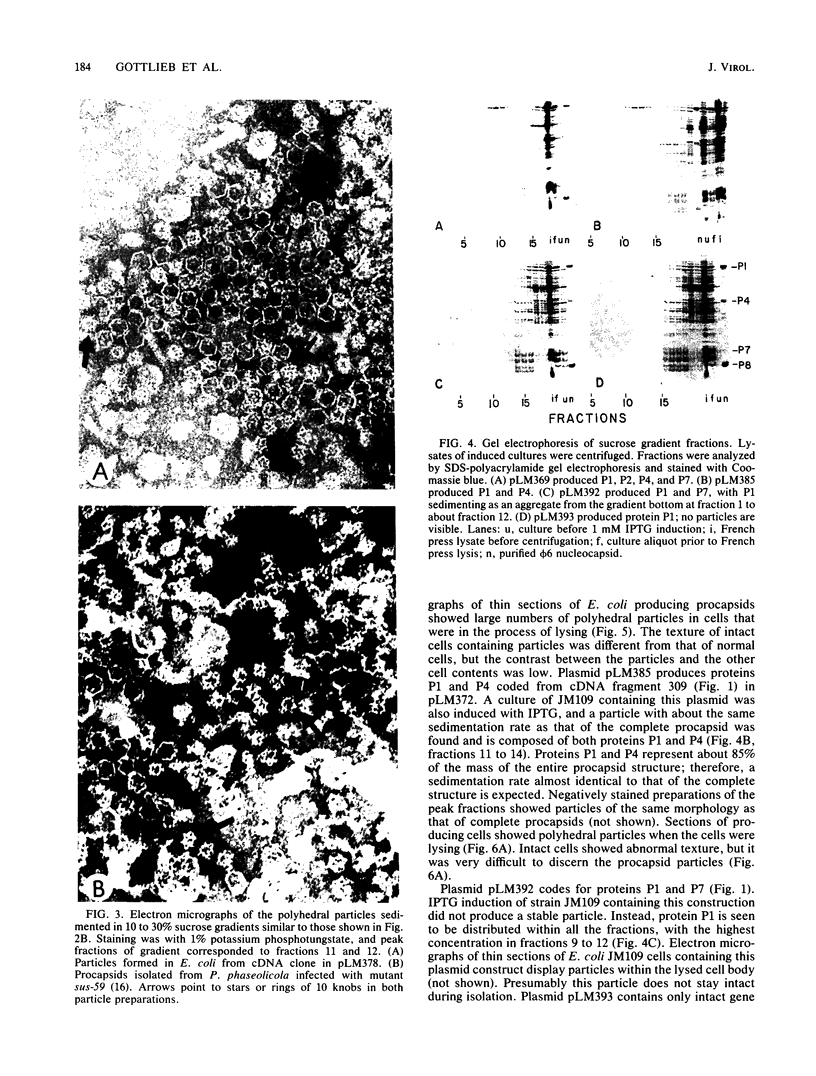
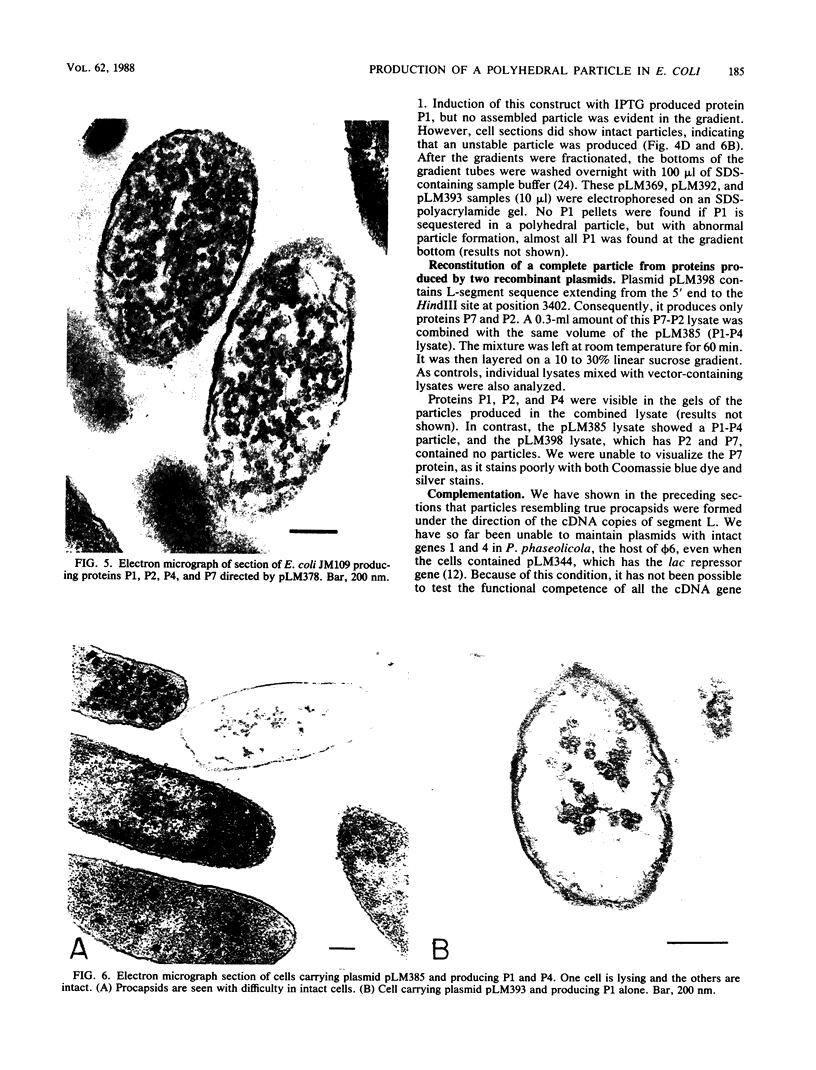
A polyhedral particle that resembles in composition and structure the procapsid of bacteriophage phi 6 was produced in Escherichia coli containing cDNA copies of the entire large genomic segment inserted into expression vector plasmids under the control of lac or tac promoters. The particles were composed of proteins P1, P2, P4, and P7 in the same stoichiometry as in the intact virion. In electron micrographs of negatively stained samples, the particles appeared as hexagons, stars, or rings of 10 knobs, which are characteristic of the five-, three-, and twofold axes of symmetry characteristic of phi 6 procapsids. Stable particles were also produced from cDNA deletions that produce only P1 and P4. Other cDNA deletions producing P1 and P7 and P1 alone resulted in unstable particles which could only be visualized in electron micrographs of thin sections of E. coli transformed by the recombinant plasmids. Our results indicate that the assembly of the phi procapsid is independent of other phage proteins and of normal phage RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Aoyama A., Hamatake R. K., Hayashi M. Morphogenesis of phi X174: in vitro synthesis of infectious phage from purified viral components. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7285–7289. doi: 10.1073/pnas.78.12.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Mindich L. Electron microscopy of cells infected with nonsense mutants of bacteriophage phi 6. Virology. 1980 Nov;107(1):222–228. doi: 10.1016/0042-6822(80)90287-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Camerini-Otero R. D., Pusey P. N., Koppel D. E., Schaefer D. W., Franklin R. M. Intensity fluctuation spectroscopy of laser light scattered by solutions of spherical viruses: R17, Q beta, BSV, PM2, and T7. II. Diffusion coefficients, molecular weights, solvation, and particle dimensions. Biochemistry. 1974 Feb 26;13(5):960–970. doi: 10.1021/bi00702a021. [DOI] [PubMed] [Google Scholar]
- Day L. A., Mindich L. The molecular weight of bacteriophage phi 6 and its nucleocapsid. Virology. 1980 Jun;103(2):376–385. doi: 10.1016/0042-6822(80)90196-8. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etten J. V., Lane L., Gonzalez C., Partridge J., Vidaver A. Comparative properties of bacteriophage phi6 and phi6 nucleocapsid. J Virol. 1976 May;18(2):652–658. doi: 10.1128/jvi.18.2.652-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis N. T., Lang D. The dodecahedral framework of the bacteriophage phi 6 nucleocapsid is composed of protein P1. J Virol. 1987 Aug;61(8):2621–2623. doi: 10.1128/jvi.61.8.2621-2623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. F., Mindich L. The isolation of new mutants of bacteriophage phi 6. Virology. 1979 Aug;97(1):164–170. doi: 10.1016/0042-6822(79)90382-9. [DOI] [PubMed] [Google Scholar]
- McGraw T., Mindich L., Frangione B. Nucleotide sequence of the small double-stranded RNA segment of bacteriophage phi 6: novel mechanism of natural translational control. J Virol. 1986 Apr;58(1):142–151. doi: 10.1128/jvi.58.1.142-151.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Lebkowski J. S., Greisen K. S., Calos M. P. Specificity of mutations induced in transfected DNA by mammalian cells. EMBO J. 1984 Dec 20;3(13):3117–3121. doi: 10.1002/j.1460-2075.1984.tb02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Bamford D., Goldthwaite C., Laverty M., Mackenzie G. Isolation of nonsense mutants of lipid-containing bacteriophage PRD1. J Virol. 1982 Dec;44(3):1013–1020. doi: 10.1128/jvi.44.3.1013-1020.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Cohen J., Weisburd M. Isolation of nonsense suppressor mutants in Pseudomonas. J Bacteriol. 1976 Apr;126(1):177–182. doi: 10.1128/jb.126.1.177-182.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Davidoff-Abelson R. The characterization of a 120 S particle formed during phi 6 infection. Virology. 1980 Jun;103(2):386–391. doi: 10.1016/0042-6822(80)90197-x. [DOI] [PubMed] [Google Scholar]
- Mindich L., MacKenzie G., Strassman J., McGraw T., Metzger S., Romantschuk M., Bamford D. cDNA cloning of portions of the bacteriophage phi 6 genome. J Bacteriol. 1985 Jun;162(3):992–999. doi: 10.1128/jb.162.3.992-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen V. M., Bamford D. H. The nucleocapsid of the lipid-containing double-stranded RNA bacteriophage phi 6 contains a protein skeleton consisting of a single polypeptide species. J Virol. 1987 Aug;61(8):2362–2367. doi: 10.1128/jvi.61.8.2362-2367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R., Ewen M. E., Brusslan J., Pagratis N. Generation of cDNA clones of the bacteriophage phi 6 segmented dsRNA genome: characterization and expression of L segment clones. Virology. 1986 Dec;155(2):402–417. doi: 10.1016/0042-6822(86)90203-5. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Tzagoloff A., Levine D., Mindich L. Proteins of bacteriophage phi6. J Virol. 1975 Sep;16(3):685–695. doi: 10.1128/jvi.16.3.685-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Semancik J. S. RNA polymerase activity associated with bacteriophage phi 6. J Virol. 1973 Sep;12(3):464–471. doi: 10.1128/jvi.12.3.464-471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Lang D. Electron microscopy of bacteriophage phi 6 nucleocapsid: three-dimensional image analysis. J Virol. 1984 Aug;51(2):484–488. doi: 10.1128/jvi.51.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]